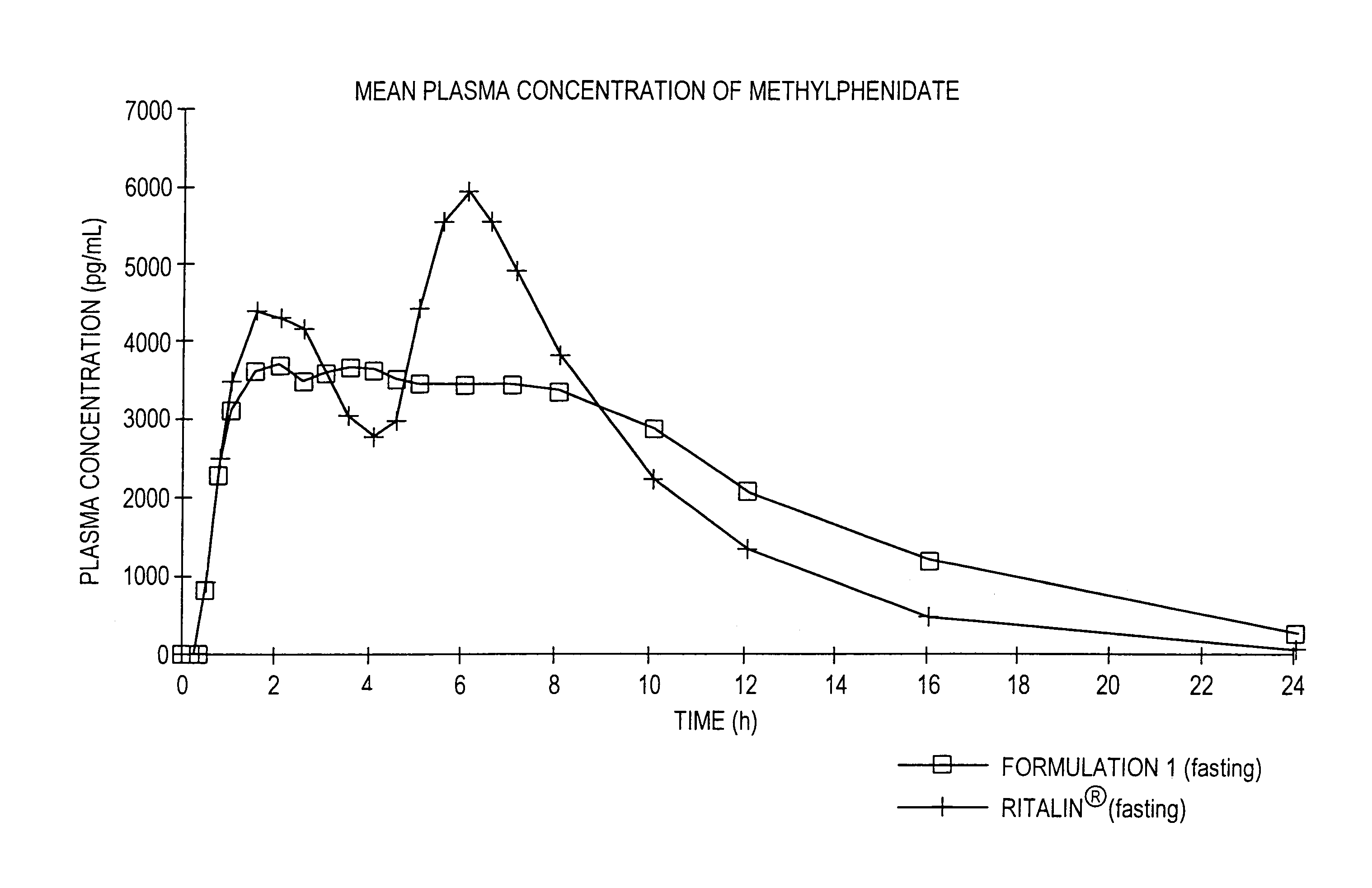Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Dose interval" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dose interval definition, dose interval meaning | English dictionary. dose. n a quantity that expresses the probability that exposure to ionizing radiation will cause biological effects. It is usually obtained by multiplying the dose by the quality factor of the radiation, but other factors may be considered.
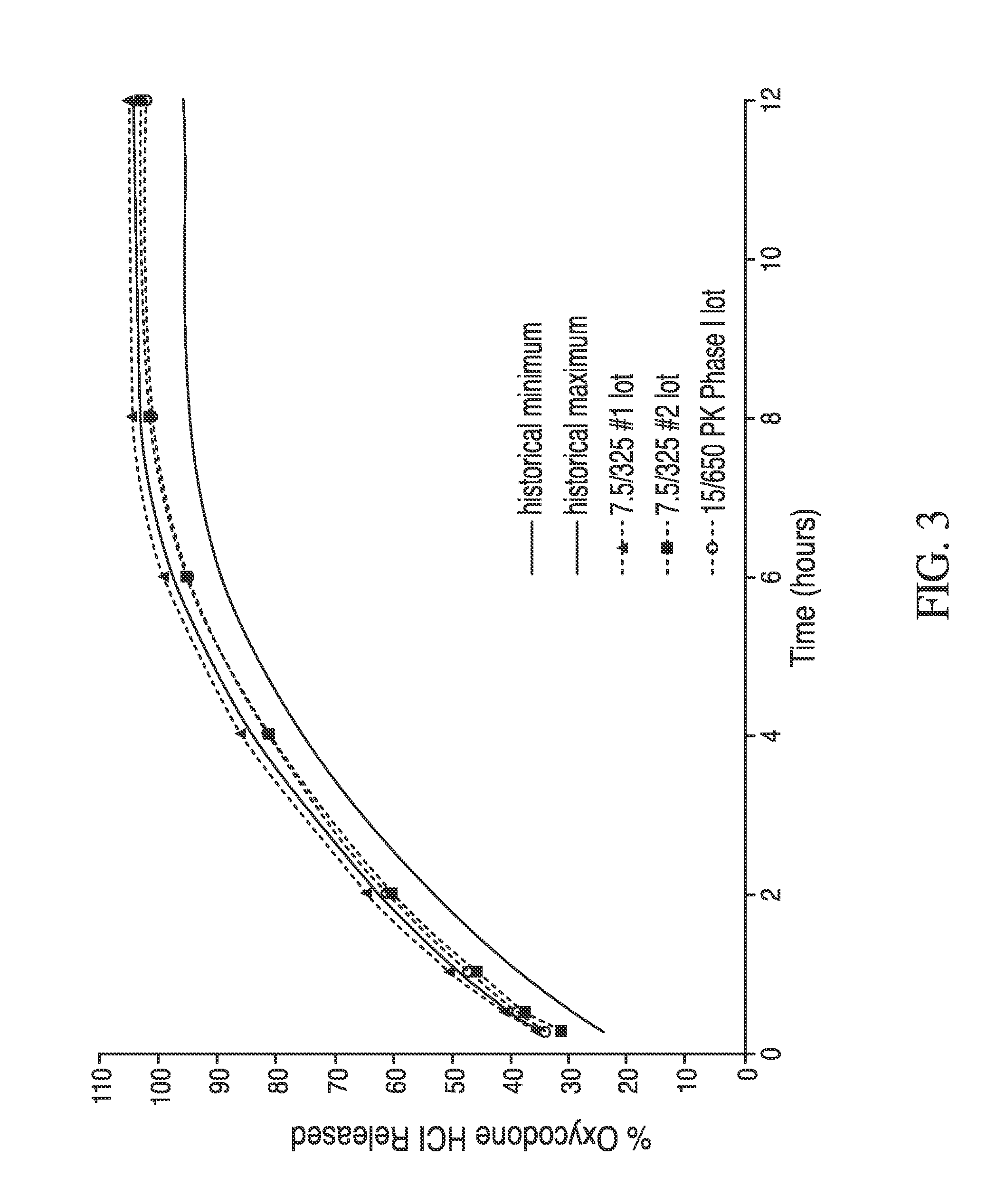
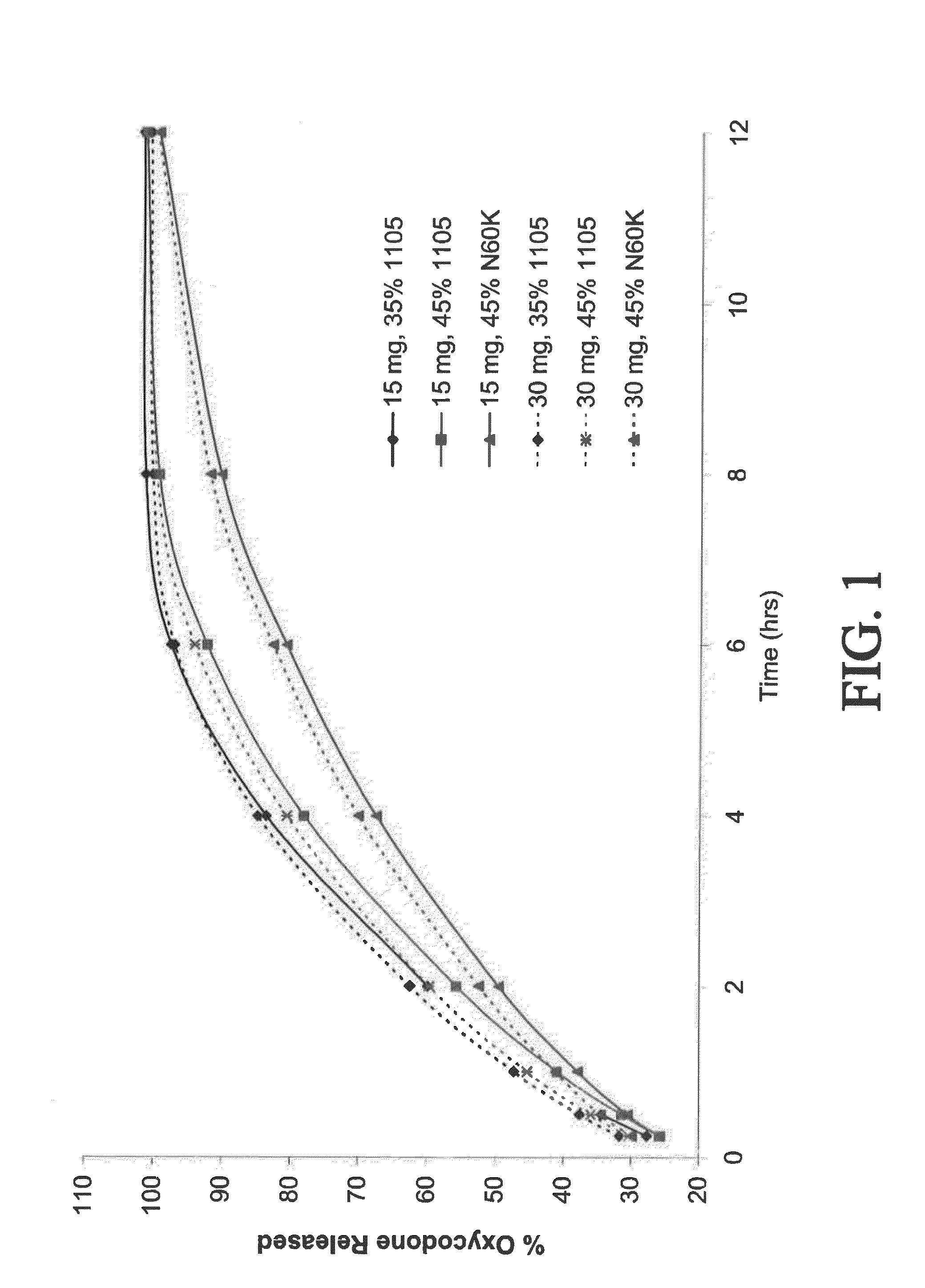
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
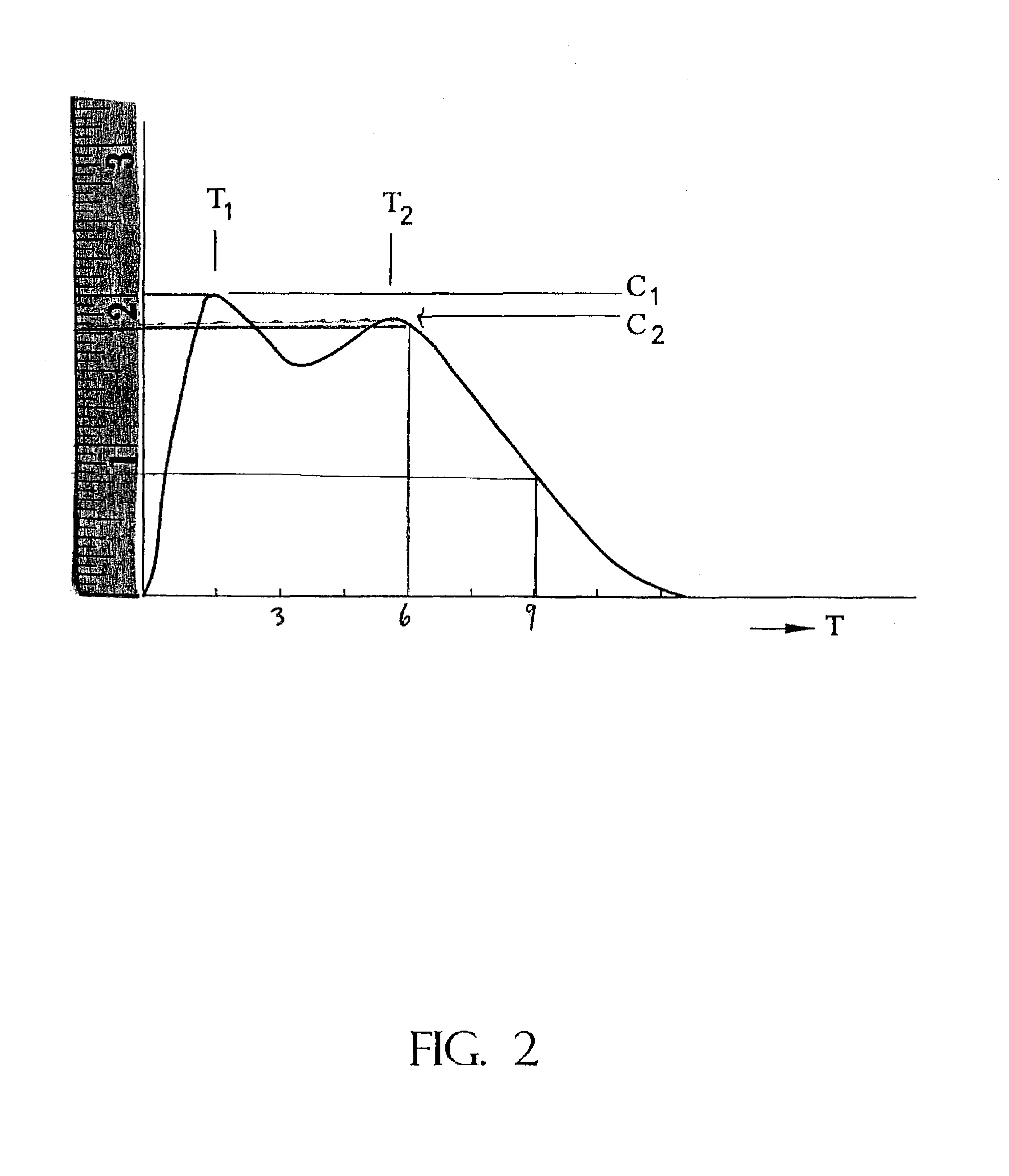
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
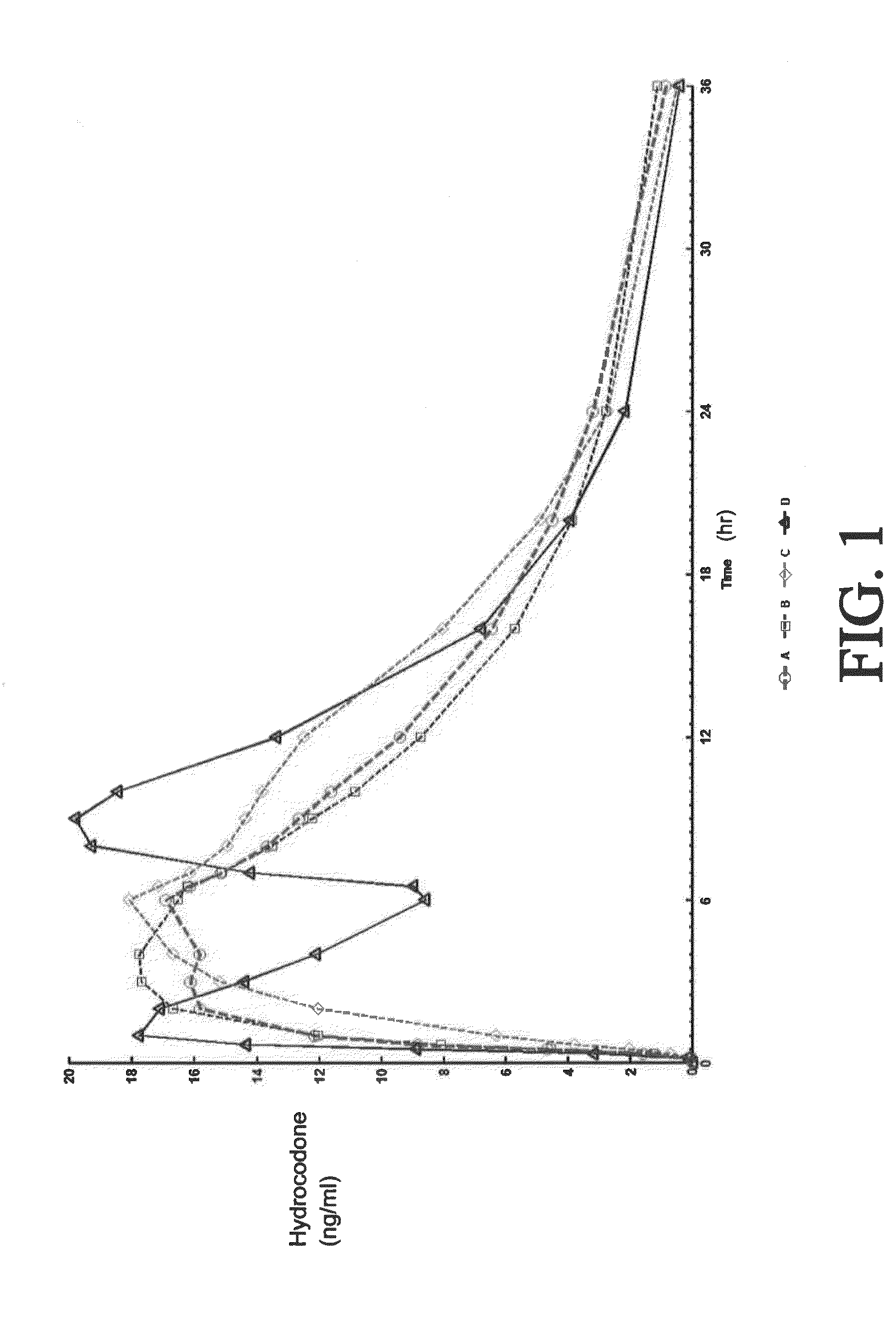
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
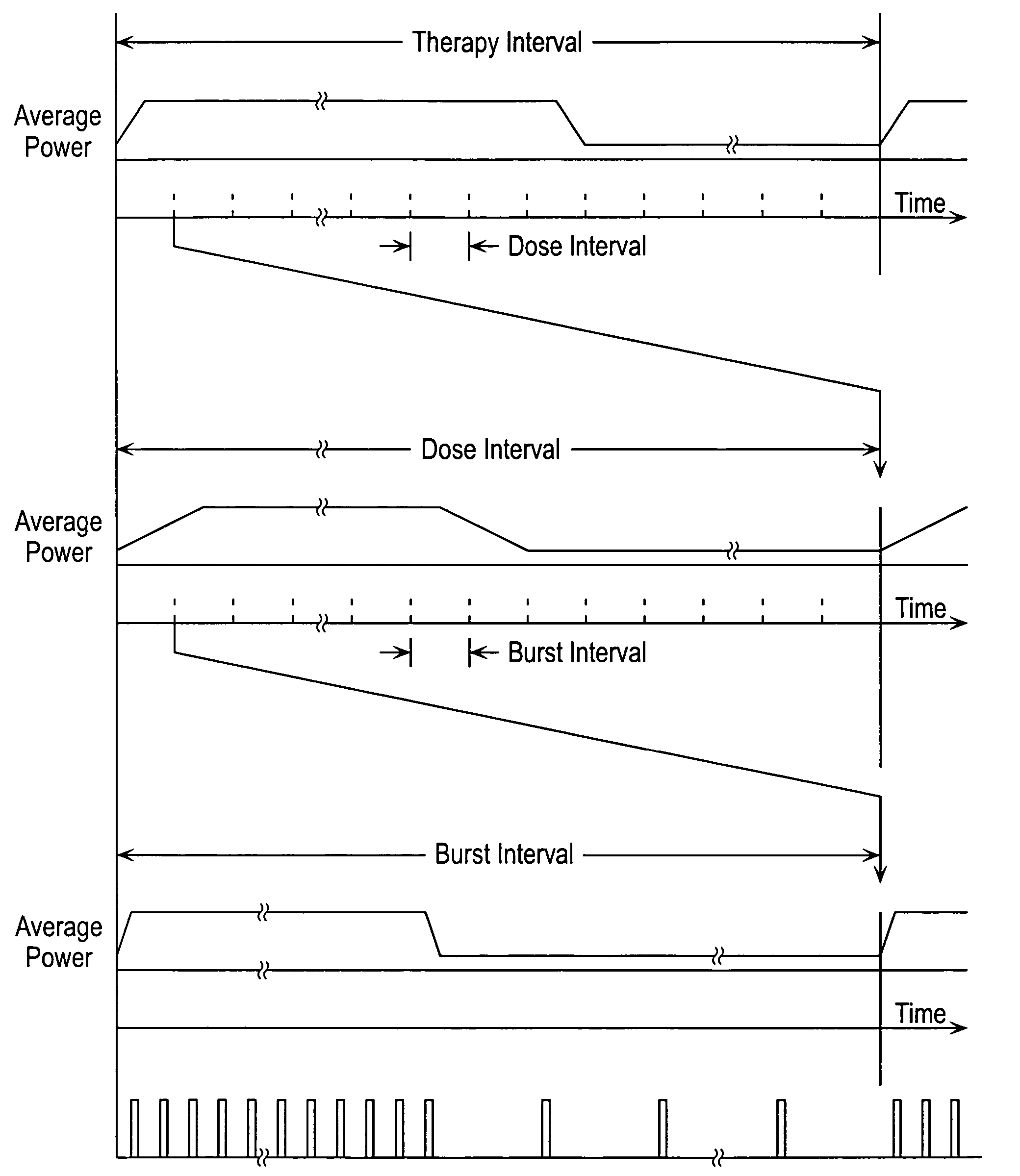
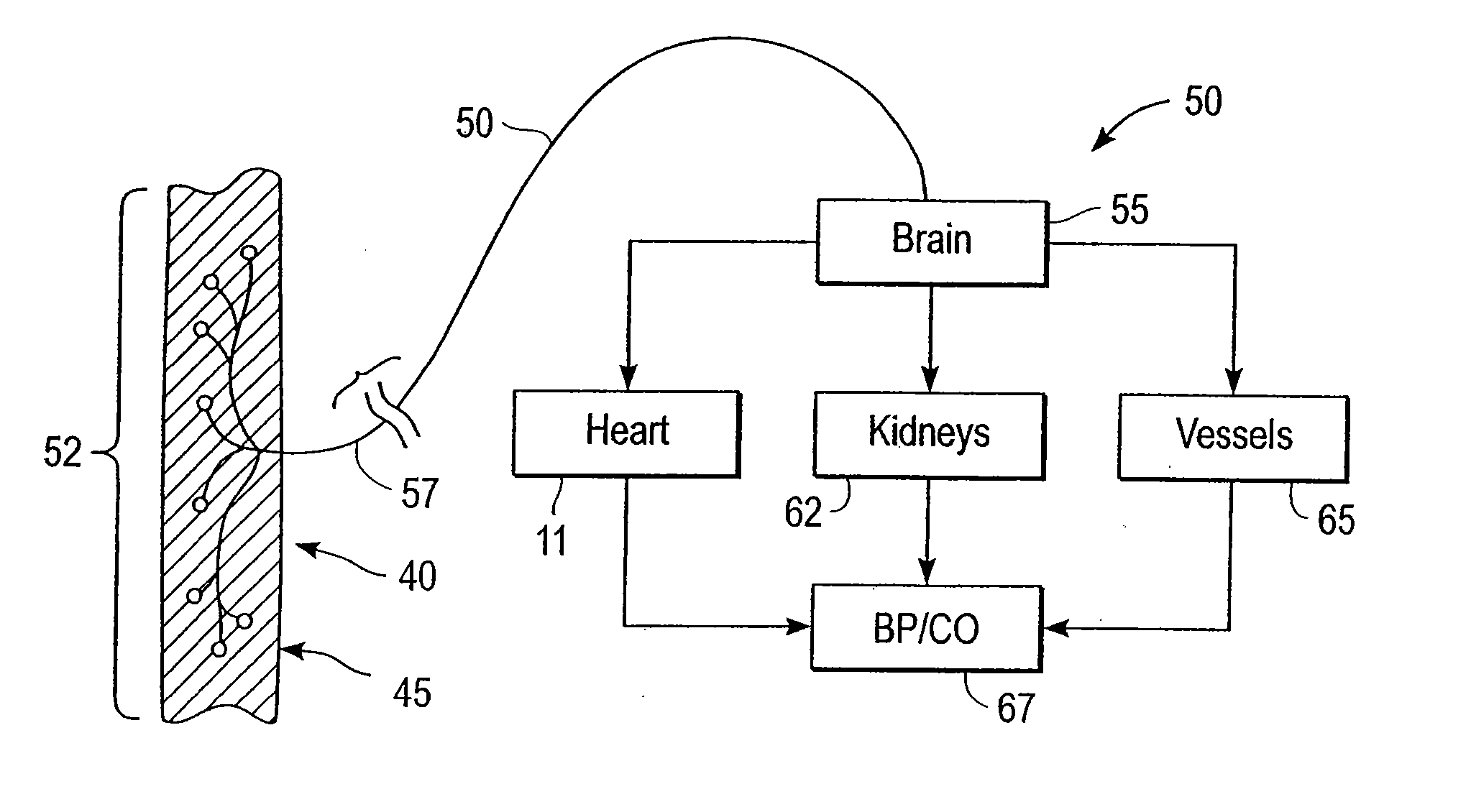
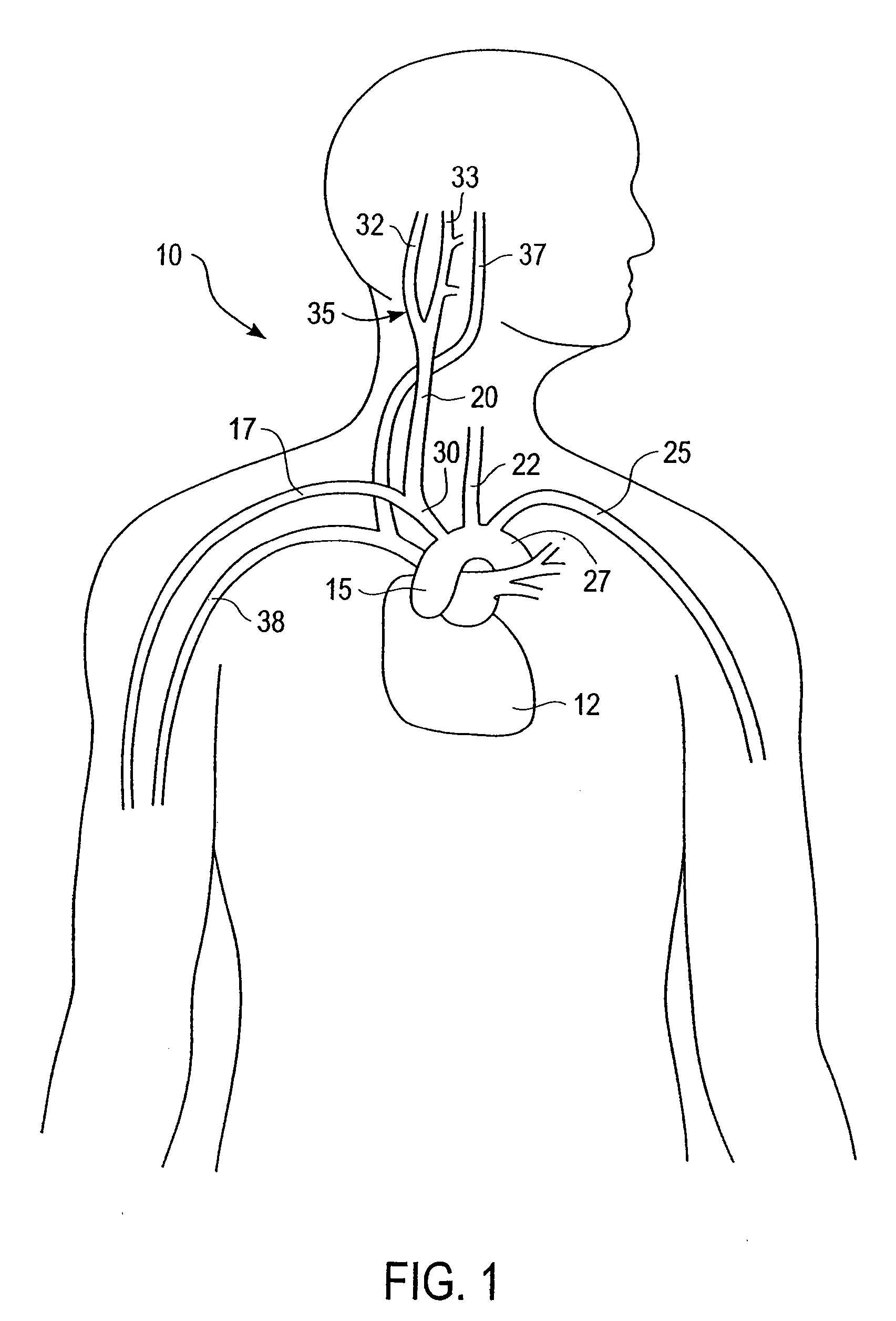
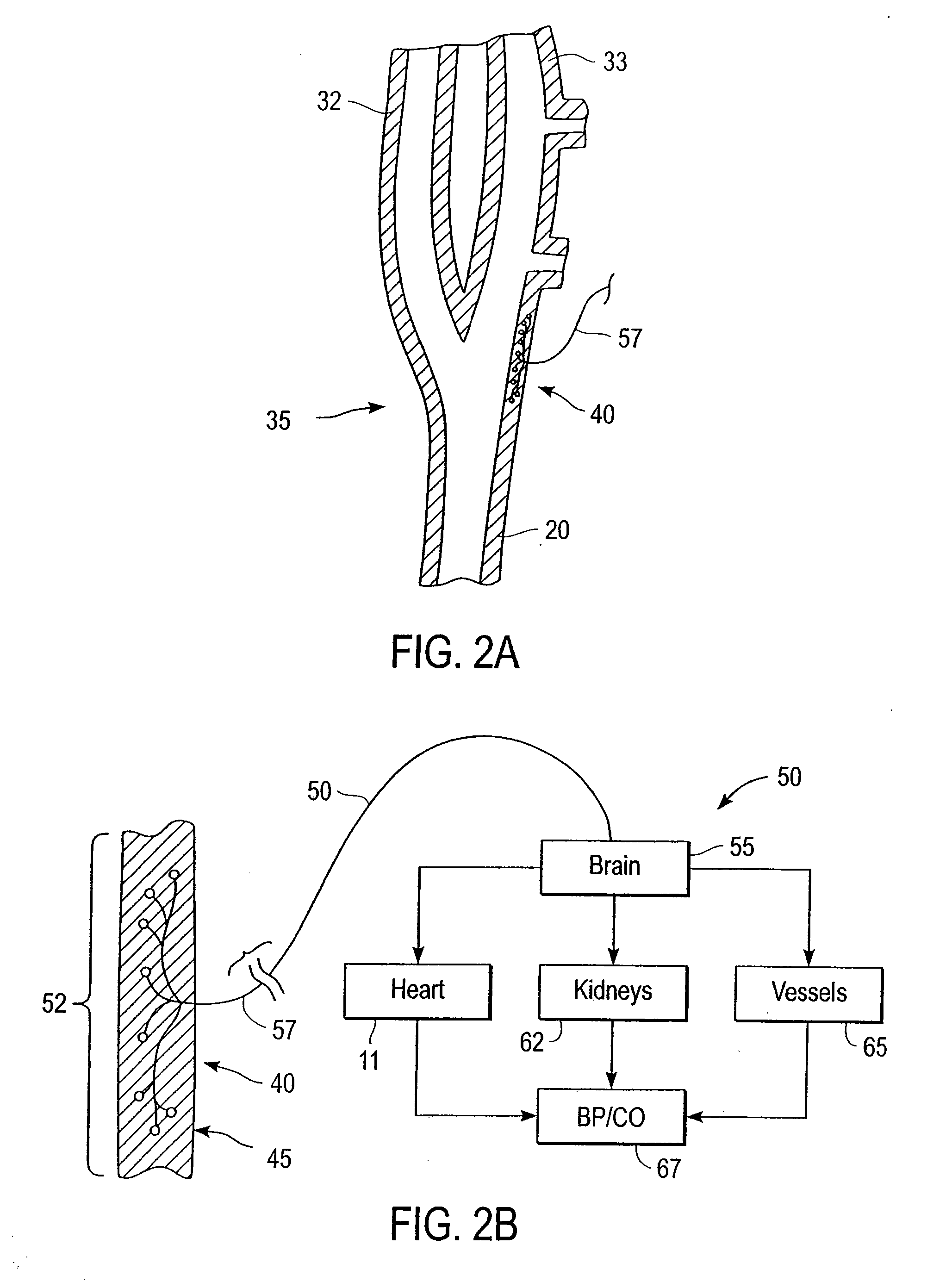
Stimulus regimens for cardiovascular reflex control
InactiveUS7623926B2Modulation of the reflex control of the patient's circulationModulate activityStentsSpinal electrodesElectricityControl signal
Baroreflex activation is achieved by providing suitable control signals to a baroreflex activation device. A method comprises establishing a therapy interval (possibly on the order of minutes to hours, or possibly of indefinite duration), within the therapy interval, establishing a plurality of dose intervals, and generating an electrical output signal. The electrical output signal has a time dependence such that the average electrical power applied to the baroreflex activation device differs between first and second portions of at least some dose intervals. Another method comprises establishing a series of therapy interval portions, during at least some therapy intervals, establishing a plurality of burst intervals (perhaps having durations commensurate with an interval between heartbeats), and generating an electrical output signal. The electrical output signal has a time dependence such that the average electrical power applied to the baroreflex activation device differs between first and second portions of the therapy intervals and also differs between first and second portions of at least some burst intervals.
Owner:CVRX
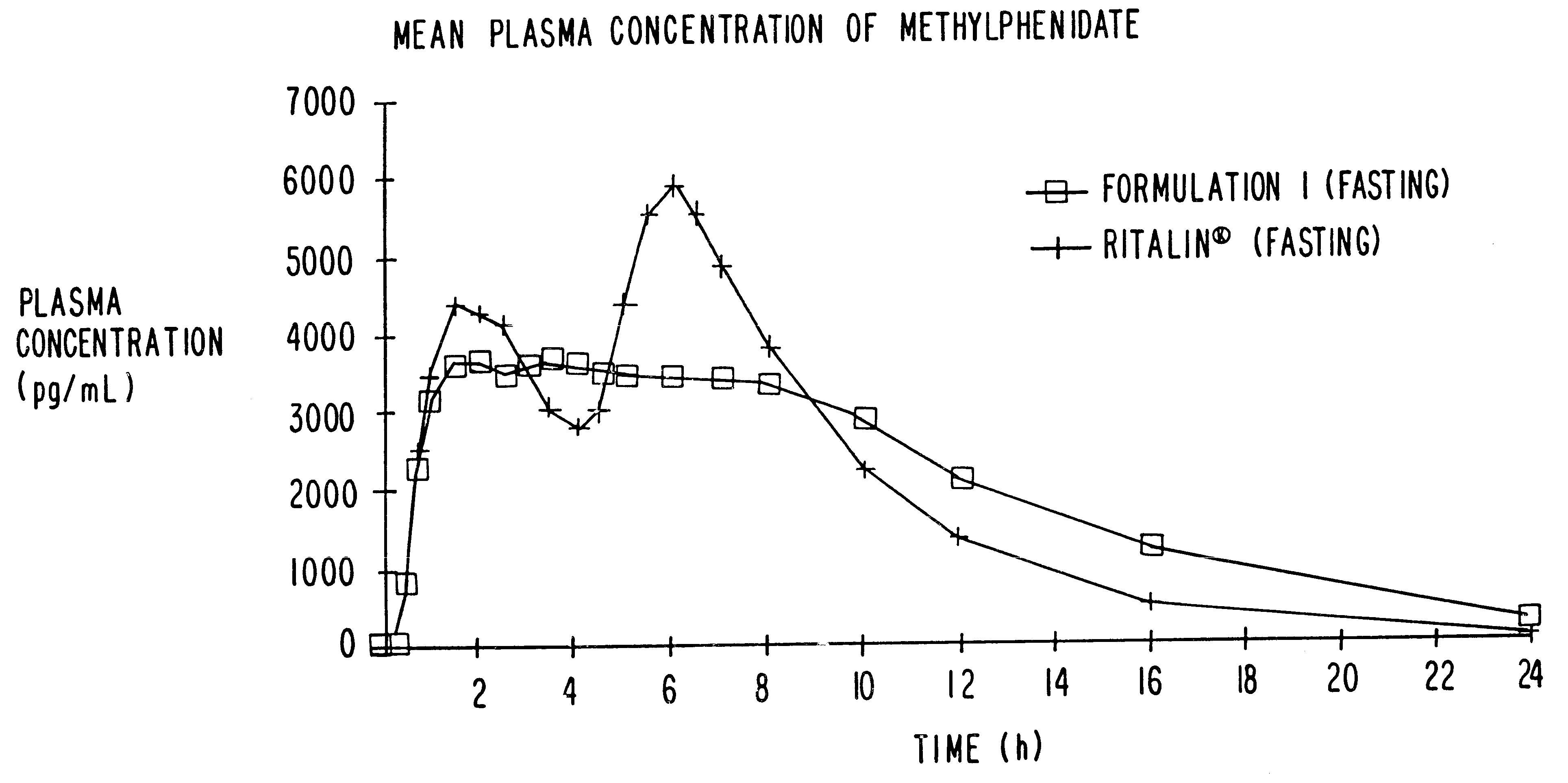
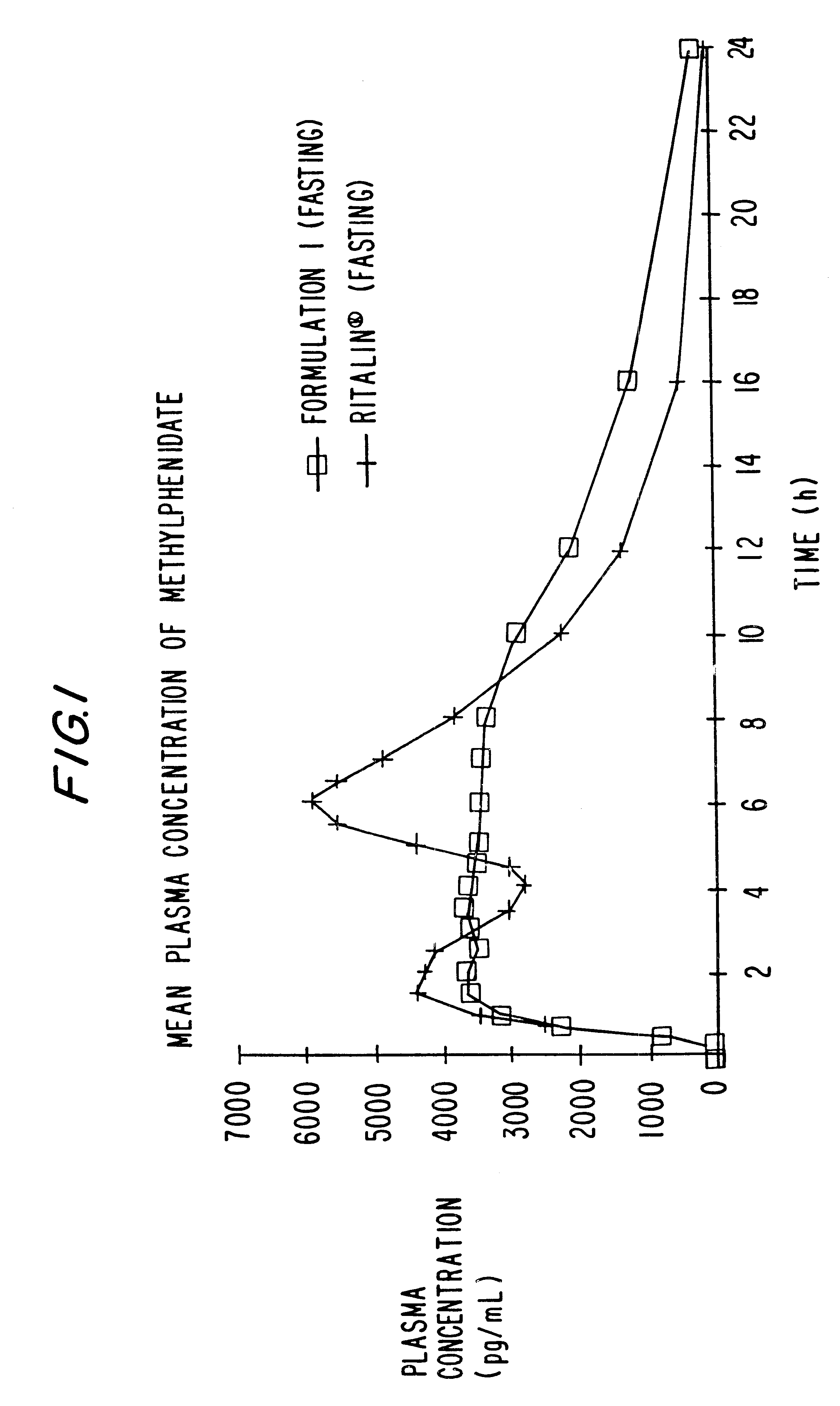
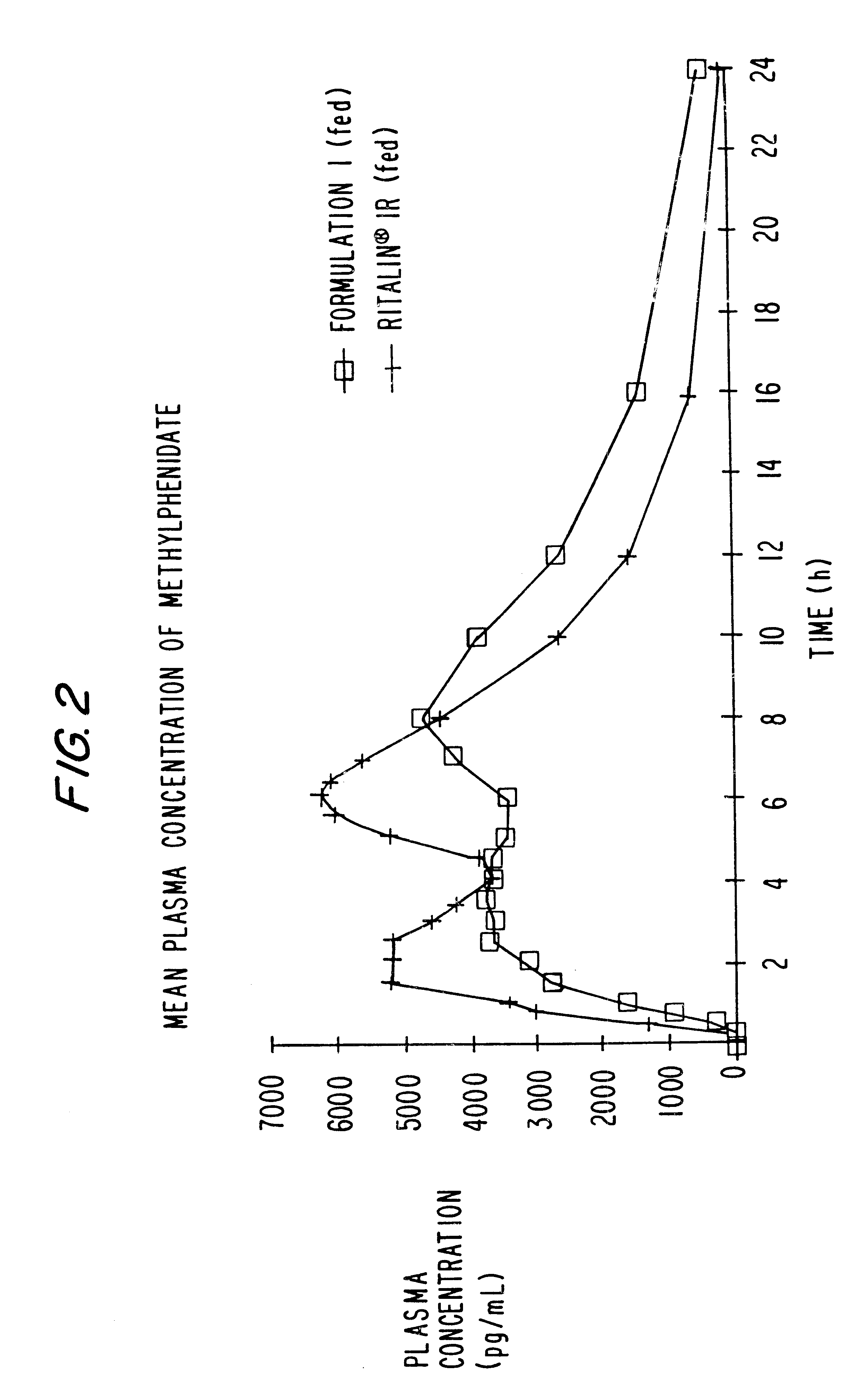
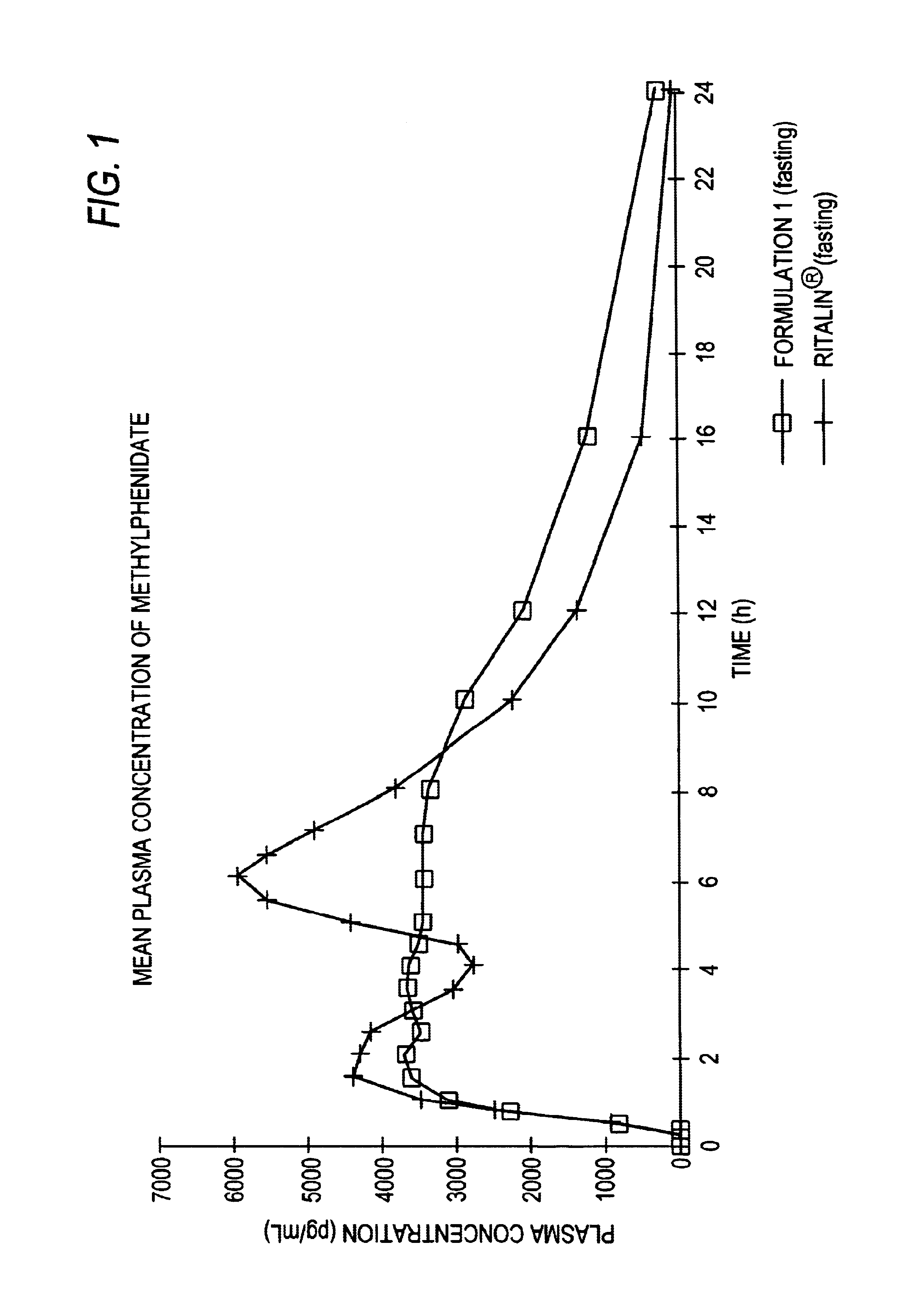
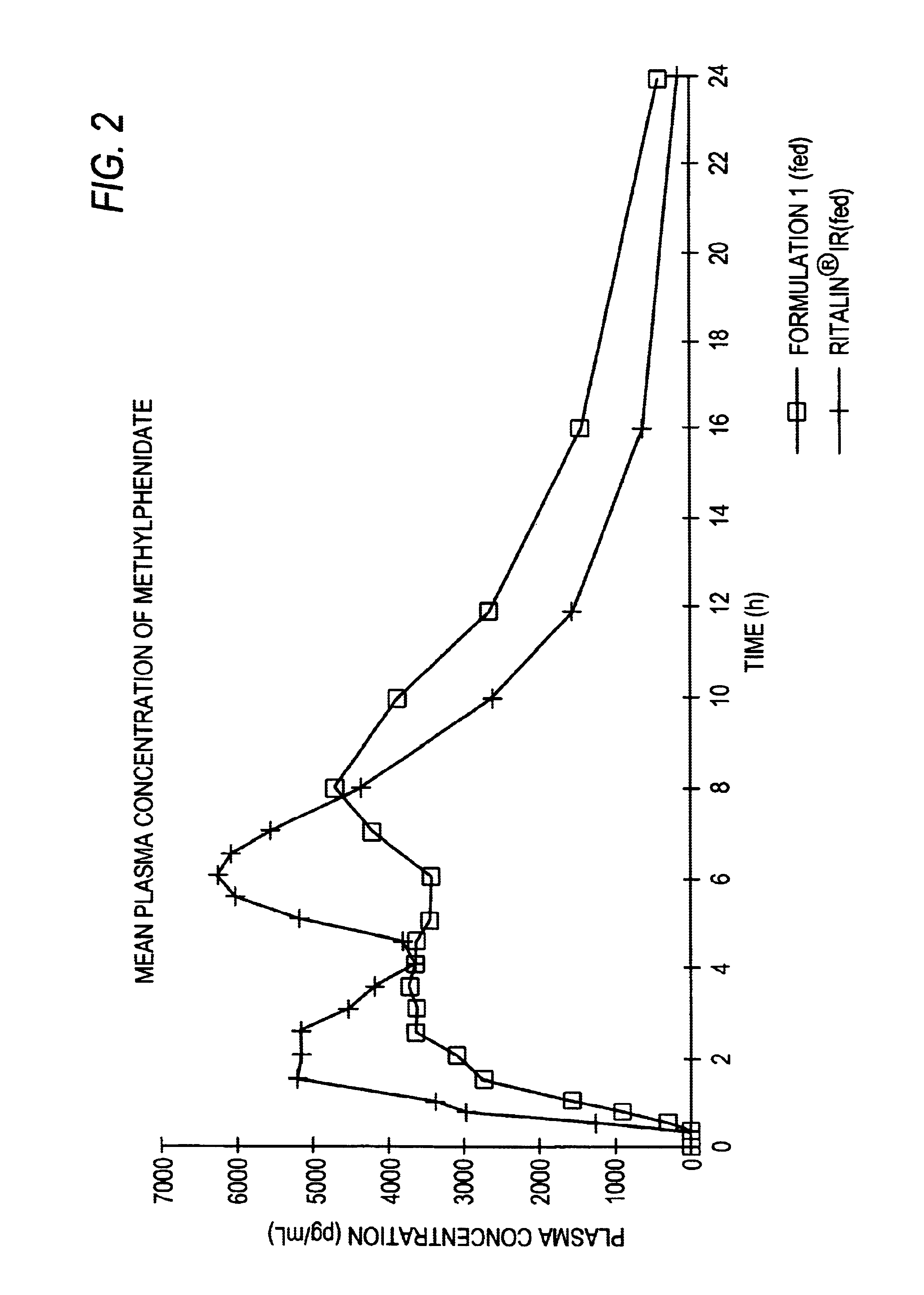
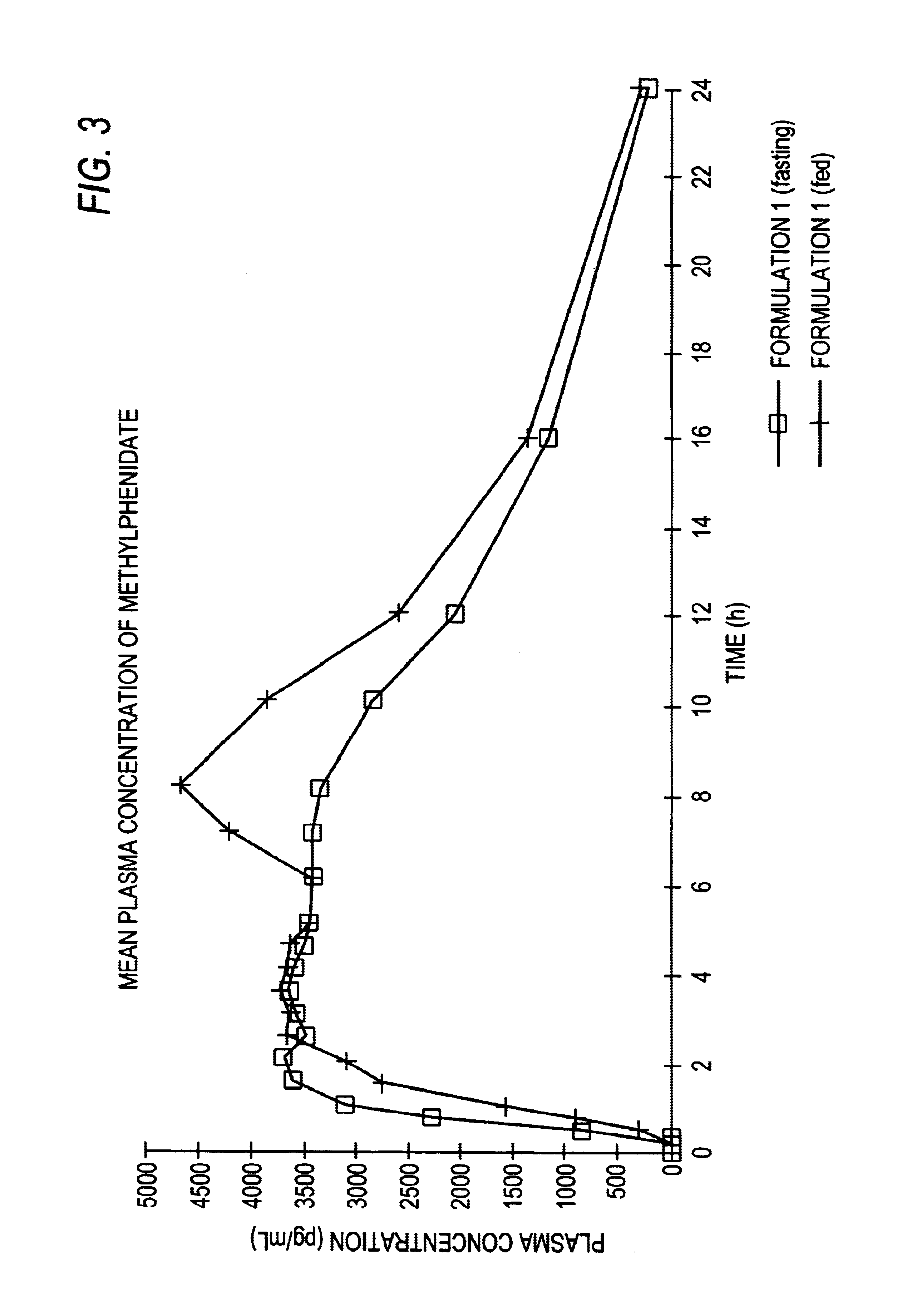
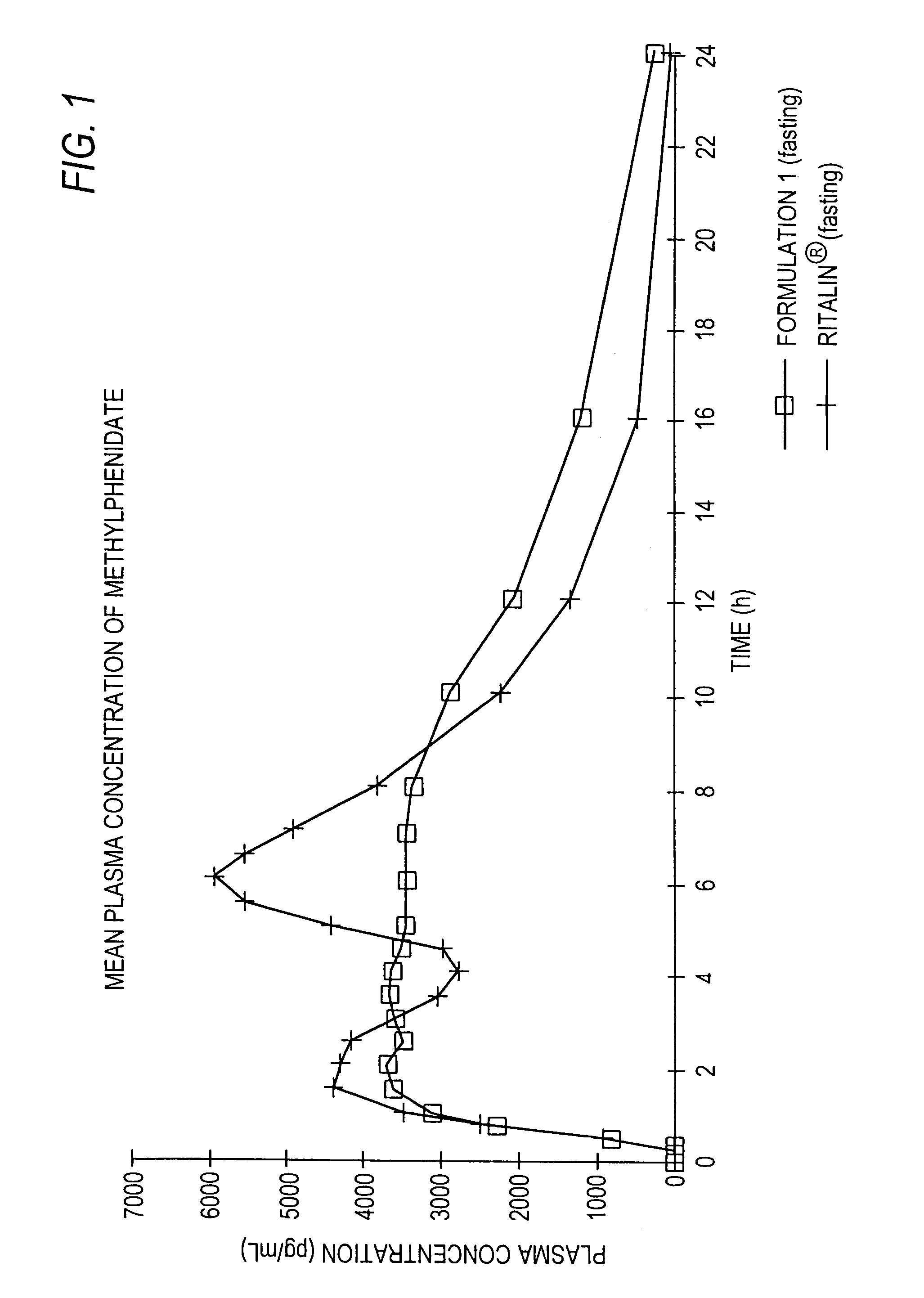
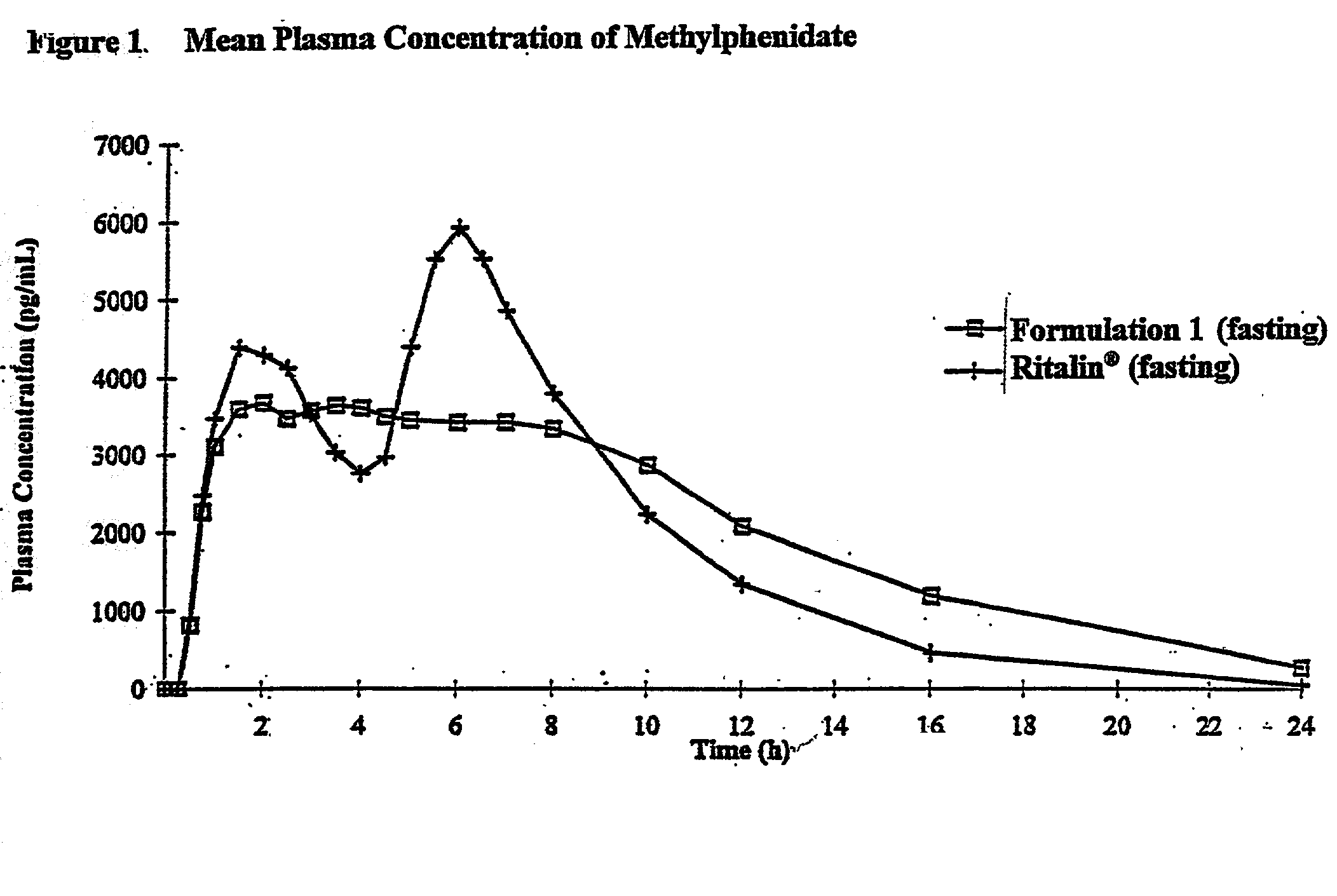
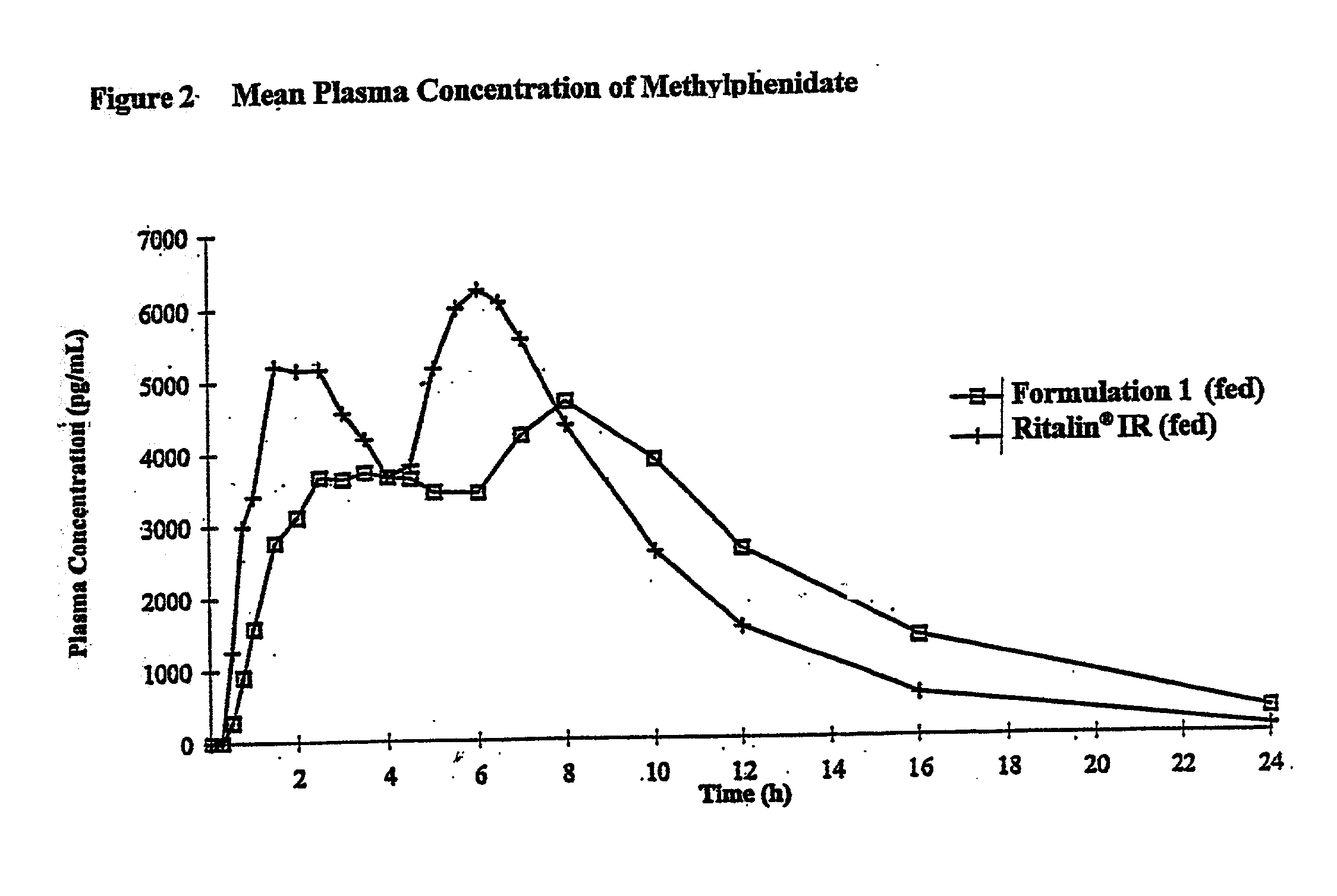
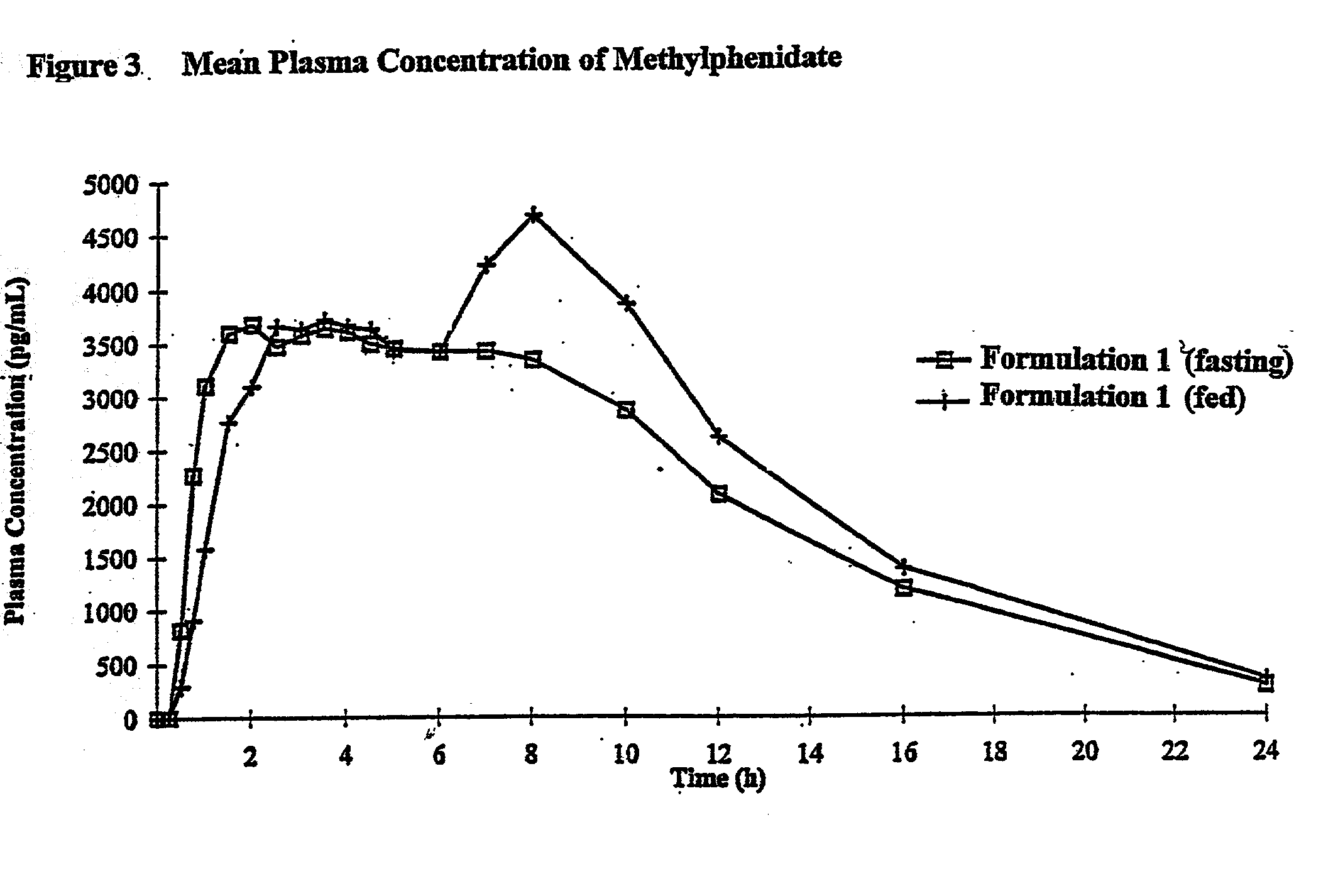
Controlled/modified release oral methylphenidate formulations
InactiveUS6673367B1Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Controlled/modified release oral methylphenidate formulations
InactiveUS7083808B2Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Stimulus regimens for cardiovascular reflex control
InactiveUS20070049989A1Modulate activityModulation of the reflex control of the patient's circulationStentsSpinal electrodesElectricityControl signal
Baroreflex activation is achieved by providing suitable control signals to a baroreflex activation device. A method comprises establishing a therapy interval (possibly on the order of minutes to hours, or possibly of indefinite duration), within the therapy interval, establishing a plurality of dose intervals, and generating an electrical output signal. The electrical output signal has a time dependence such that the average electrical power applied to the baroreflex activation device differs between first and second portions of at least some dose intervals. Another method comprises establishing a series of therapy interval portions, during at least some therapy intervals, establishing a plurality of burst intervals (perhaps having durations commensurate with an interval between heartbeats), and generating an electrical output signal. The electrical output signal has a time dependence such that the average electrical power applied to the baroreflex activation device differs between first and second portions of the therapy intervals and also differs between first and second portions of at least some burst intervals.
Owner:CVRX
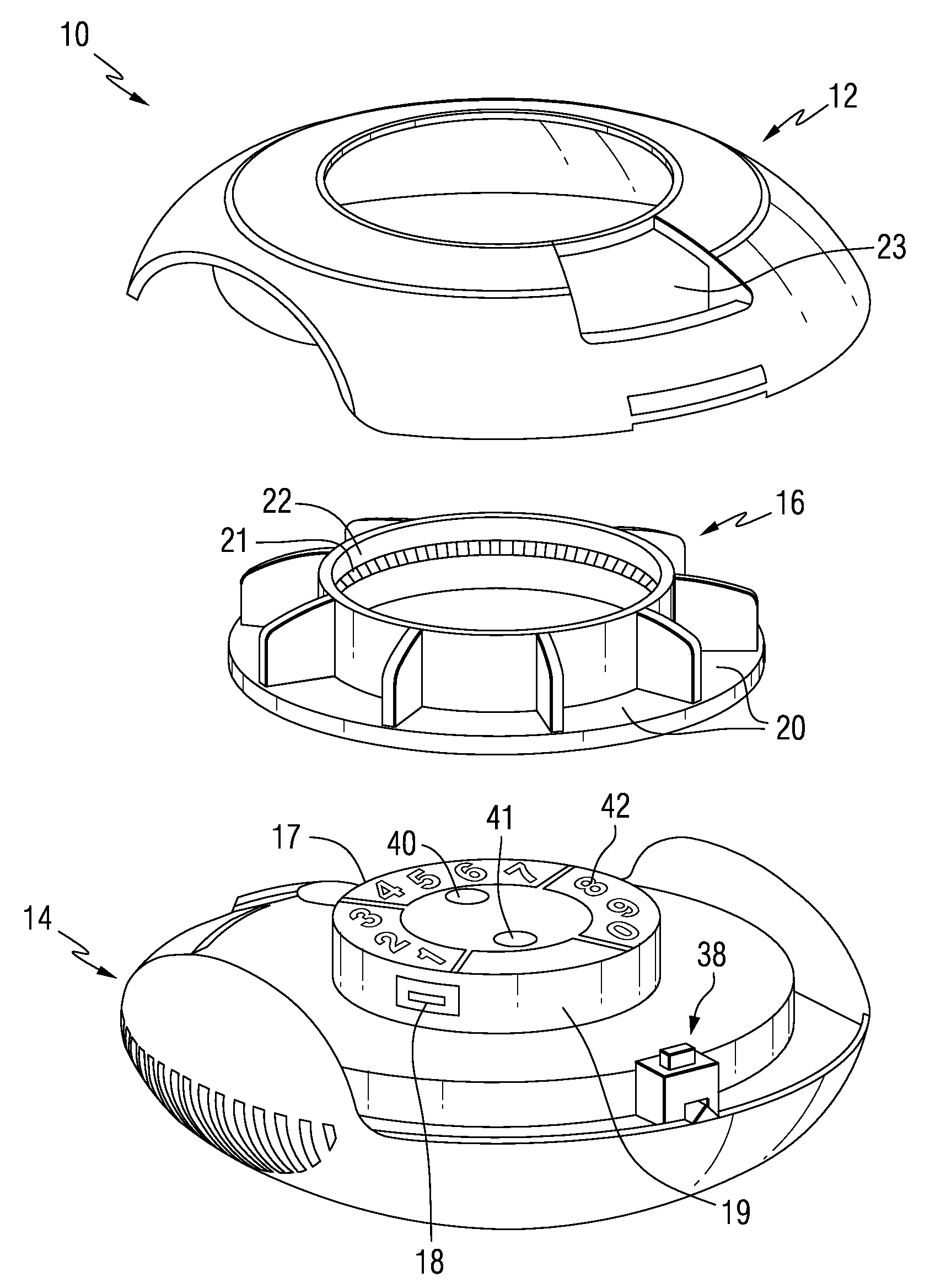
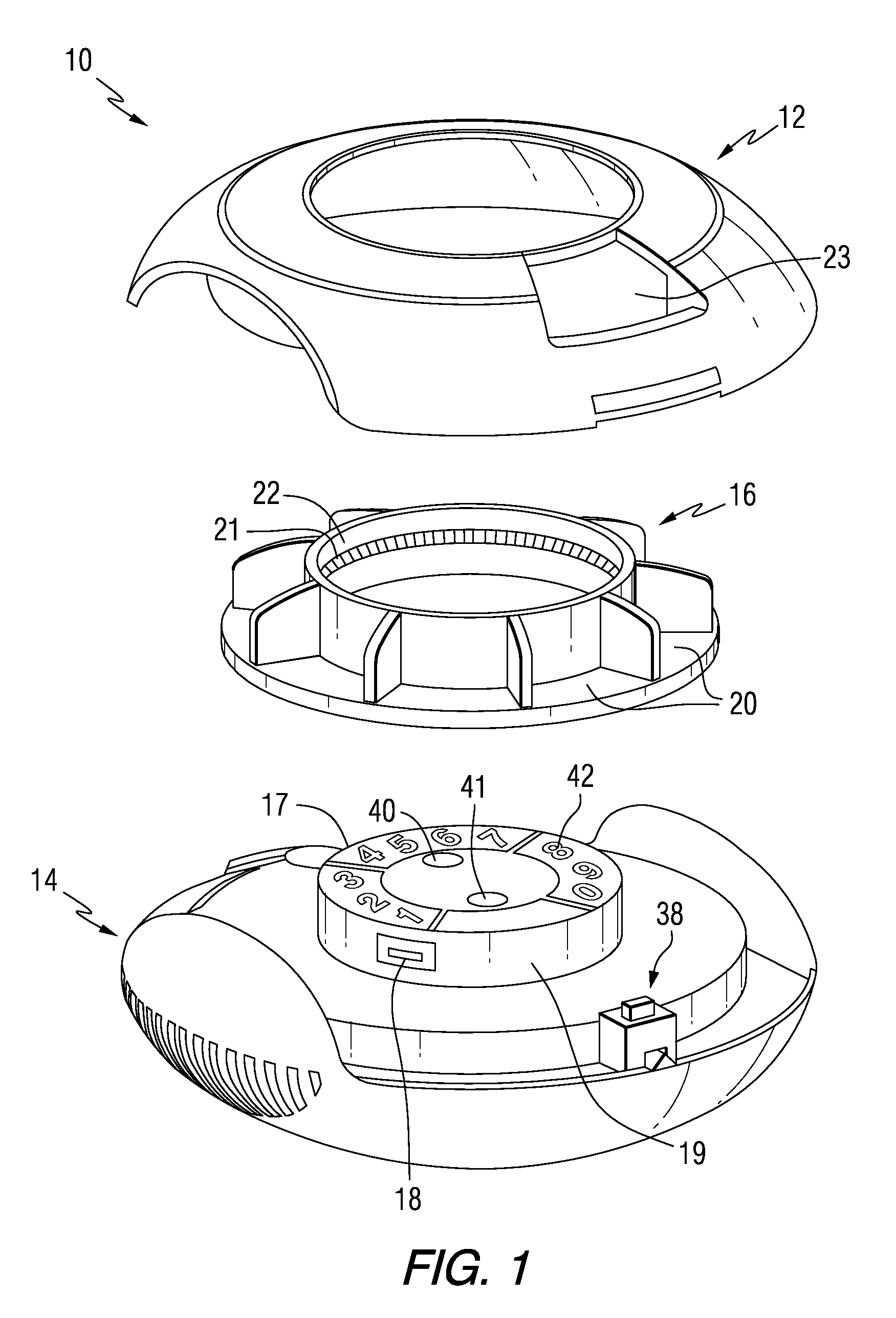
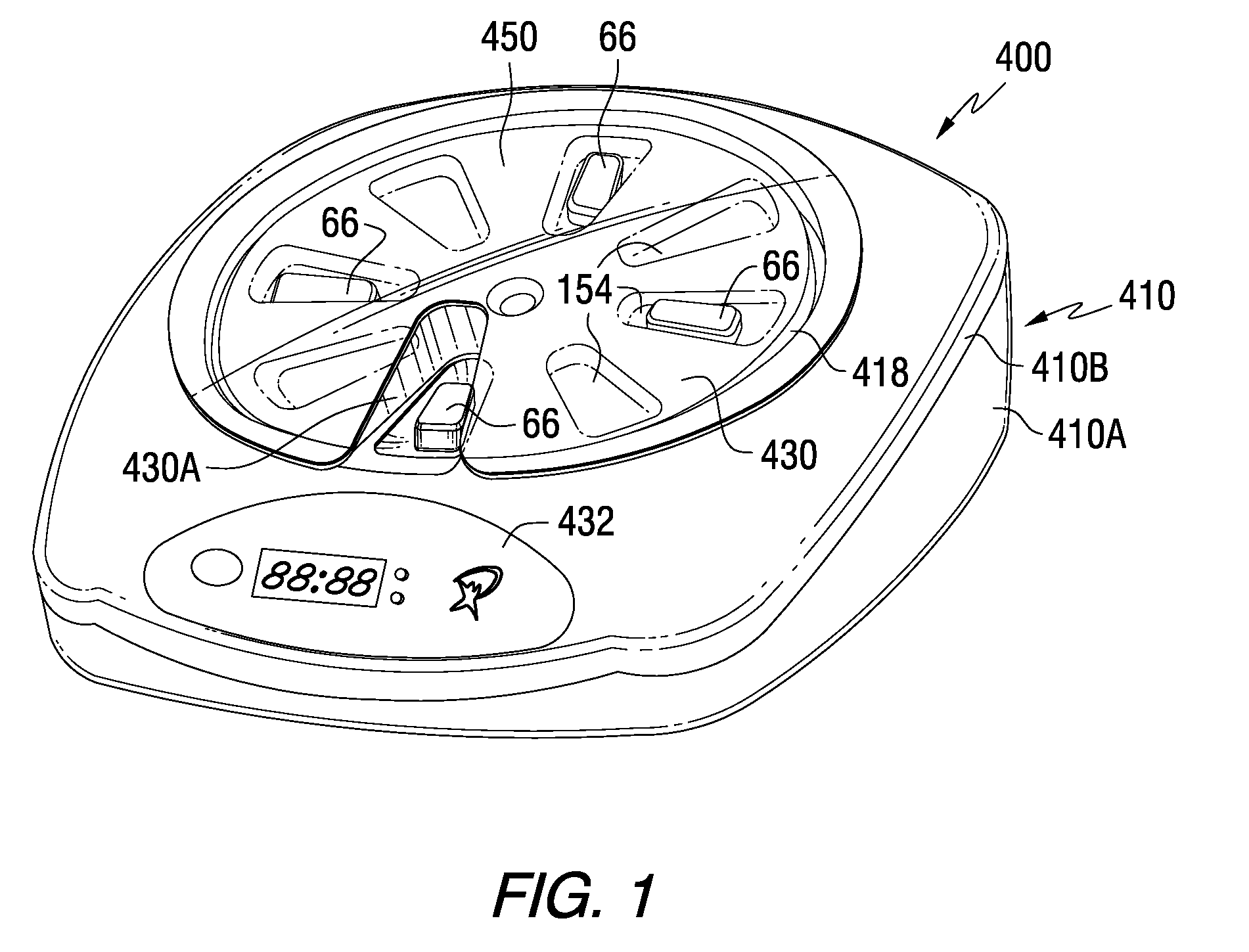
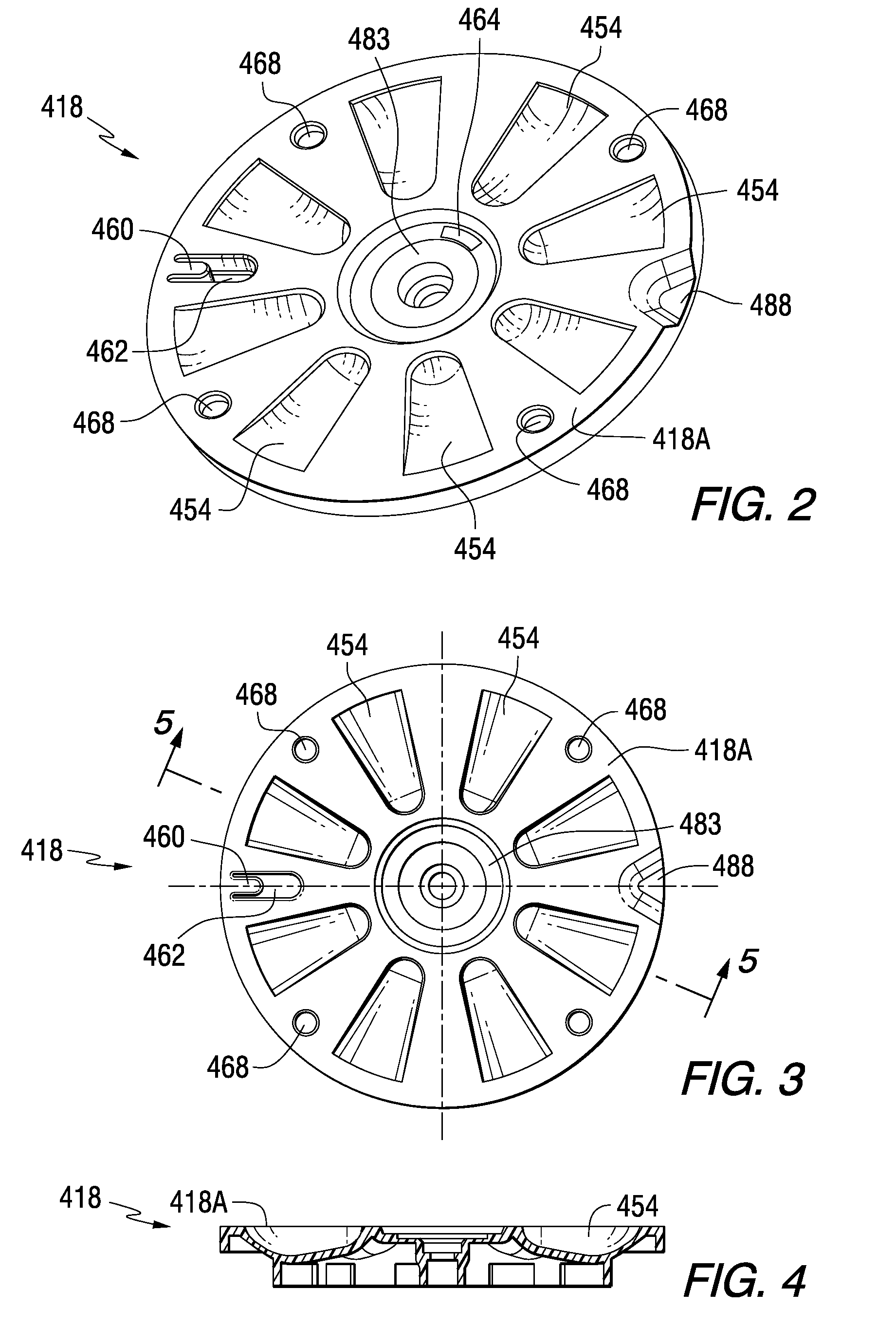
Patient Controlled Timed Medication Dispenser
InactiveUS20100305750A1Small article dispensingElectric signal transmission systemsMedication DispenserMedication dose
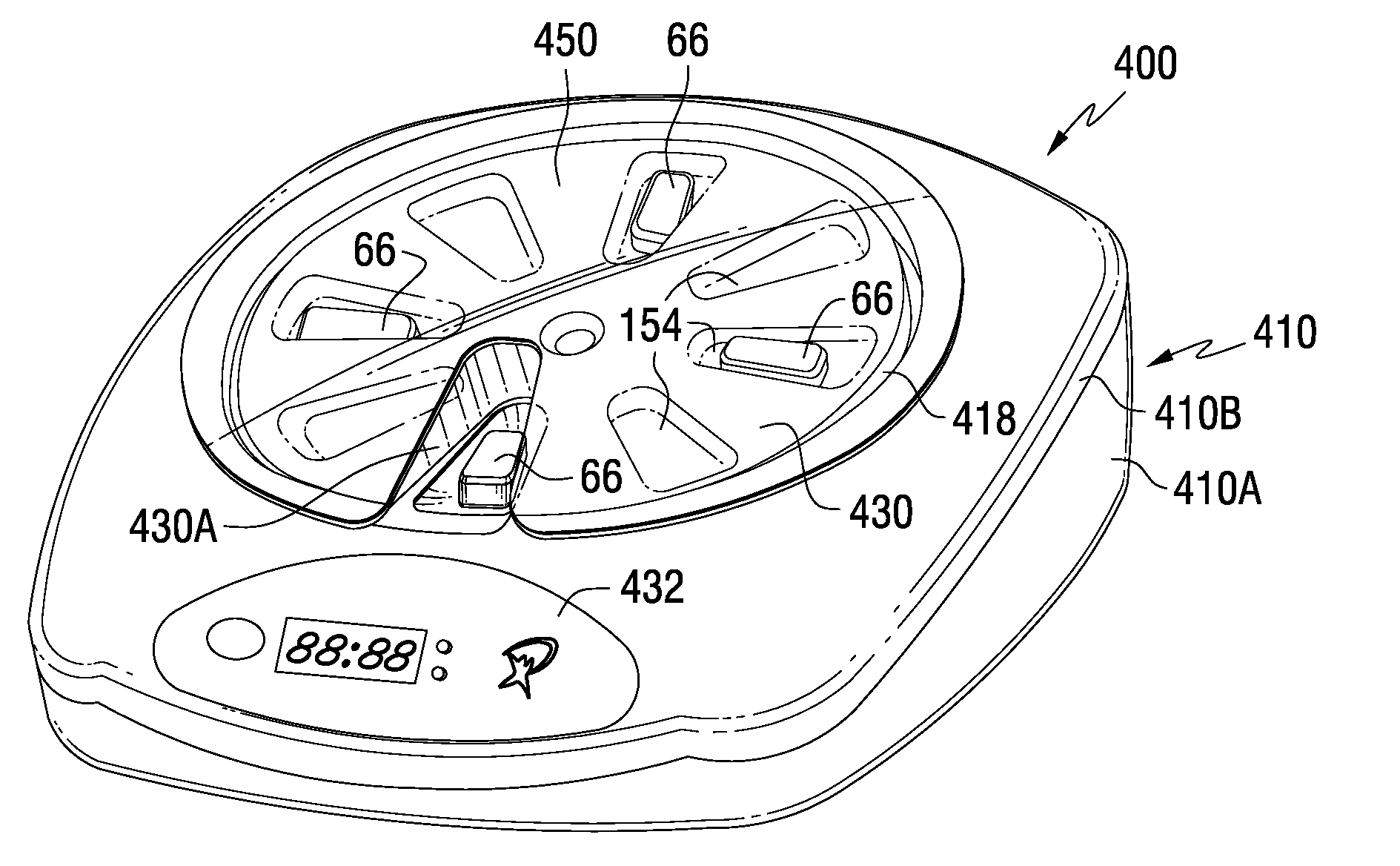
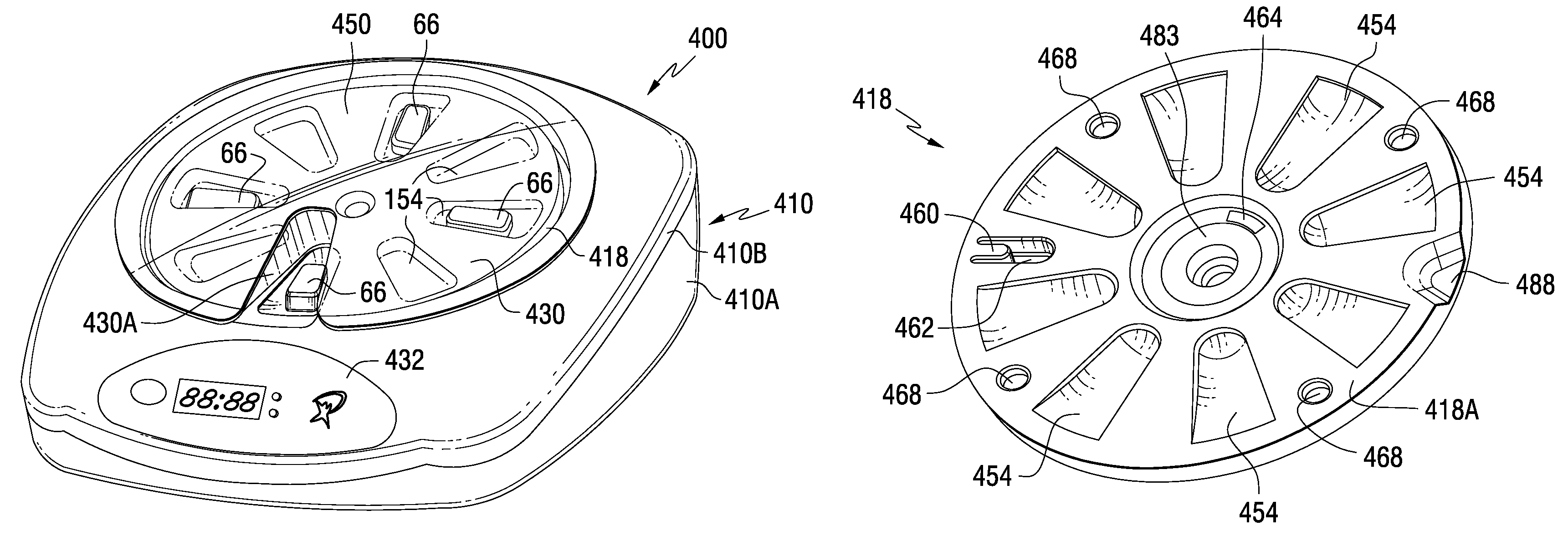
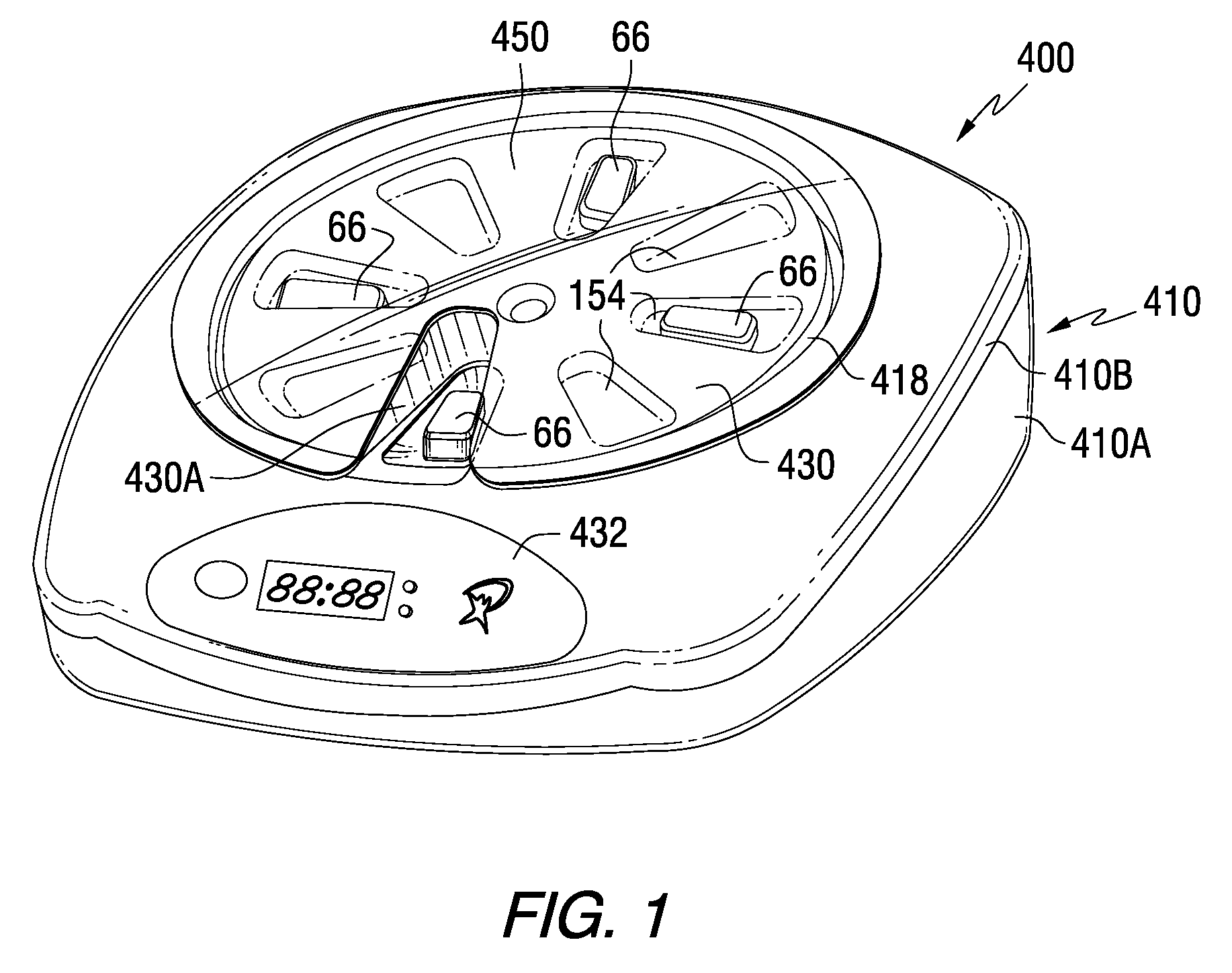
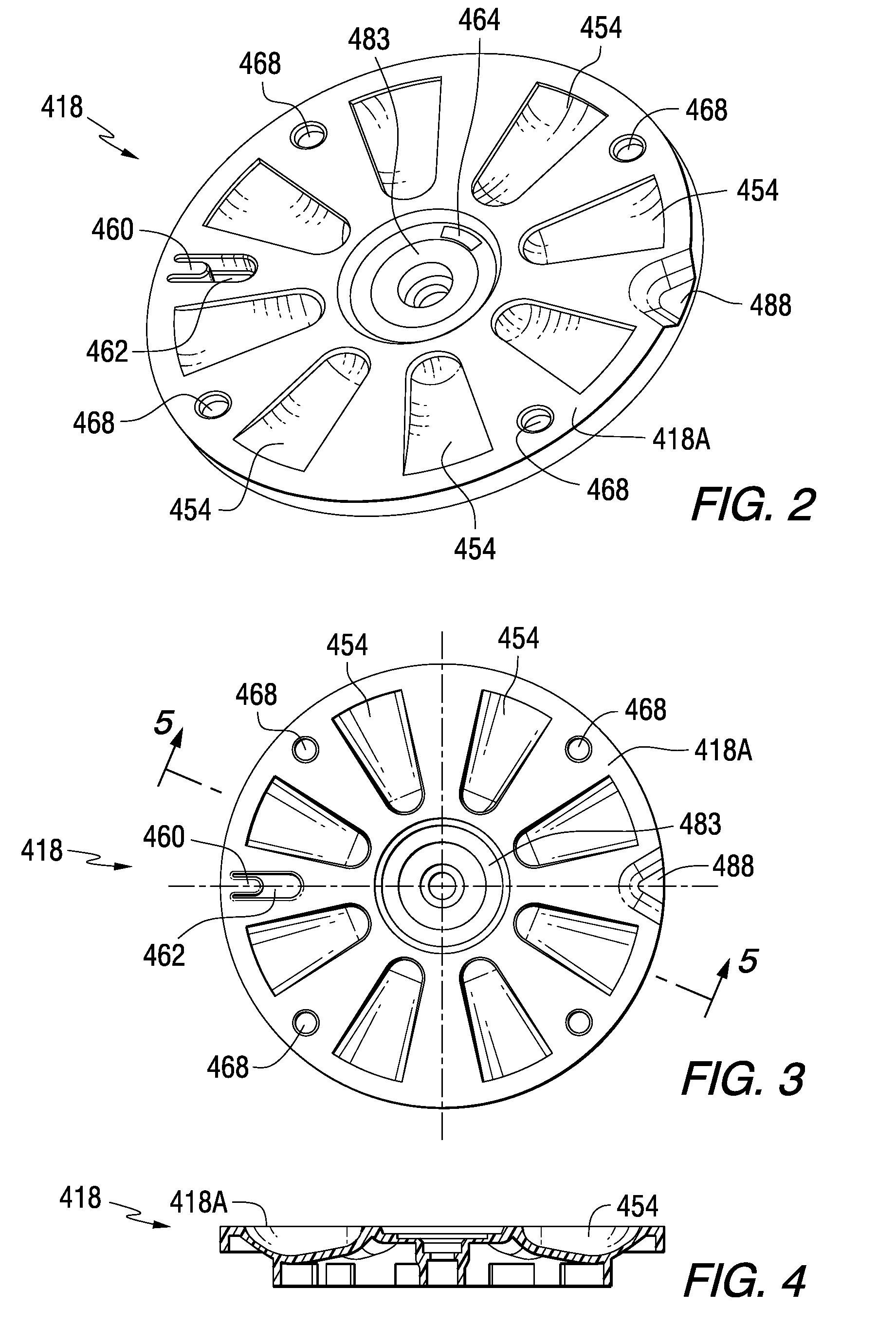
A medication dispenser for permitting administration of a medication dose to an authorized user only after a predetermined minimum-dosing interval has elapsed. The medication dispenser comprises: a medication tray having a substantially circular shape and comprising a plurality of compartments containing a medication dose, the compartments disposed about a circumference thereof; regions between two consecutive dose-carrying compartments comprising an empty compartment or a blank region; an enclosure for supporting the dispenser, the enclosure including an opening; and a controller for controlling the medication tray to align one of the plurality of compartments containing a medication dose with the opening after the minimum dosing interval has elapsed and after a person has been authenticated as an authorized user, thereby permitting the authorized user to access the medication dose through the opening, wherein the medication dispenser remains in a dose-accessible configuration for a predetermined hold period, after which the medication tray is controlled to align a next successive empty compartment or blank region with the opening.
Owner:CONLEY N SHARON
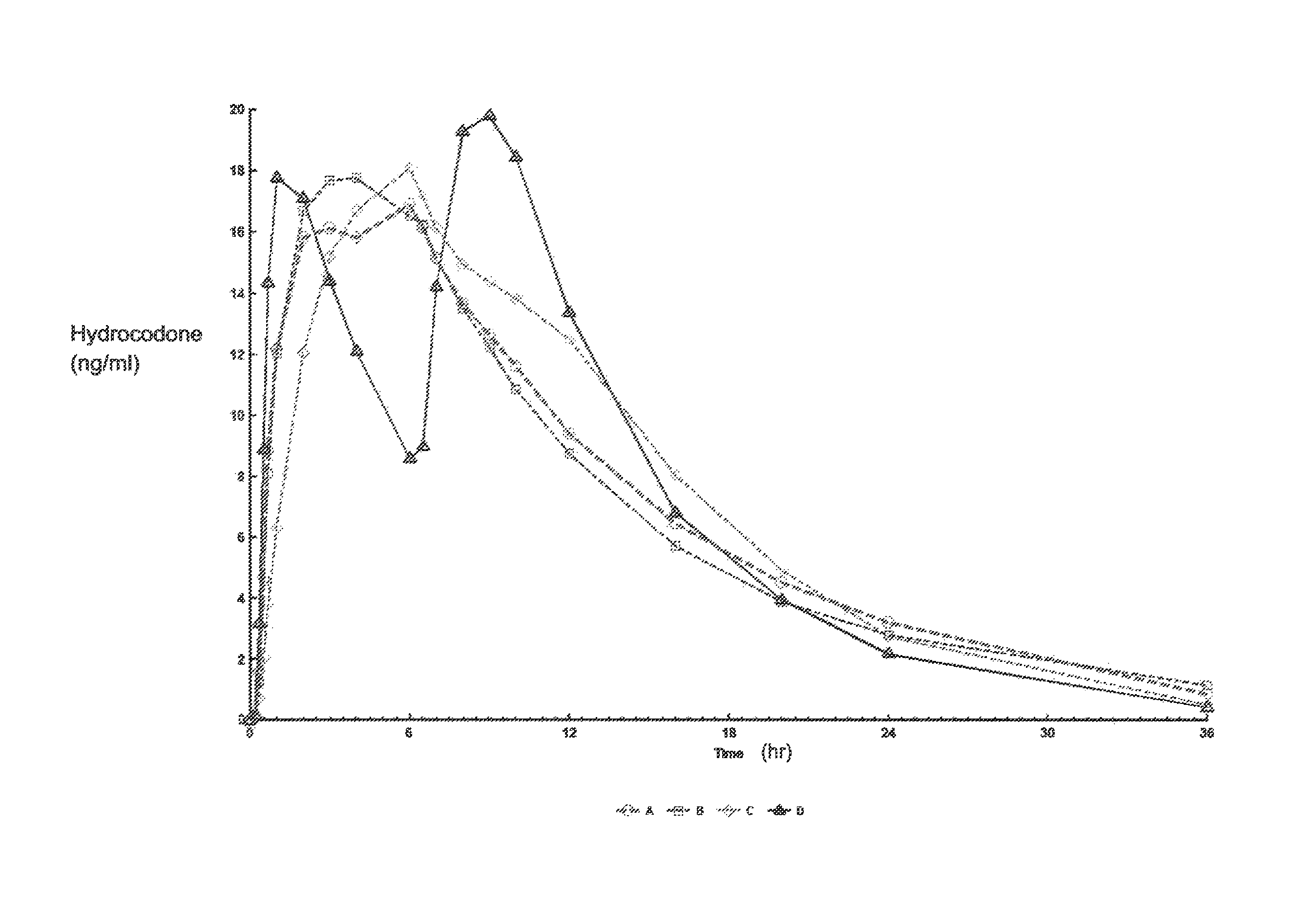
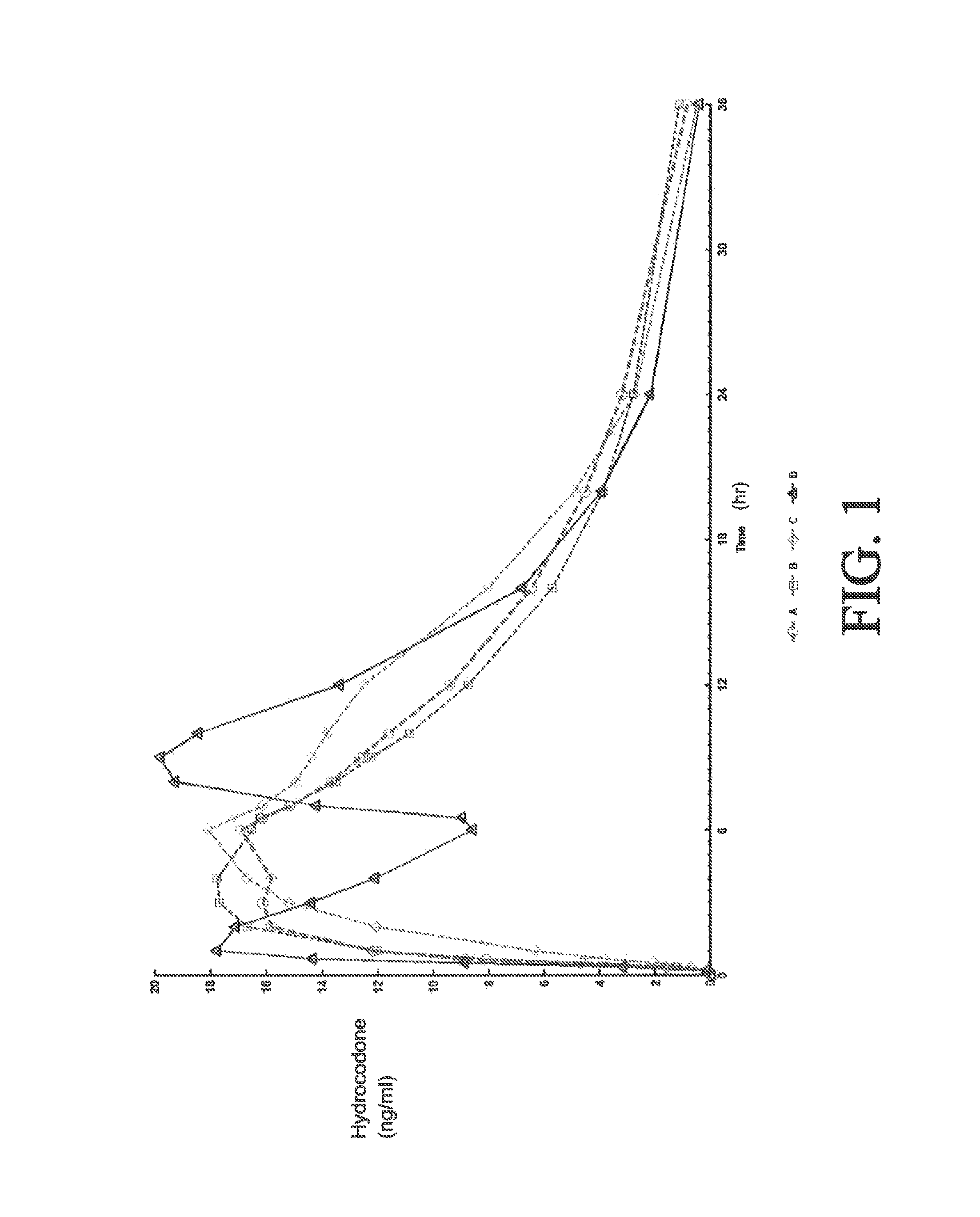
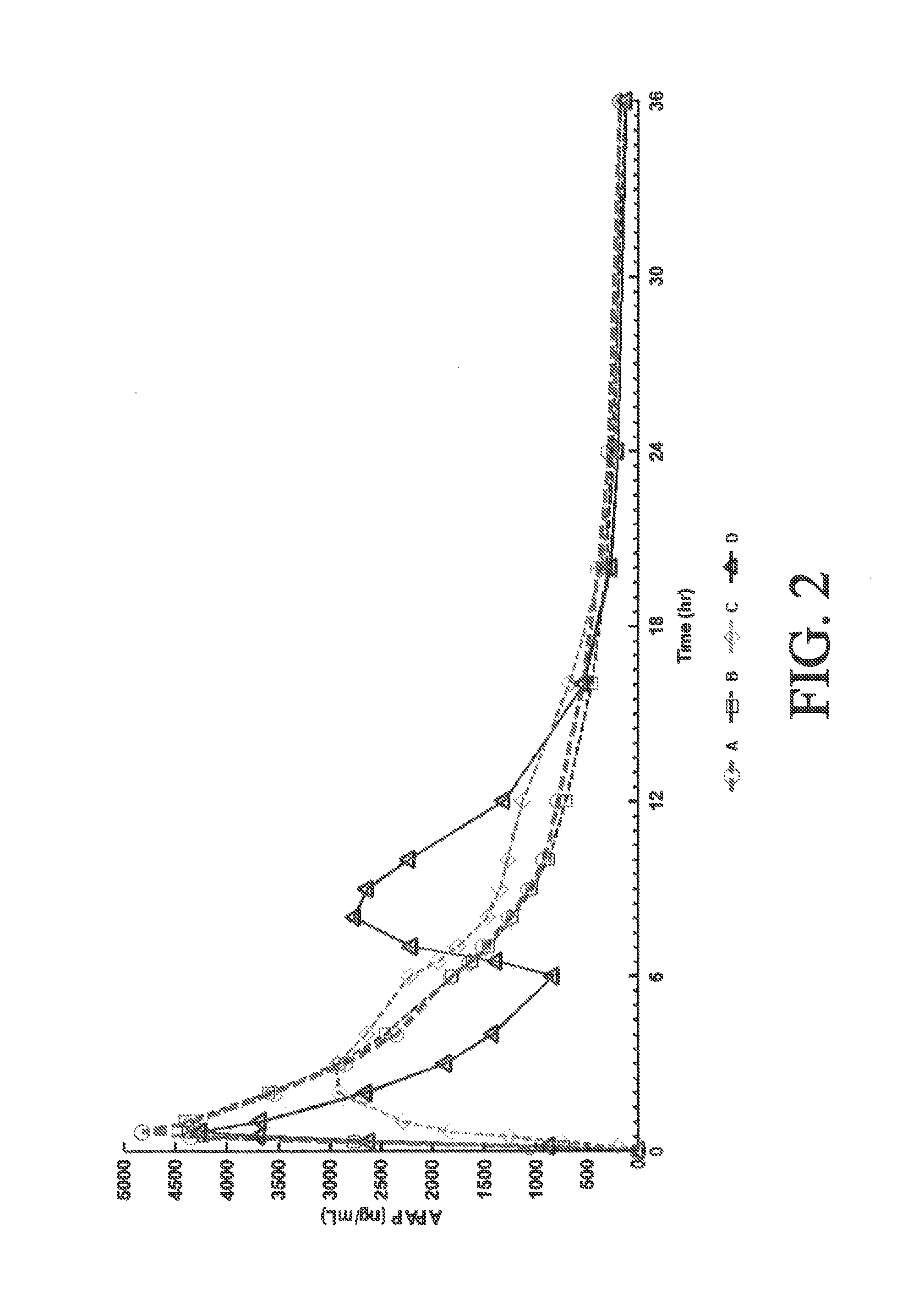
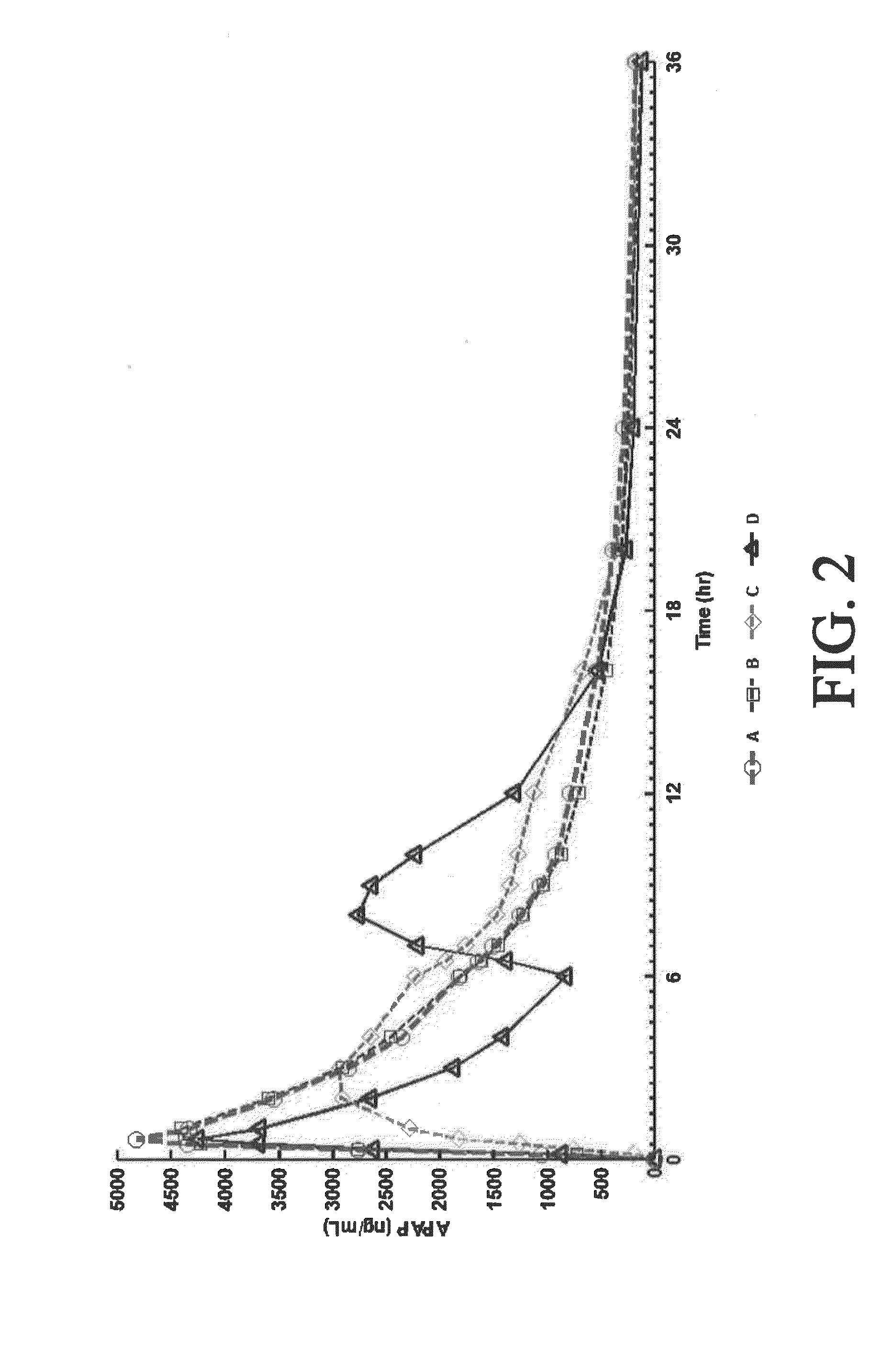
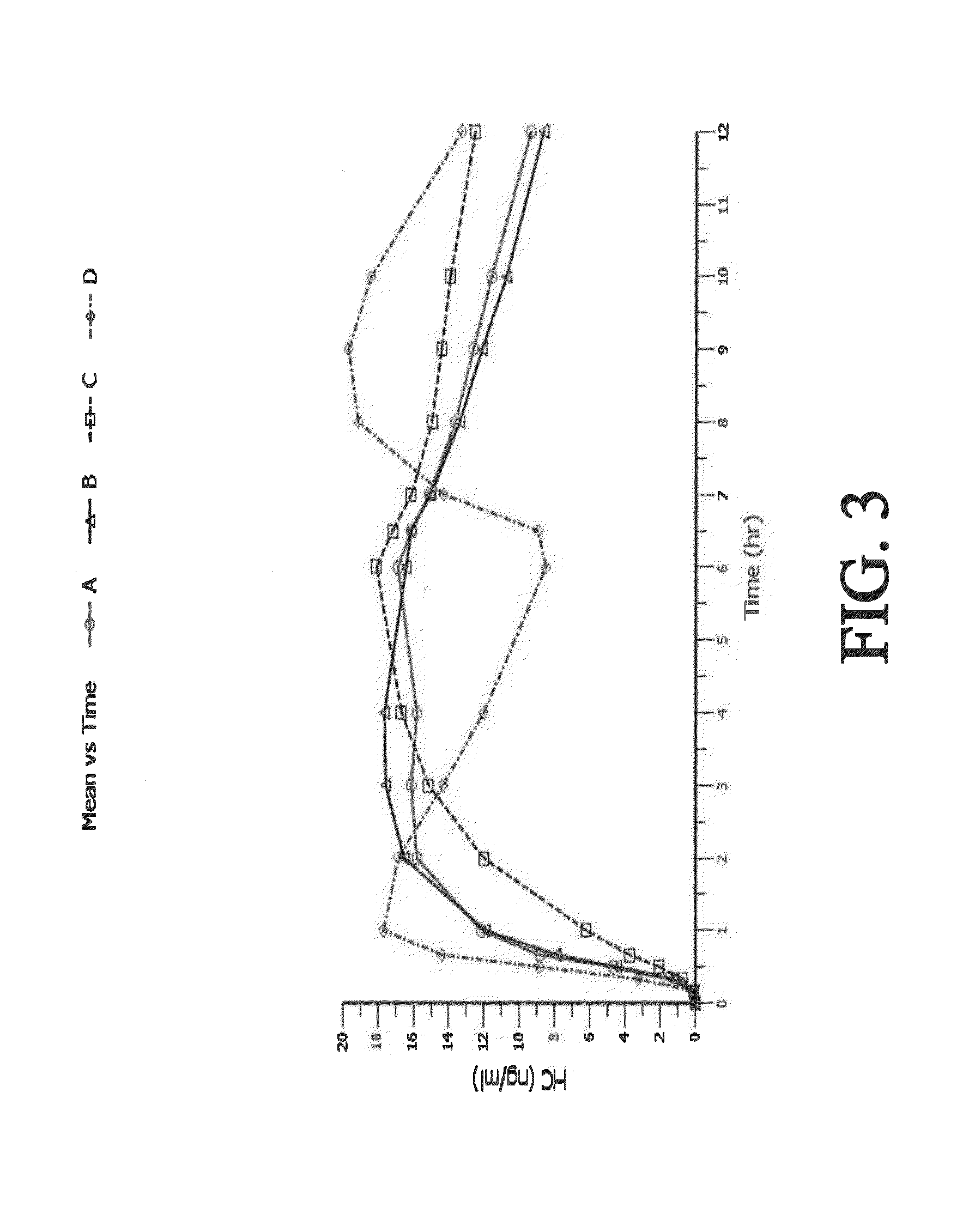
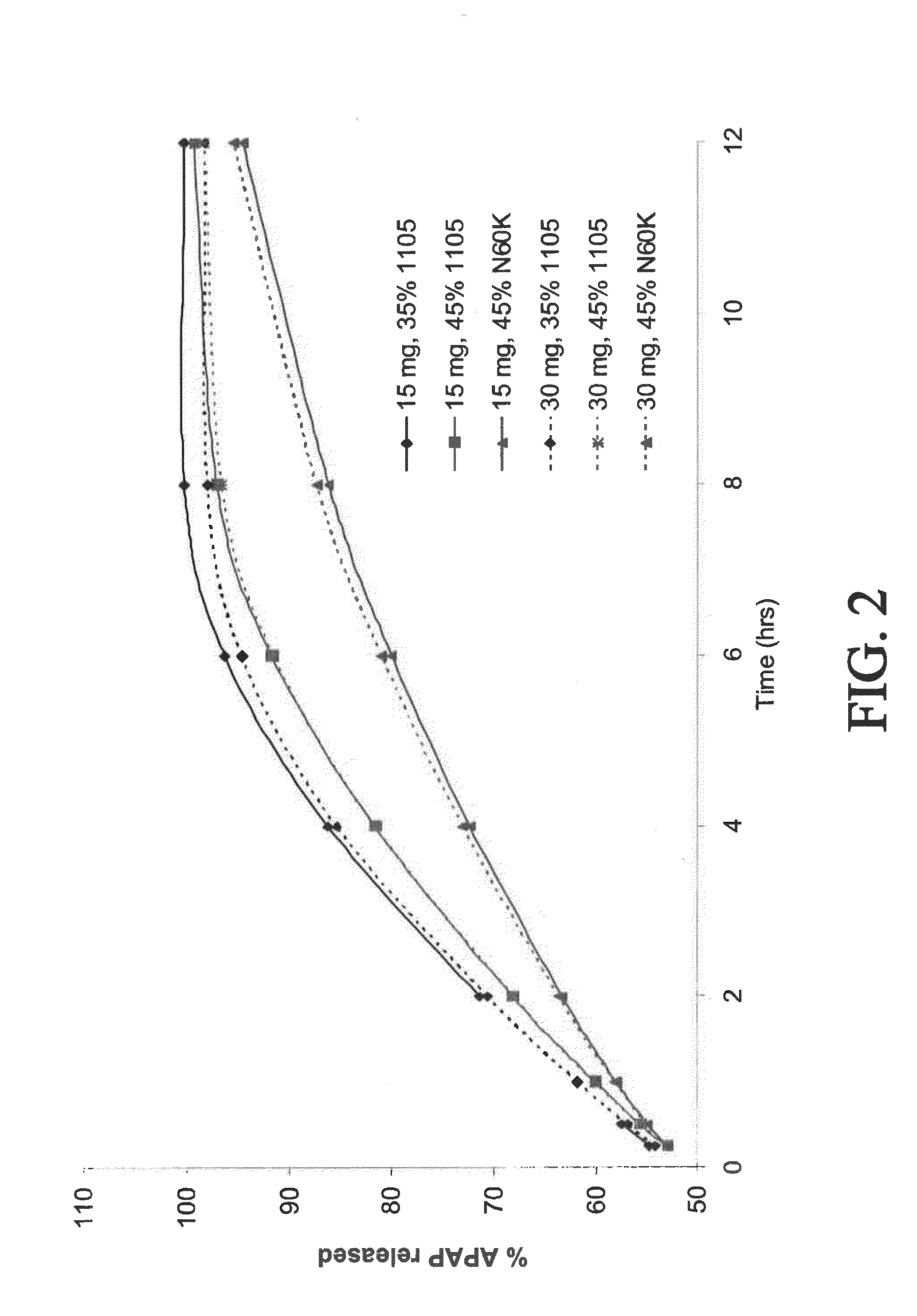
Tamper resistant composition comprising hydrocodone and acetaminophen for rapid onset and extended duration of analgesia
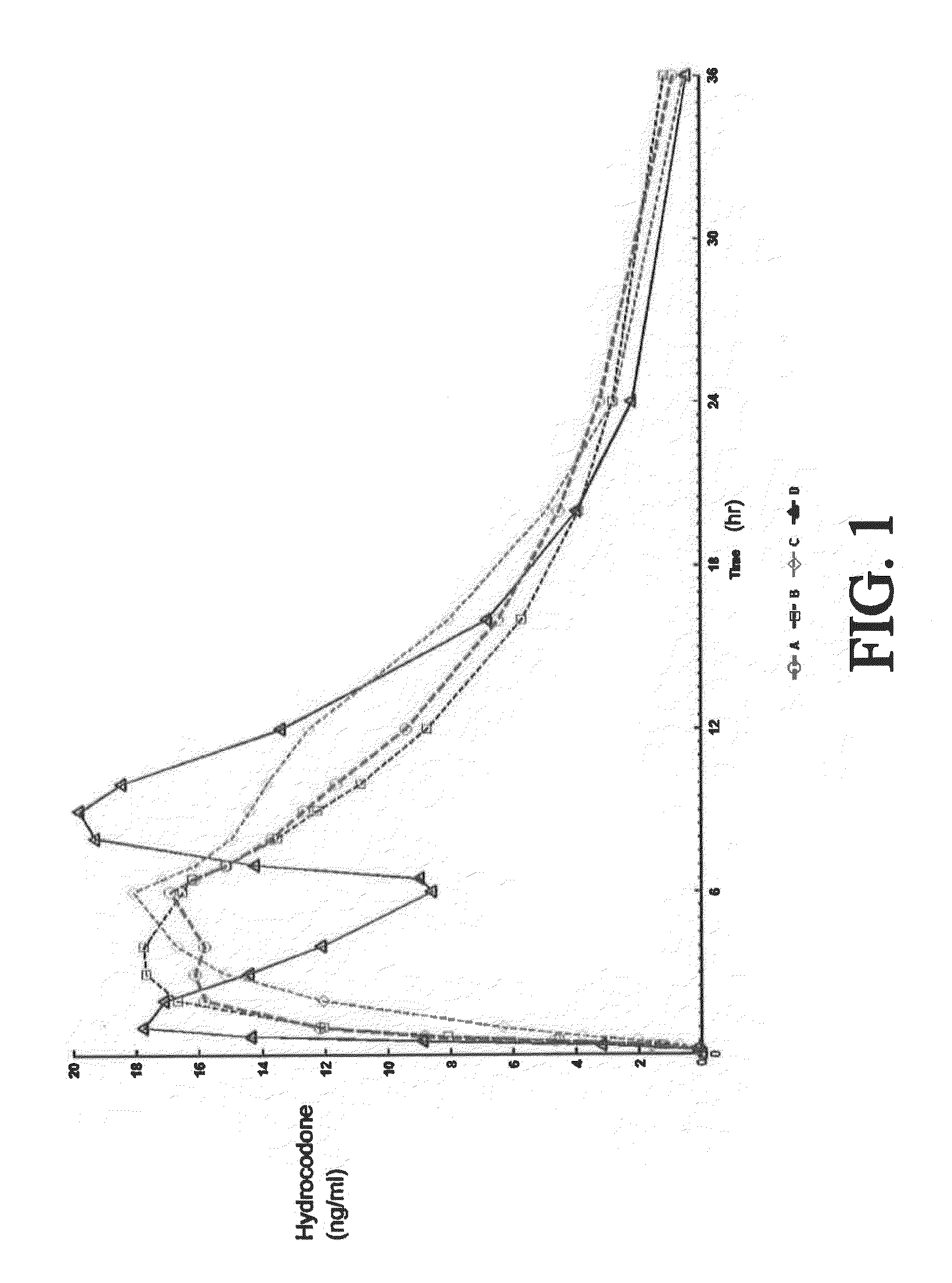
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Methods of providing sustained treatment with opioids
InactiveUS6231886B1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationPlasma glucose
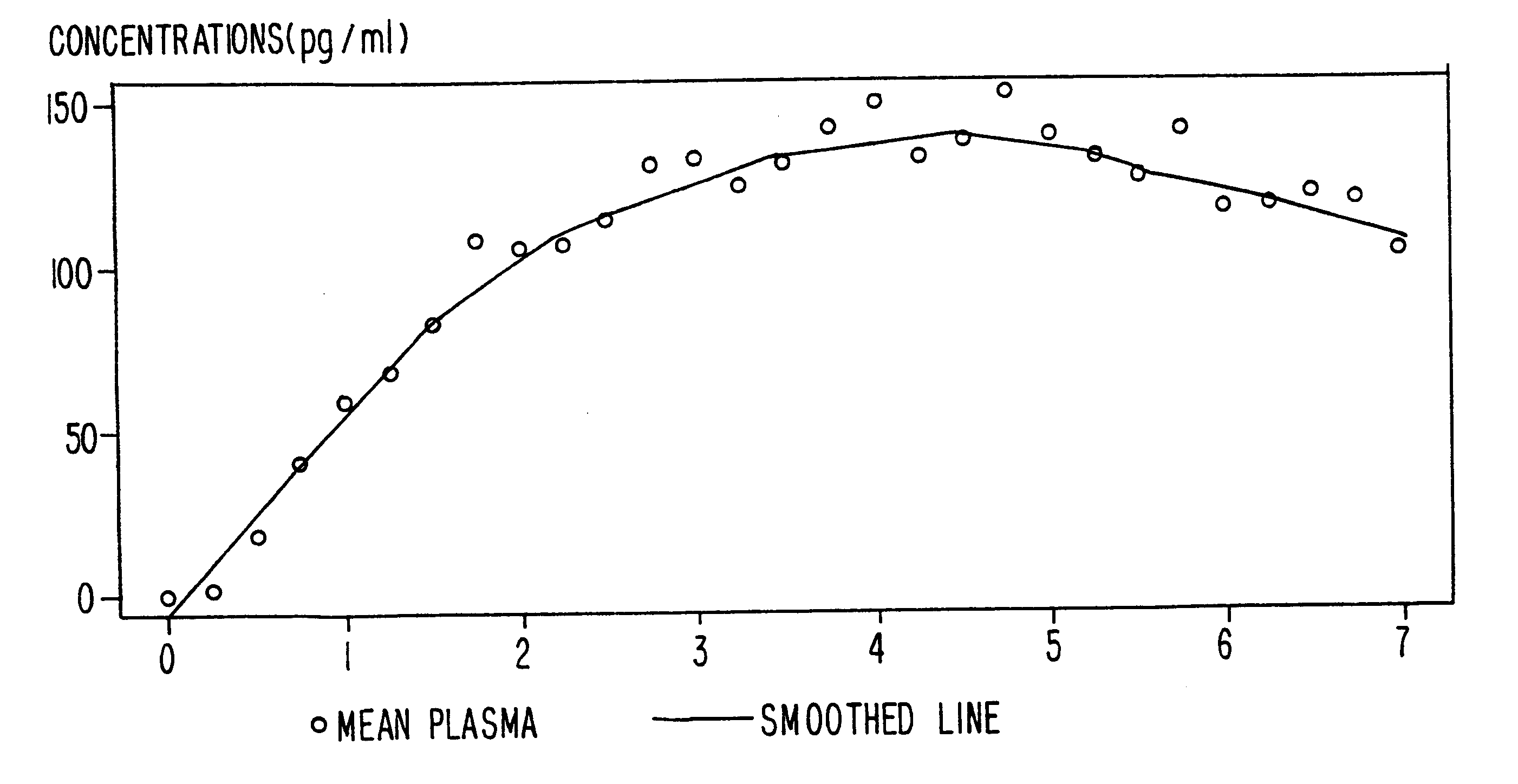
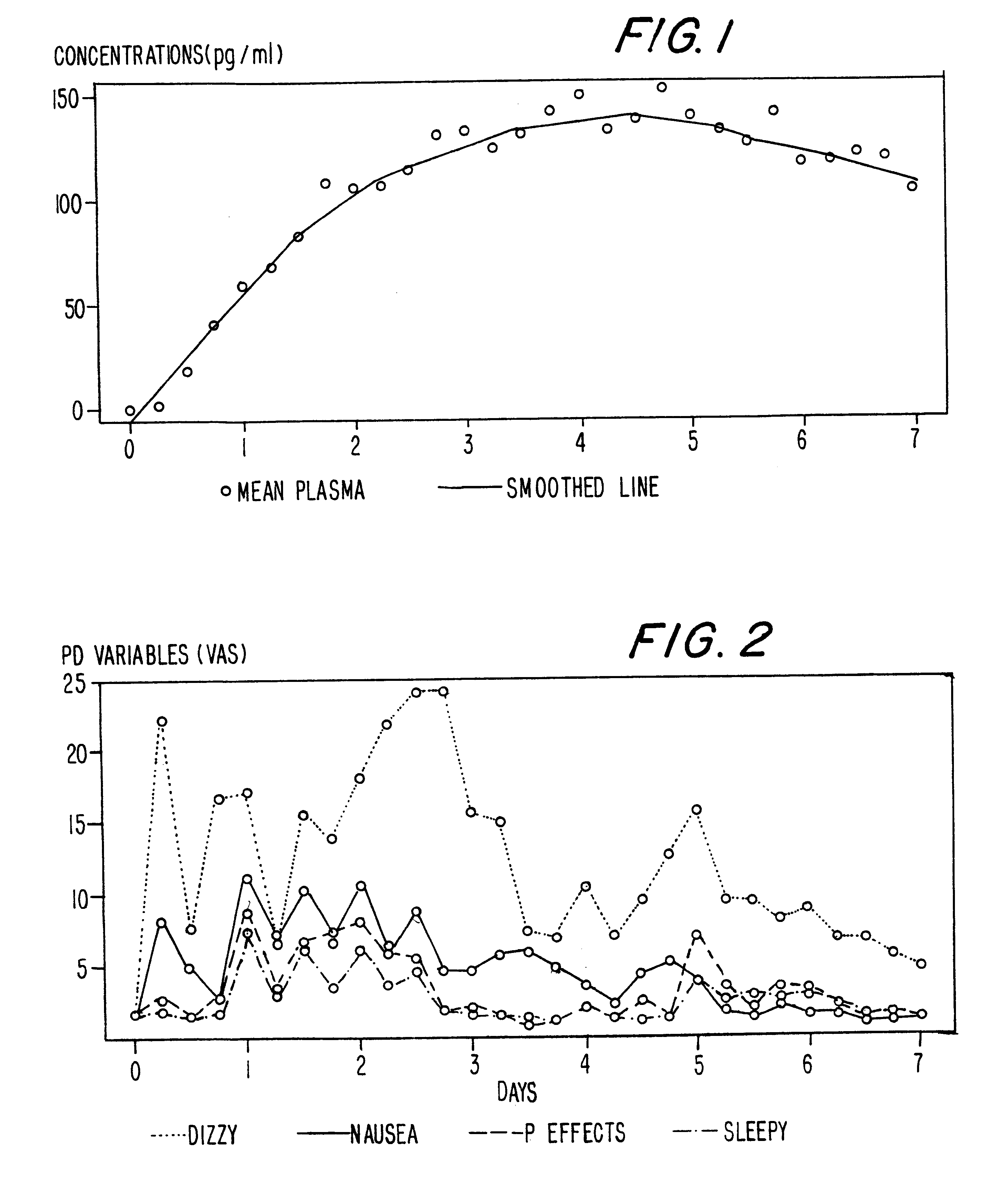
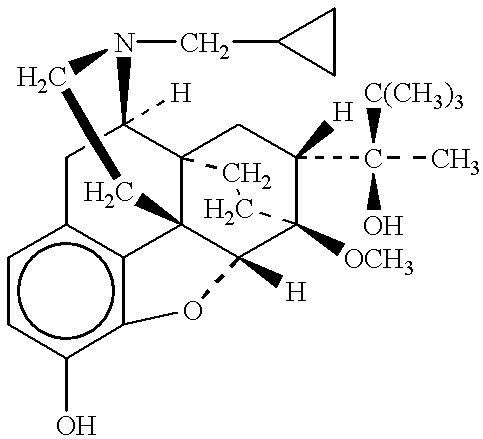
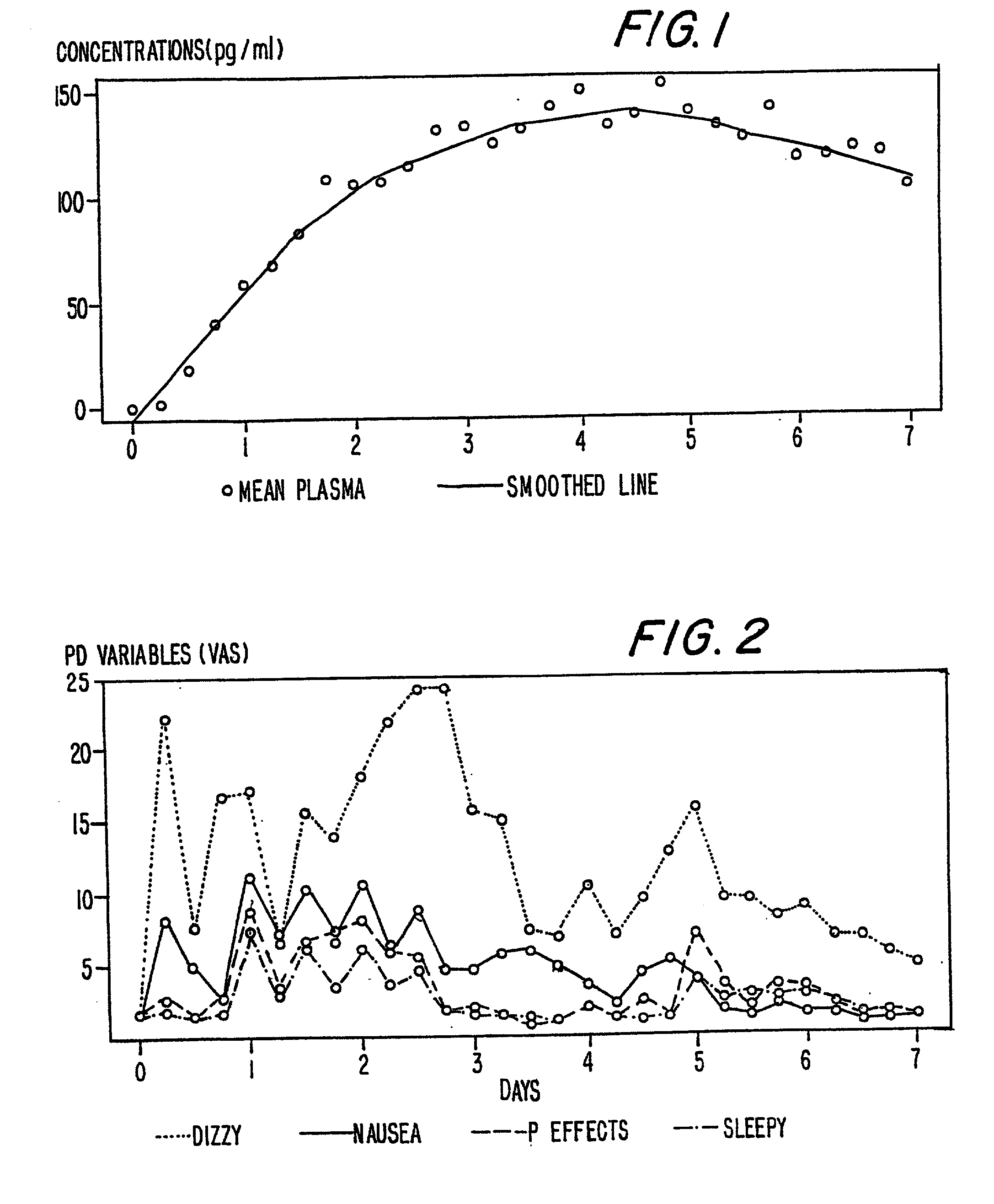
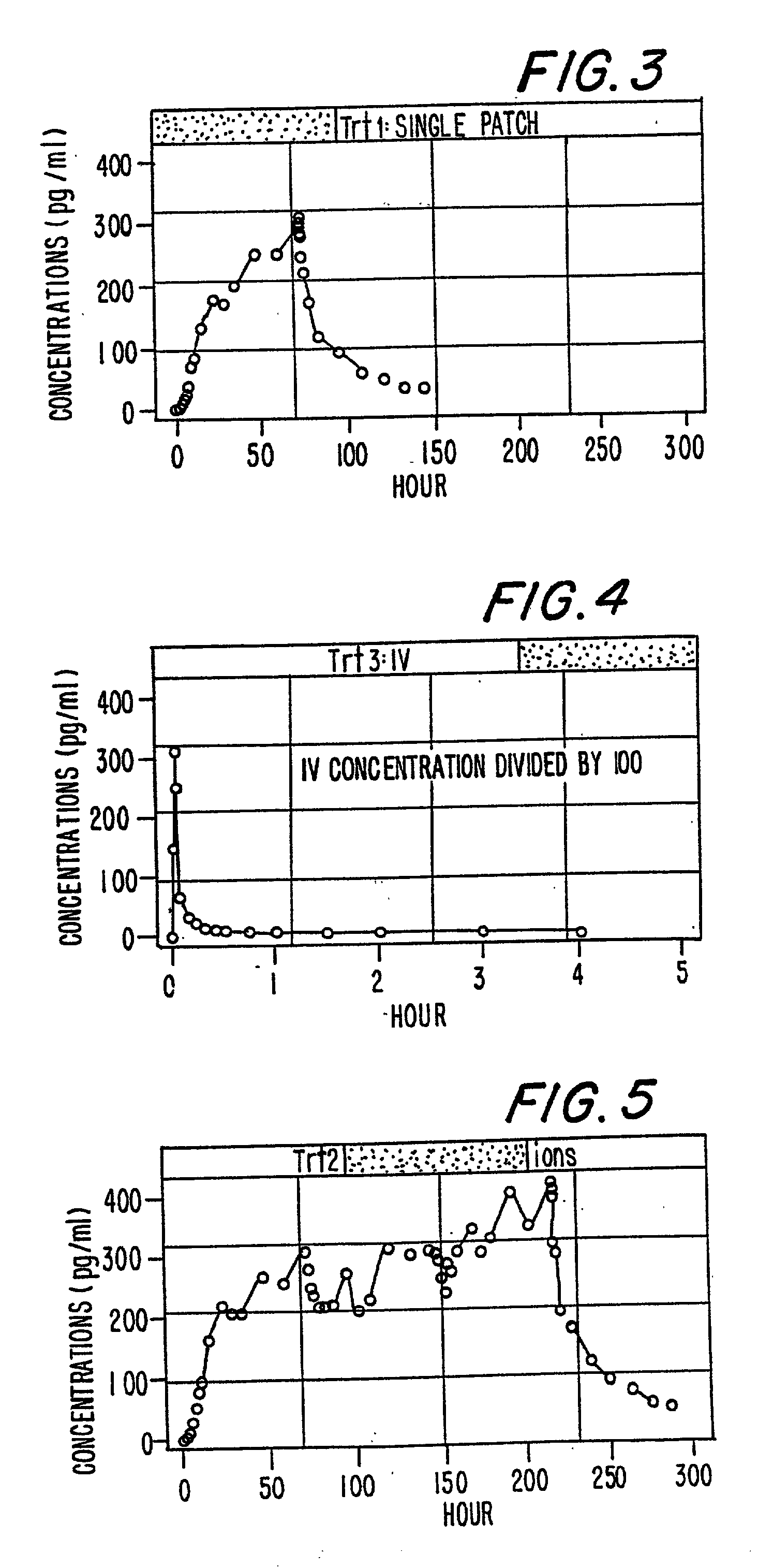
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an addition two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Tamper Resistant Composition Comprising Hydrocodone And Acetaminophen For Rapid Onset And Extended Duration Of Analgesia
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Compositions Comprising An Opioid And An Additional Active Pharmaceutical Ingredient For Rapid Onset And Extended Duration Of Analgesia That May Be Administered Without Regard To Food
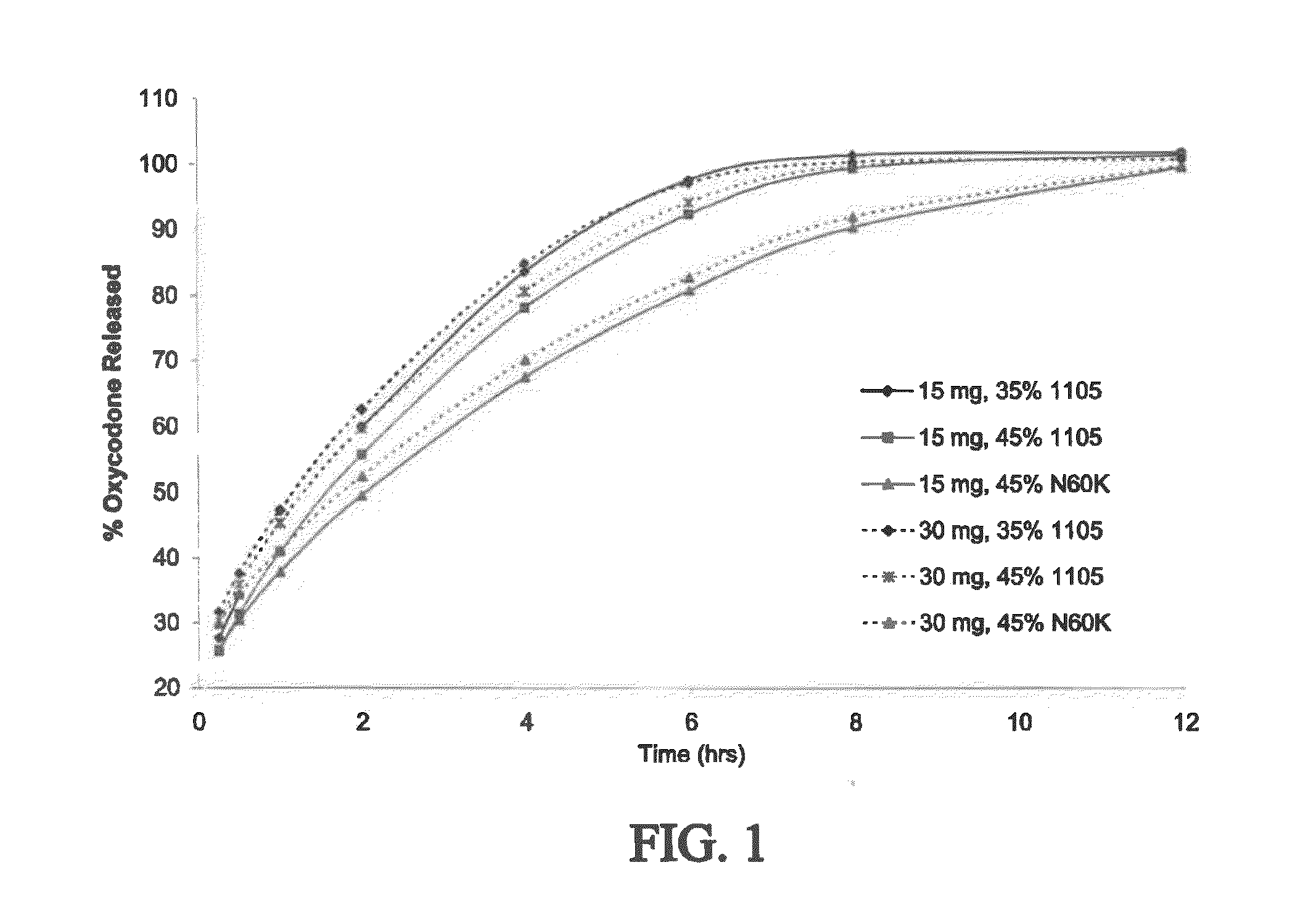
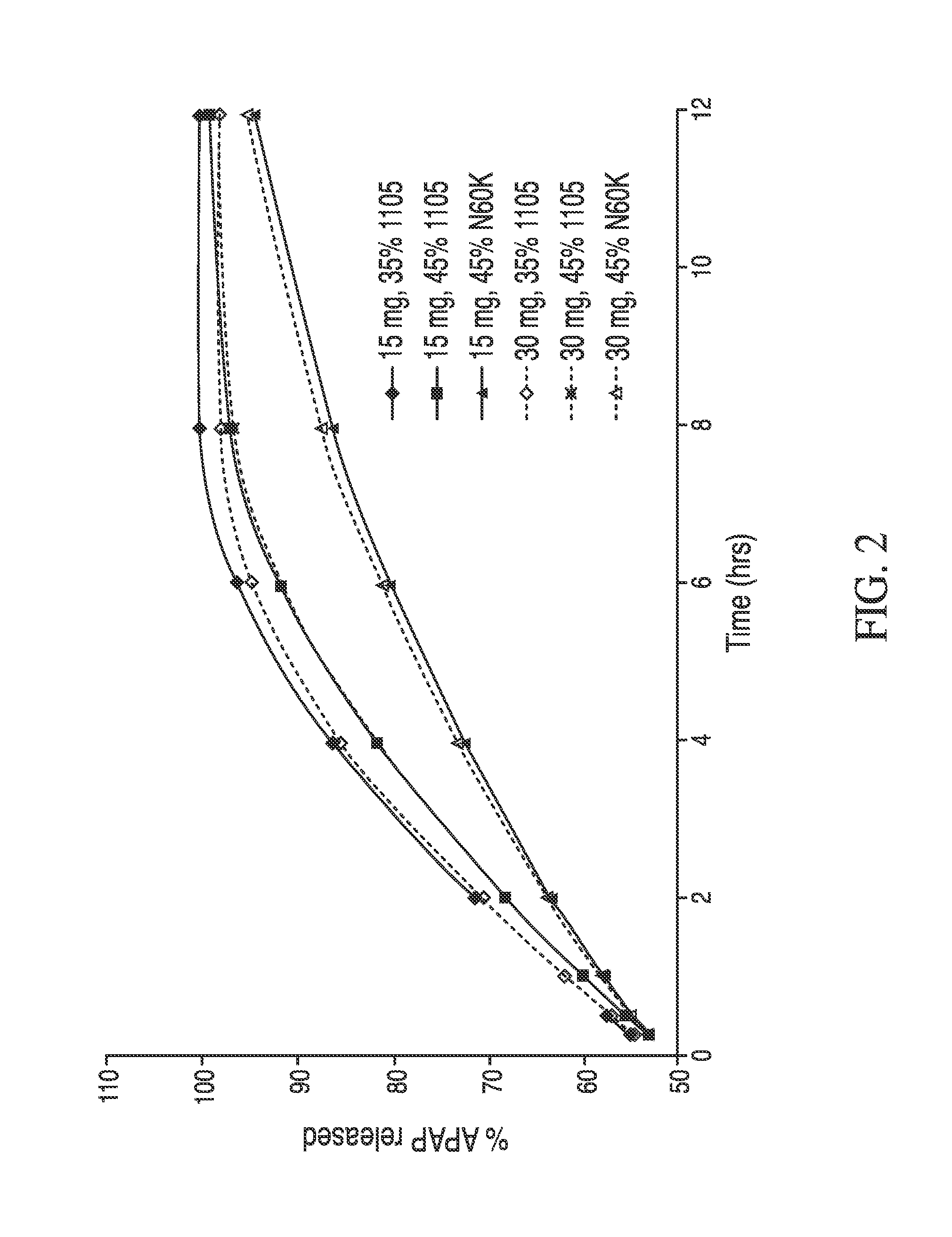
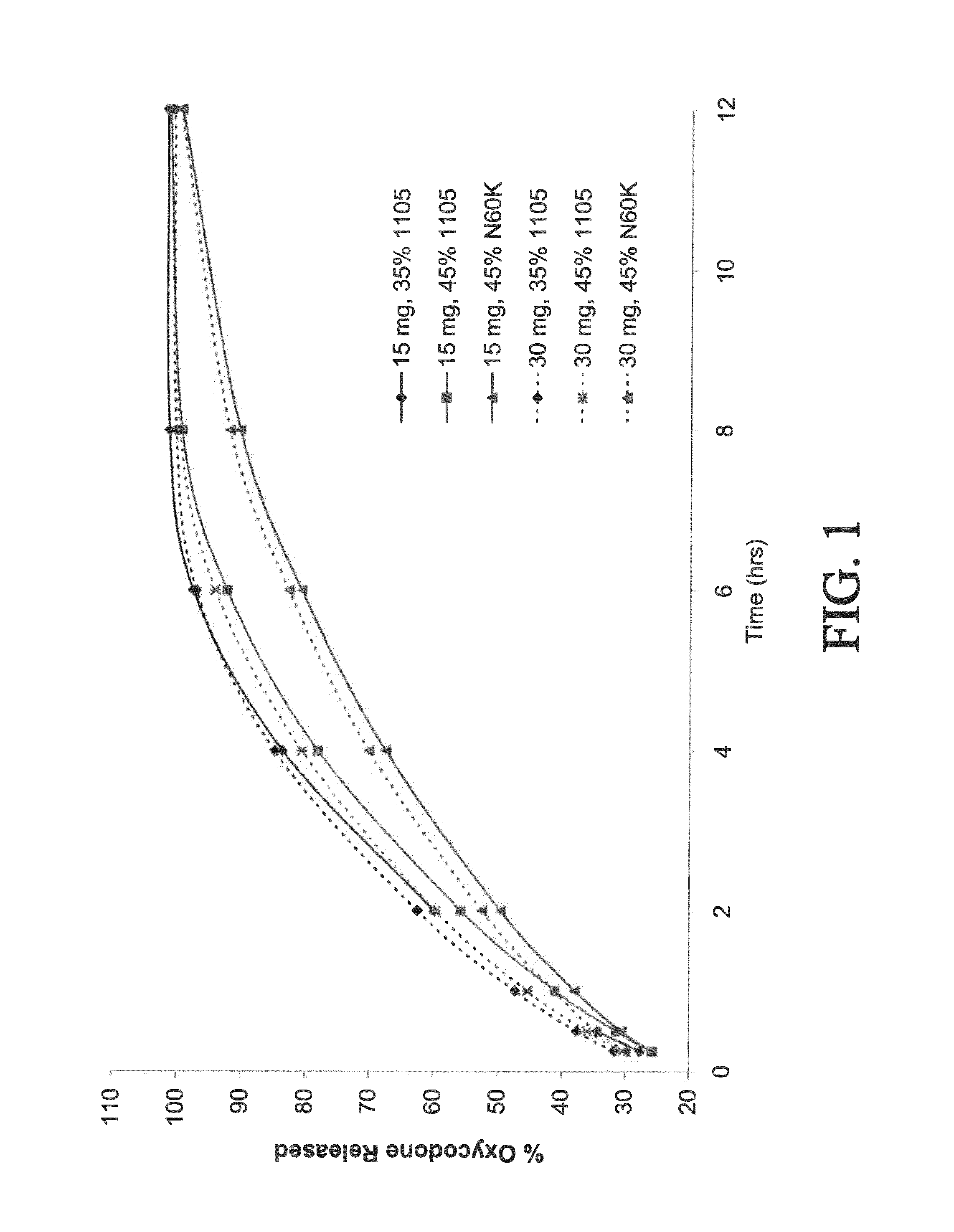
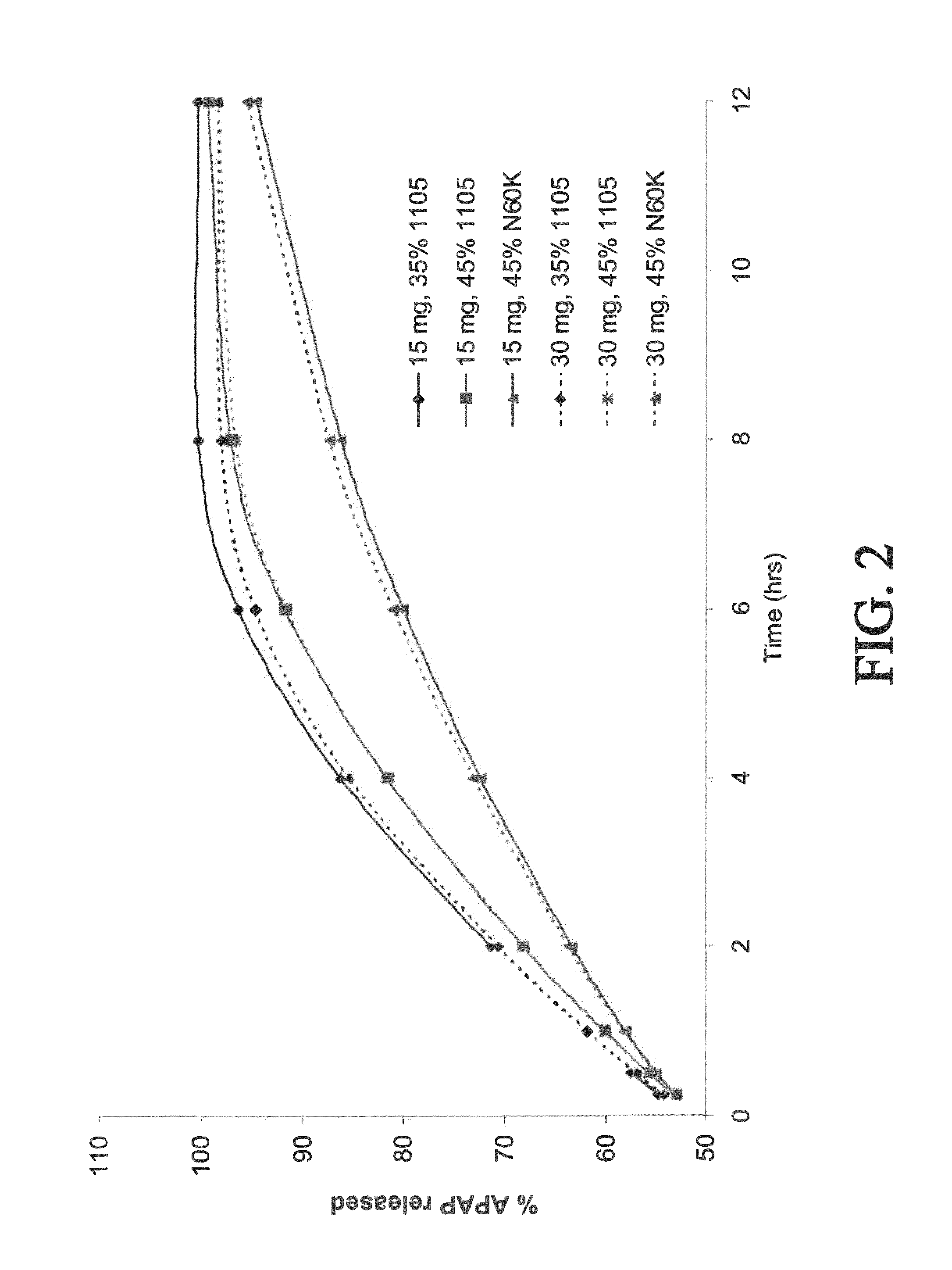
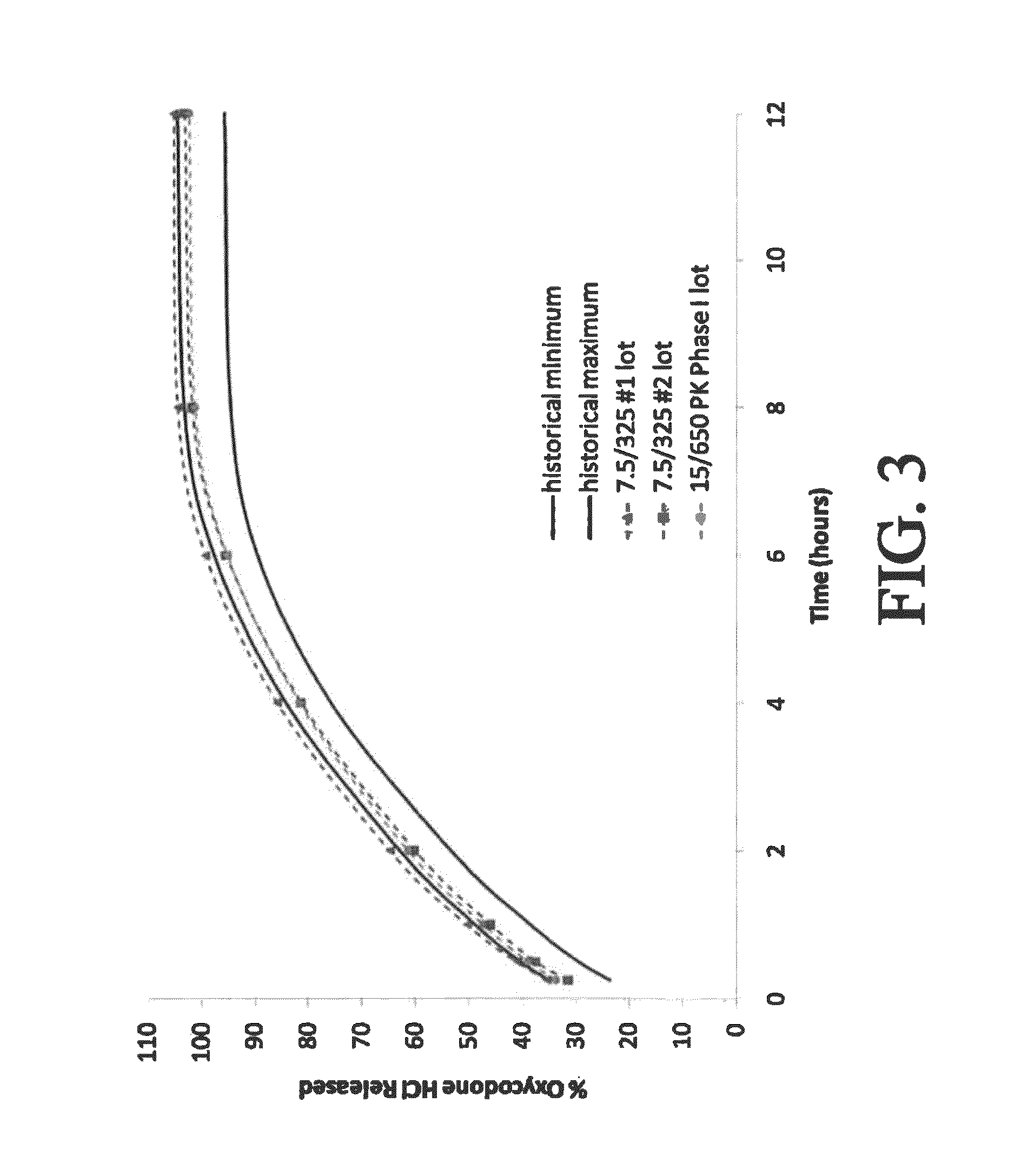
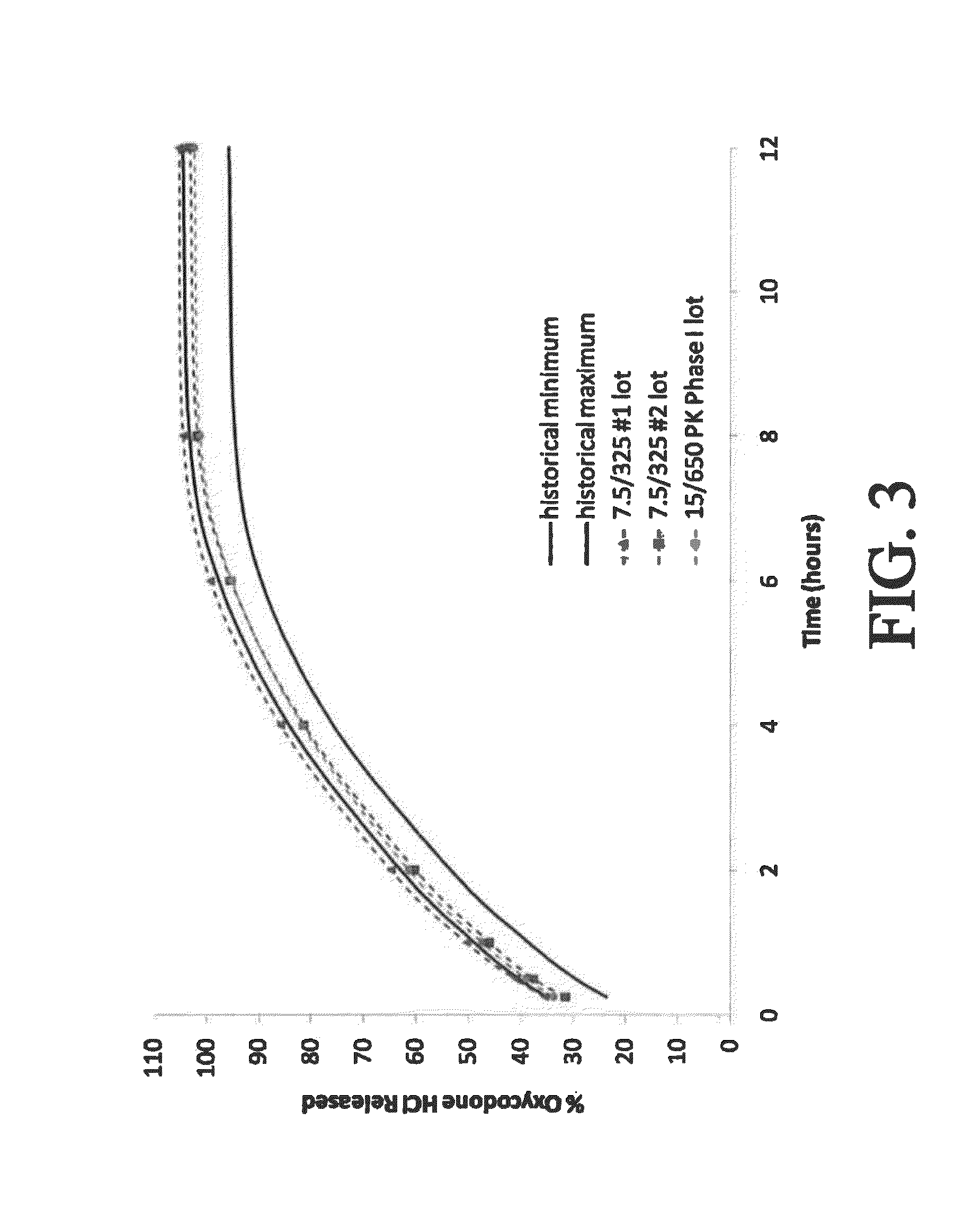
The present disclosure provides pharmaceutical compositions comprising an opioid and an additional active pharmaceutical ingredient, wherein the composition exhibits gastric retentive properties which are achieved by a combination of a physical property of the composition and release of the opioid, wherein upon administration to a subject, the composition has at least one pharmacokinetic parameter that differs by less than about 30% when the subject is in a fasted state as compared to a fed state. The present disclosure further provides pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides reduced abuse potential.
Owner:MALLINCKRODT INC
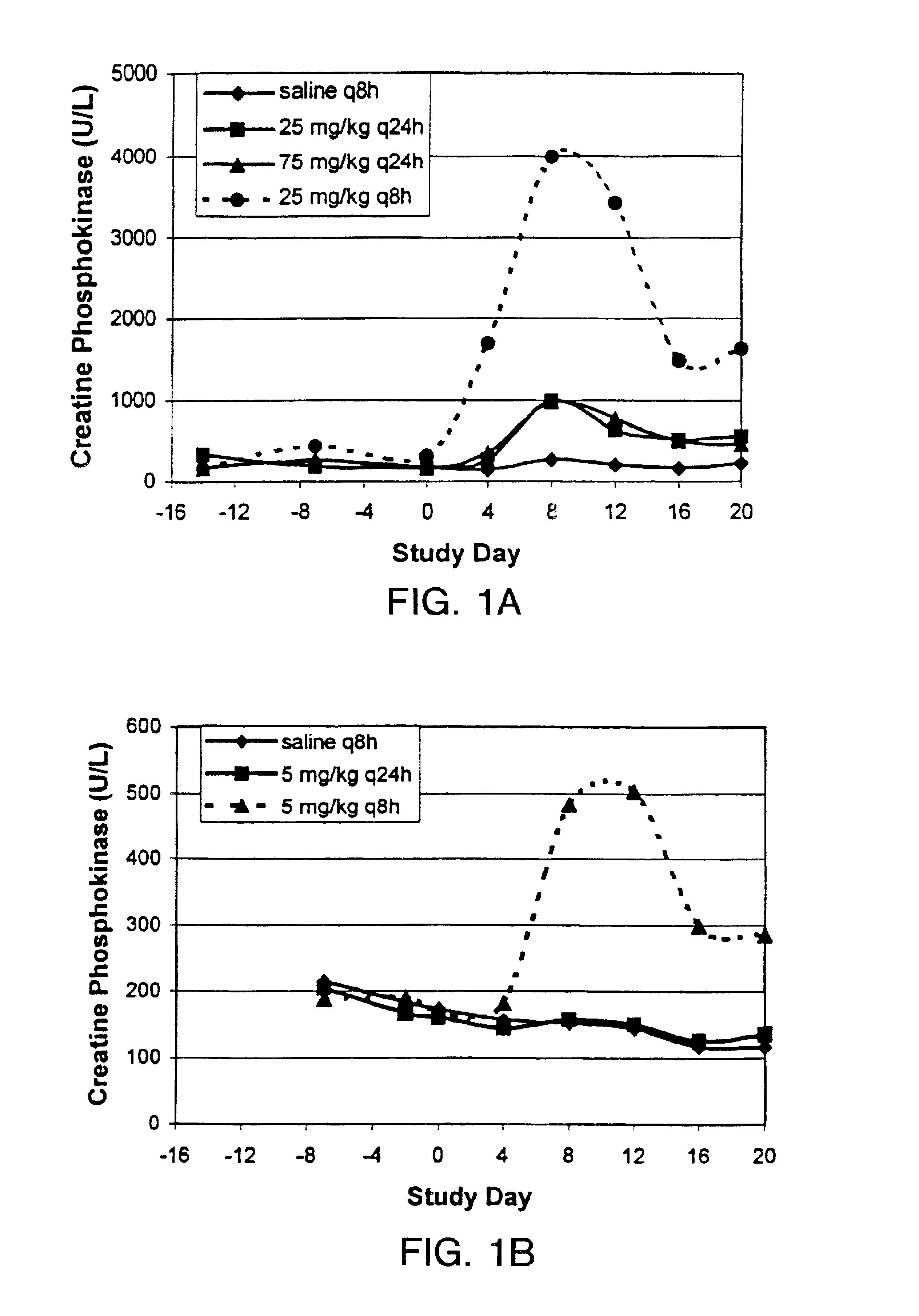
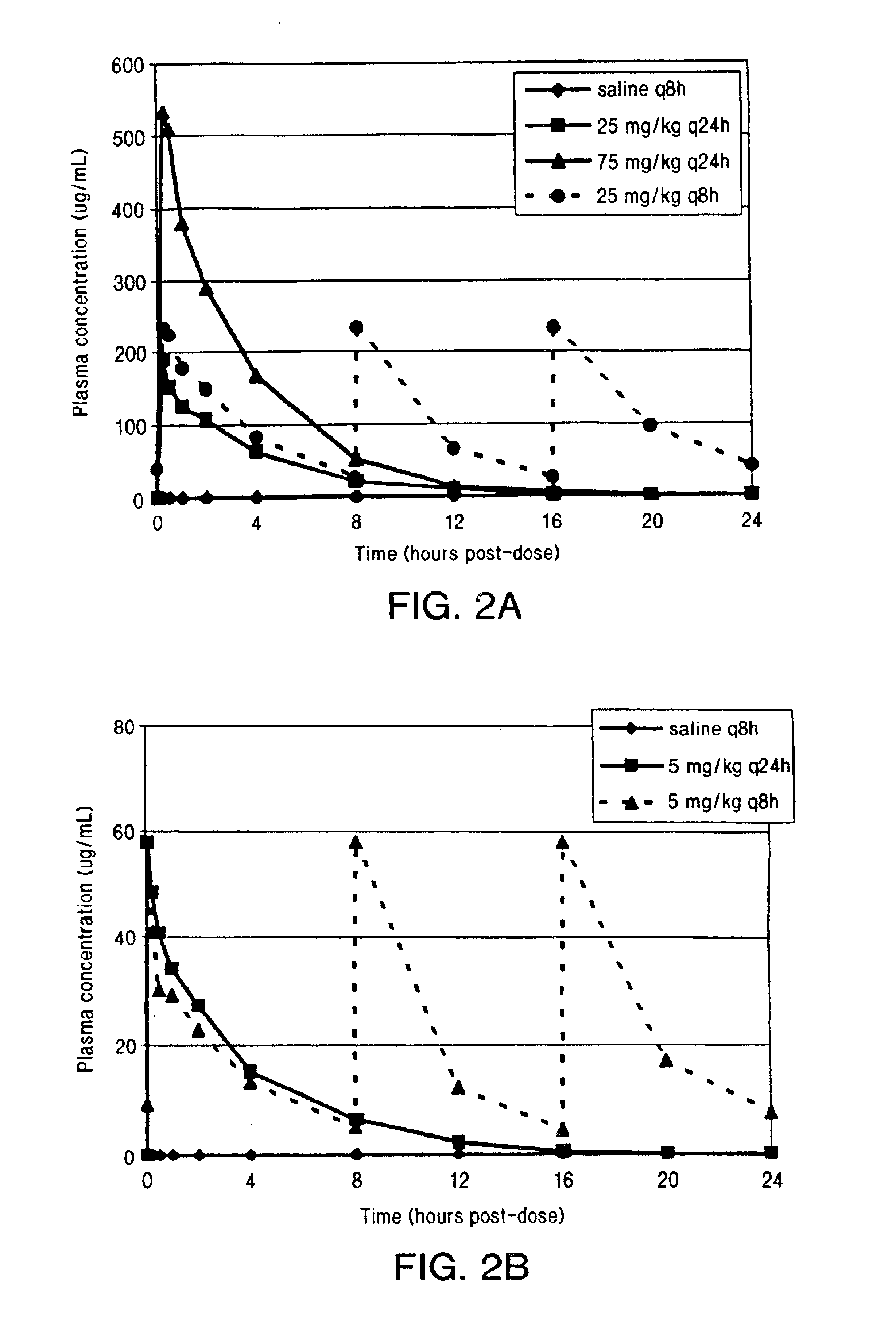
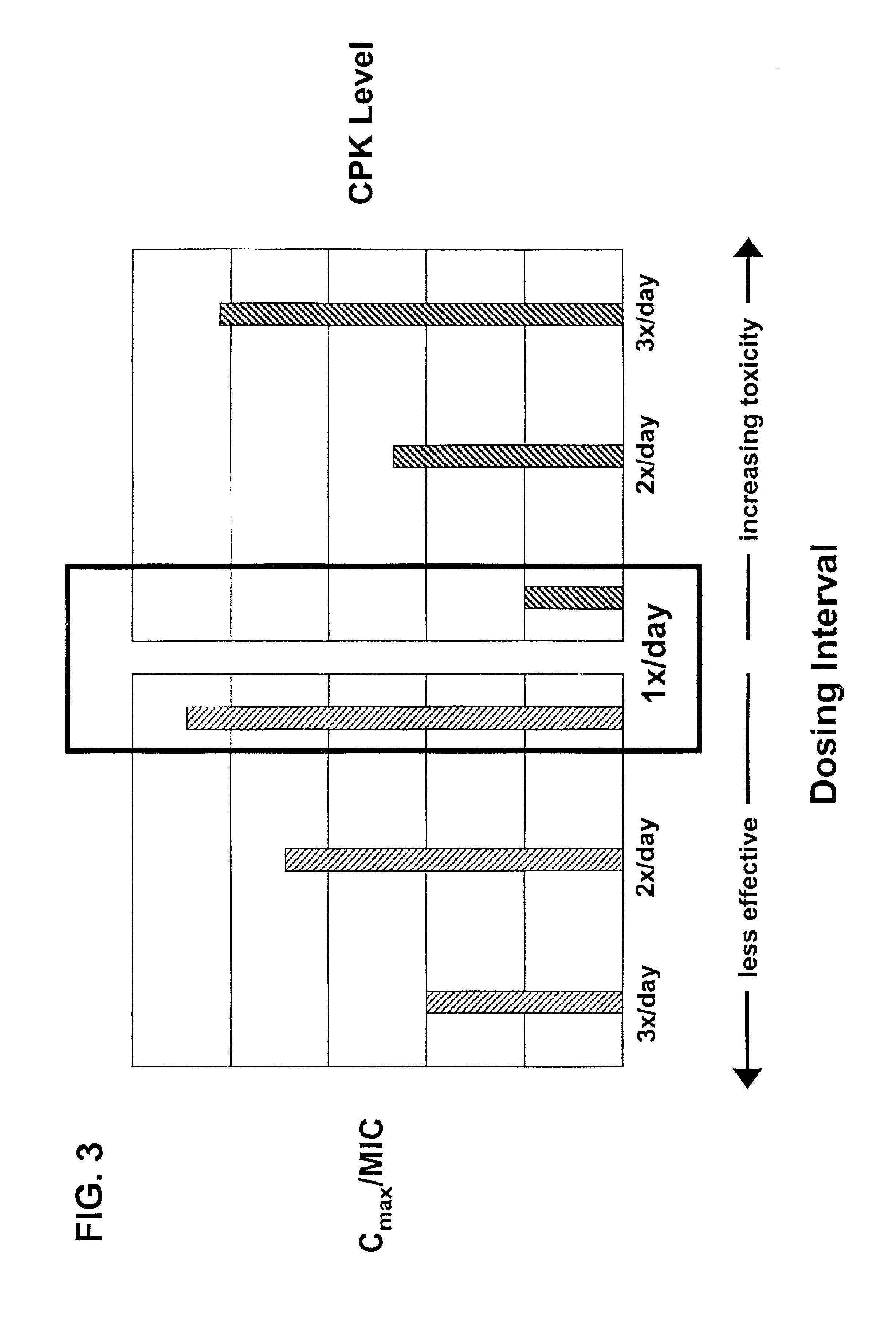
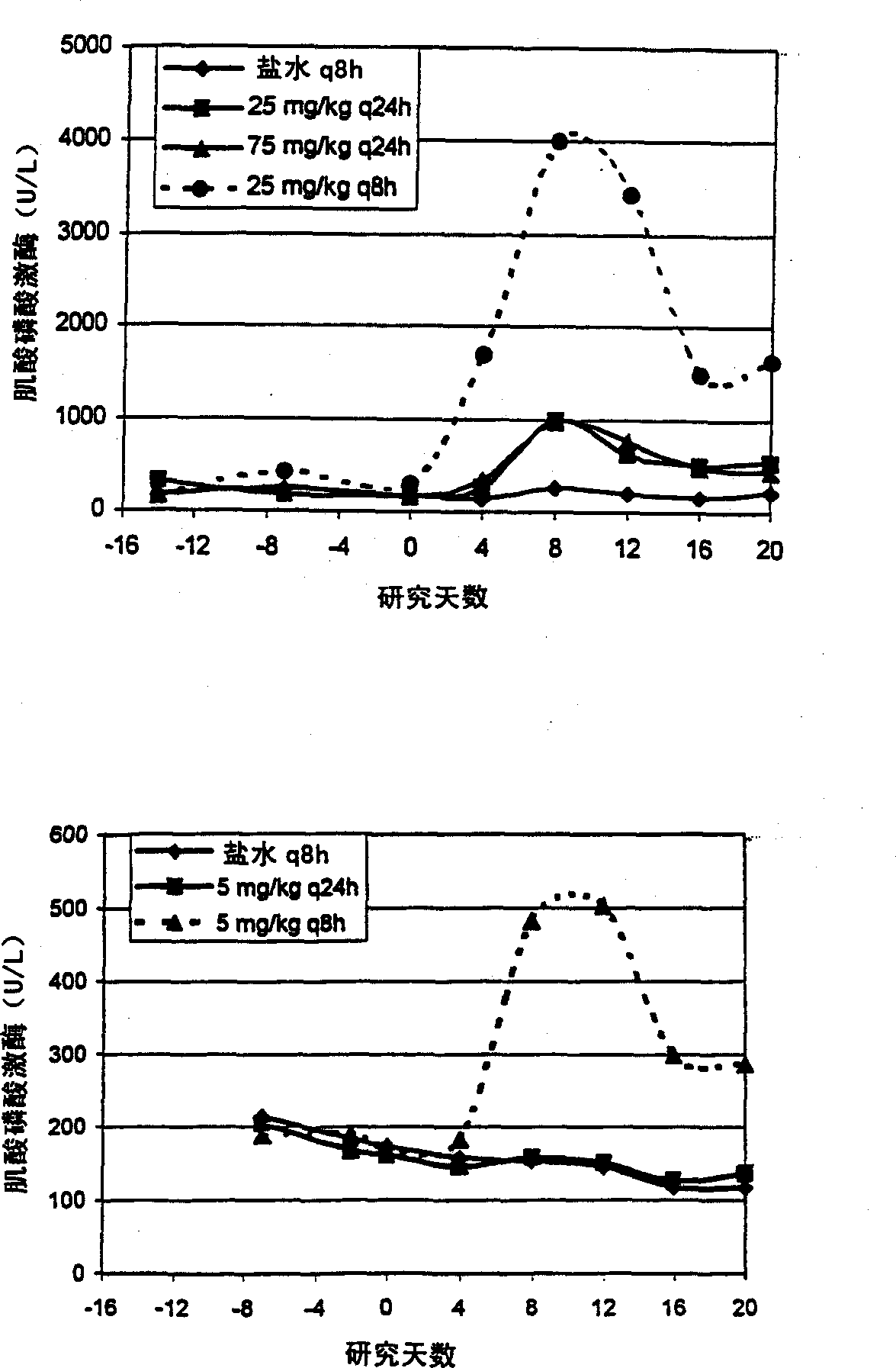
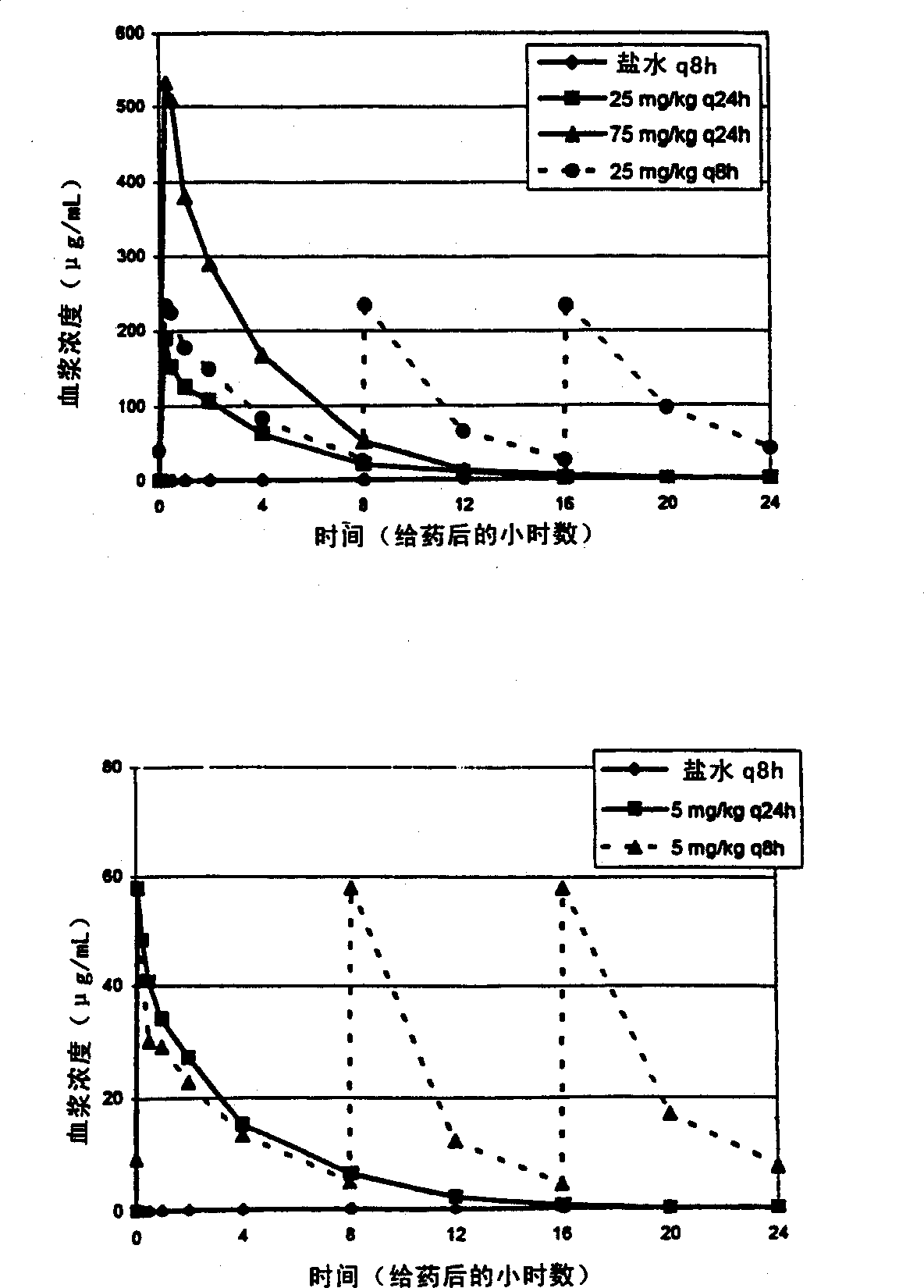
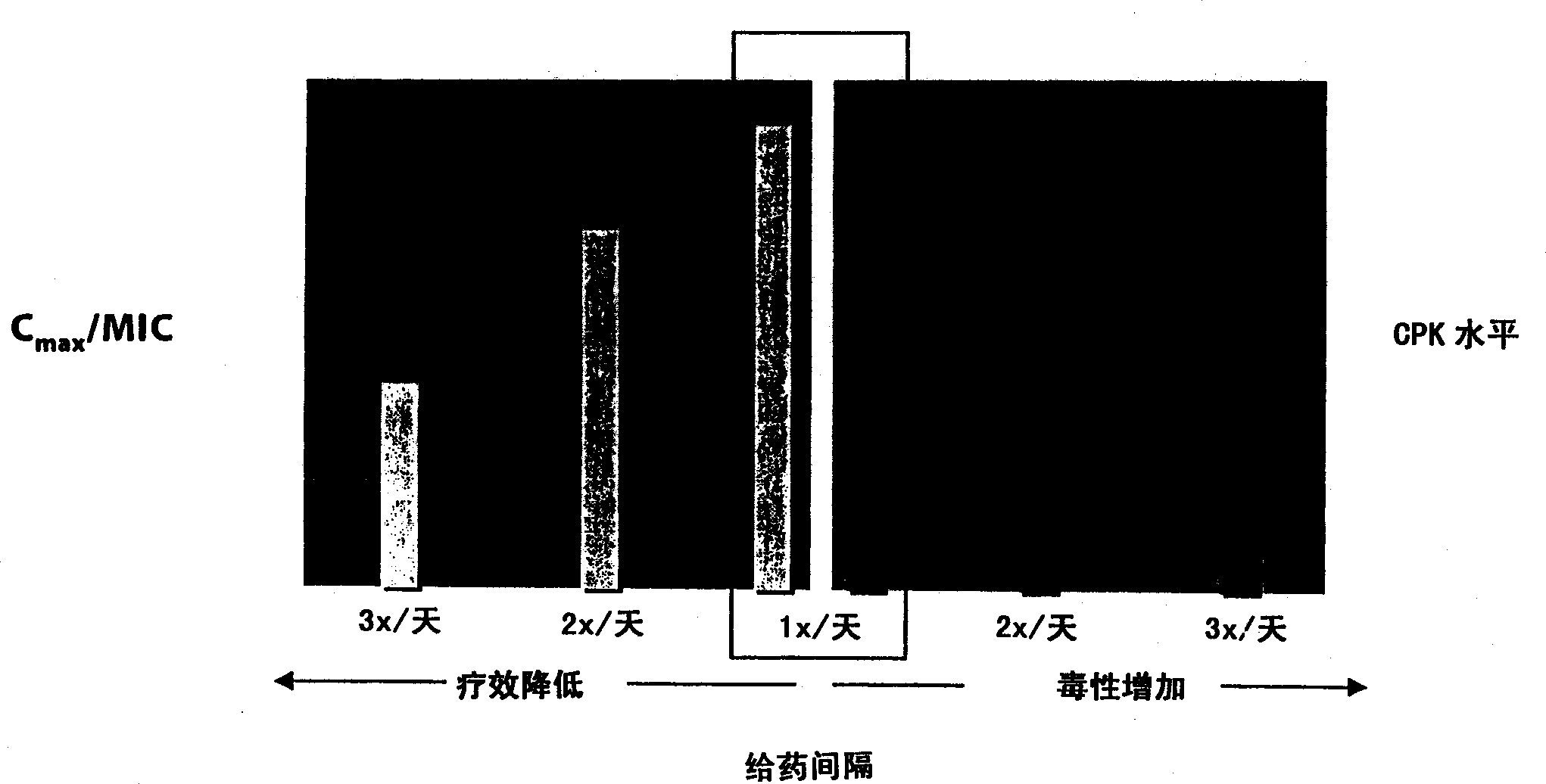
Methods for administration of antibiotics
InactiveUS6852689B2Minimizes skeletal muscle toxicitySufficient efficacy levelAntibacterial agentsBiocideAntibiotic YSkeletal muscle
The invention provides methods for administering a therapeutically effective amount of daptomycin while minimizing skeletal muscle toxicity. The methods provide daptomycin administration at a dosing interval of 24 hours or greater. This long dosing interval minimizes skeletal muscle toxicity and allows for higher peak concentrations of daptomycin, which is related to daptomycin's efficacy. The invention also provides methods of administering lipopeptide antibiotics other than daptomycin while minimizing skeletal muscle toxicity by administering a therapeutically effective amount of the lipopeptide antibiotic at a dosage interval that does not result in muscle toxicity. The invention also provides methods of administering quinupristin / dalfopristin while minimizing skeletal muscle toxicity by administering a therapeutically effective amount of quinupristin / dalfopristin at a dosage interval that dos not result in muscle toxicity.
Owner:CUBIST PHARMA INC
Method of providing sustained analgesia with buprenorphine
InactiveUS20010002259A1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationZero order kinetics
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an additional two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Tray insert for medication on demand device
InactiveUS20090078606A1Small article dispensingCoin-freed apparatus detailsMedication DispenserMedication dose
In one embodiment, the invention comprises a tray insert for a medication dispenser, the dispenser permitting access to a medication dose after a minimum dosing interval has elapsed since presentation of an immediately previous dose. The dispenser comprises a dispenser cover having an opening through which a patient accesses the medication dose presented through the opening and a carousel for receiving the tray insert. The tray insert comprises a substrate, a plurality of medication retention areas in the substrate each for holding at least one medication dose, each medication retention area received within a depression of the carousel and a cover removably affixed to an upper surface of the substrate for retaining the medication doses in the medication retention areas prior to use, wherein after the tray insert is mated with the carousel the cover.
Owner:AVANCEN MOD
Combination composition comprising oxycodone and acetaminophen for rapid onset and extended duration of analgesia
The present disclosure provides an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
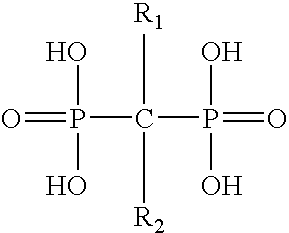
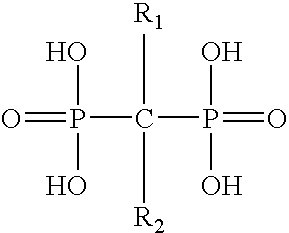
Pharmaceutical formulation for oral delivery of bisphosphates
InactiveUS20050182028A1Minimizing potential esophageal irritationMinimize irritationBiocidePhosphorous compound active ingredientsDosing regimenPharmacy
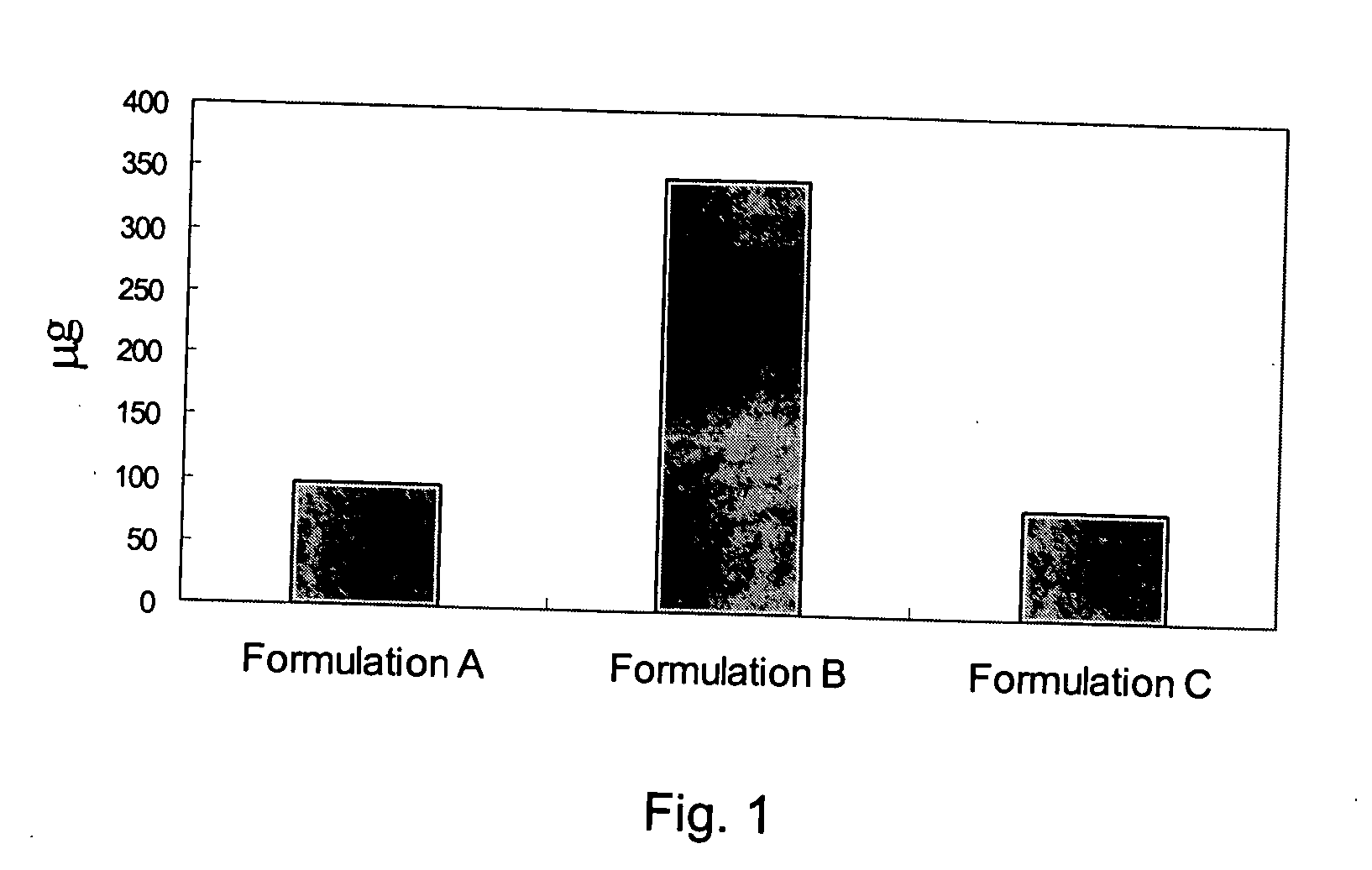
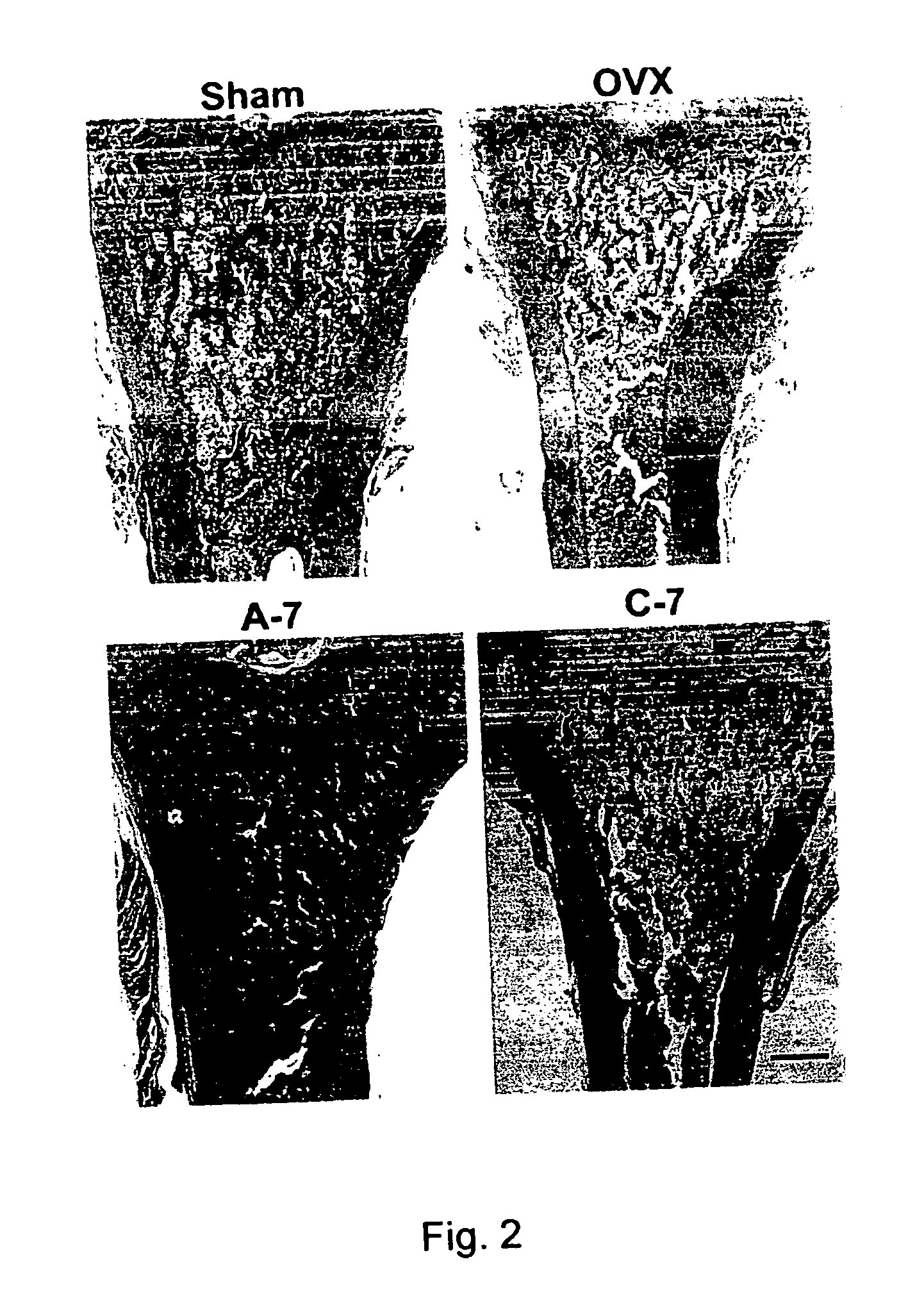
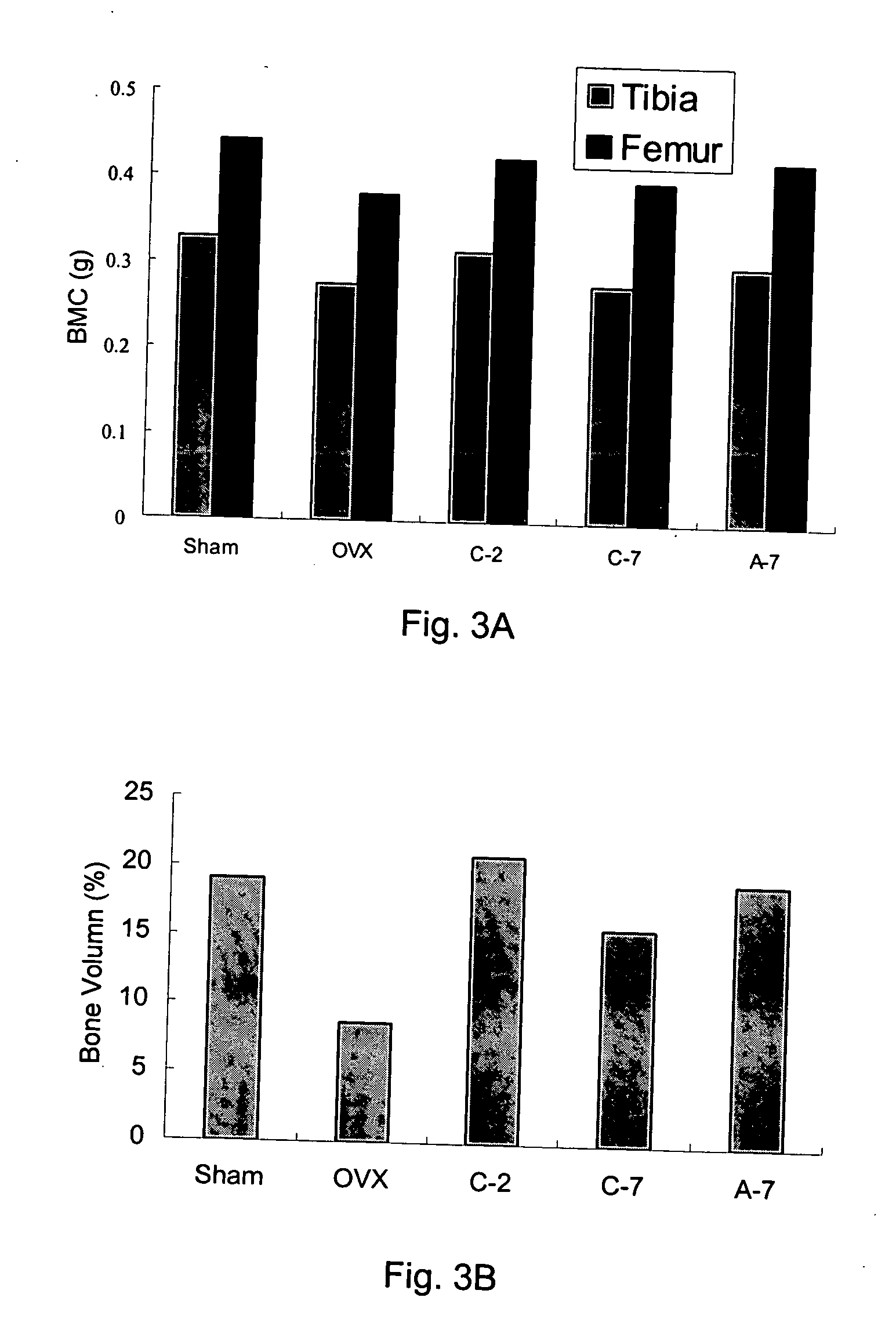
The present invention discloses a method for treating or preventing a bone disorder in a mammal in need thereof comprising orally administering to said mammal a pharmaceutically effective amount of a pharmaceutical composition of at least one bisphosphonate, or a pharmaceutically acceptable salt or esters thereof, and at least one aminoalky methacrylate copolymer, according to a dosing schedule having a dosing interval selected from once-weekly dosing, twice-monthly dosing, once-monthly, once-quarterly and once-annually dosing. The present invention further discloses a method for treating or preventing a bone disorder in a mammal in need thereof comprising continuously orally administering a unit dosage per-day to said mammal in a short time for a long time therapy.
Owner:CHEN CHIH MING JAMES
Methods and compositions for dosing of allergens
ActiveUS20050175638A1Easy to manageRelieve painAnthropod material medical ingredientsSnake antigen ingredientsImmunotherapyPharmacology
Owner:GREER LAB
Preparation of SRB (sulfate reducing bacterium) biological inhibitor and method for inhibiting SRB in crude oil
ActiveCN103114085AGrowth inhibitionInhibitionDrilling compositionOn/in organic carrierPolyvinyl alcoholNitrifying bacteria
The invention relates to a preparation method of an SRB (sulfate reducing bacterium) biological inhibitor, which comprises the following steps: by taking polyvinyl alcohol, sodium alginate, denitrifying bacterium suspension, calcium carbonate, boric acid, calcium chloride, silicon dioxide and water as raw materials, preparing an embedding agent, then preparing a microbial mixed solution, and finally preparing to obtain immobilized denitrifying bacterium balls. The prepared SRB biological inhibitor can be used in the inhibition of SRB in crude oil. Sodium nitrate particles are firstly added into an oil well casing port; then, the SRB biological inhibitor is added; and finally, water is added to flush the two chemicals to the bottom of a well, and the dosing interval of each time is 15 days, thus achieving the purpose of inhibiting the growth of SRB for a long time. According to the invention, by using the sodium nitrate particles to provide a nitrogen source for the denitrifying bacteria, an oil layer origin and exogenous denitrifying bacterium microorganisms are activated, and the original and exogenous microorganisms are utilized to change the growth environment of the system so as to quickly inhibit the growth of SRB in the oil well, thereby inhibiting the generation of hydrogen sulfide gas from the source and reducing corrosion and sulfide hazard caused by SRB.
Owner:SHAANXI URSTAR ENVIRONMENTAL SCI & TECH
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS20030054033A1Patient compliance is goodOrganic active ingredientsNervous disorderImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
Methods and compositions for dosing of allergens
ActiveUS8491909B2Anthropod material medical ingredientsViral antigen ingredientsImmunotherapyPharmacology
The present invention comprises methods and compositions for immunotherapy. An aspect of the invention comprises administration of one or more allergens in compositions via oral and sublingual routes. Allergen compositions are administered in dosing intervals wherein the increase in the one or more allergens administered to the patient are provided in increasing volumes of a single concentration of at least one allergen.
Owner:GREER LAB
Method for inhibiting bone resorption with an alendronate and vitamin d formulation
Composition and method for preventing or treating abnormal bone resorption in mammals, the composition characterized as containing, a supplementary effective amount of a non-activated metabolite of vitamin D2 and / or D3 and a pharmaceutically effective amount of bisphosphonate to provide vitamin D nutrition during treatment to facilitate normal bone formation and mineralization, while minimizing the occurrence of or potential for the complications associated with vitamin D insufficiency, such as hypocalcemia and osteomalacia. The method of preventing or treating may be further characterized by concomitantly administering the components simultaneously or alternately at dosing intervals selected from once-weekly, twice-weekly, bi-weekly, monthly, and bimonthly.
Owner:DAIFOTIS ANASTASIA G +2
Combination composition comprising oxycodone and acetaminophen for rapid onset and extended duration of analgesia
InactiveUS20140170217A1Reduce riskBiocidePharmaceutical non-active ingredientsRapid onsetAcetaminophen
Owner:MALLINCKRODT INC
Ambulatory Medication on Demand Dipsenser
ActiveUS20150310186A1Drug and medicationsOral administration deviceComputer hardwareMedication Dispenser
A medication dispenser. The dispenser comprises a sensor for receiving biometric information from a user, a memory for storing a minimum dosing interval, a controller for determining whether the user is an authorized user, a belt having medication-carrying slots, a dose opening defined in an exterior surface of the dispenser, and a signaling device. The controller activates the signaling device and the sensor only when the minimum dosing interval has elapsed as measured from a last successful user authentication. The sensor is responsive to user biometric information provided any time after activation of the signaling device and the sensor, but not before activation of the signaling device and the sensor. After determining the use is an authorized user, a medication-carrying slot is aligned with the dose opening.
Owner:ADVANCEN MOD
Tray insert for medication on demand device
InactiveUS7661532B2Small article dispensingCoin-freed apparatus detailsMedication DispenserMedication dose
Owner:AVANCEN MOD
Extended Release Compositions Comprising Hydrocodone And Acetaminophen For Rapid Onset And Prolonged Analgesia That May Be Administered Without Regard To Food
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Giant knotweed rhizome active ingredient emodin extraction method
InactiveCN1800122AAbsolute reductionReduce consumptionHydrocarbon purification/separationHydrocarbonsAlcoholPOLYGONUM CUSPIDATUM
The invention provides a method for extracting the effective component archin of the giant knotweed rhizome, which puts the drugs into five extracting tanks after shattering them to in turn extract the E, D, C, B, A tank with the extracting solution: 30%-70% alcohol whose amount is 8-12 volume of the drug's quality, the temperature: 35-65 deg. and the time: 20-50 minutes, so that the effect component of the extracting solution of the five tanks descendent, it then discharges opeing the tank which has the highest effect component and discharges the dross of the tank which has been five solution extracted, it dose interval removal to the other extracting solution and dose four cycles and then amalgamates the extracting solutions.
Owner:ZHEJIANG UNIV
Novel ceftriaxone sodium for injection
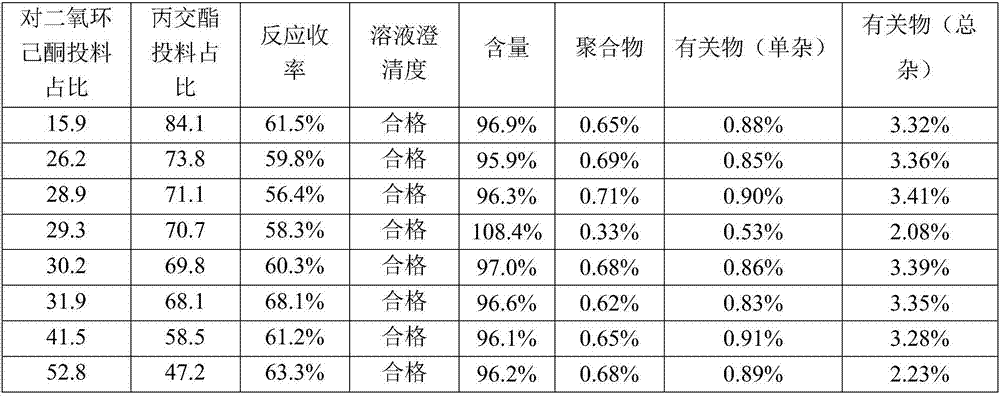
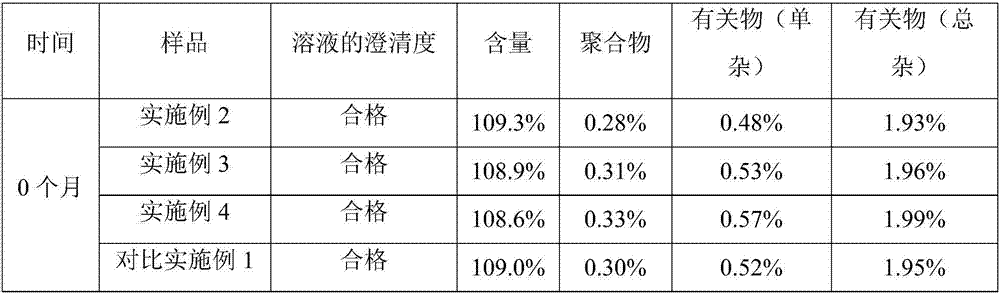
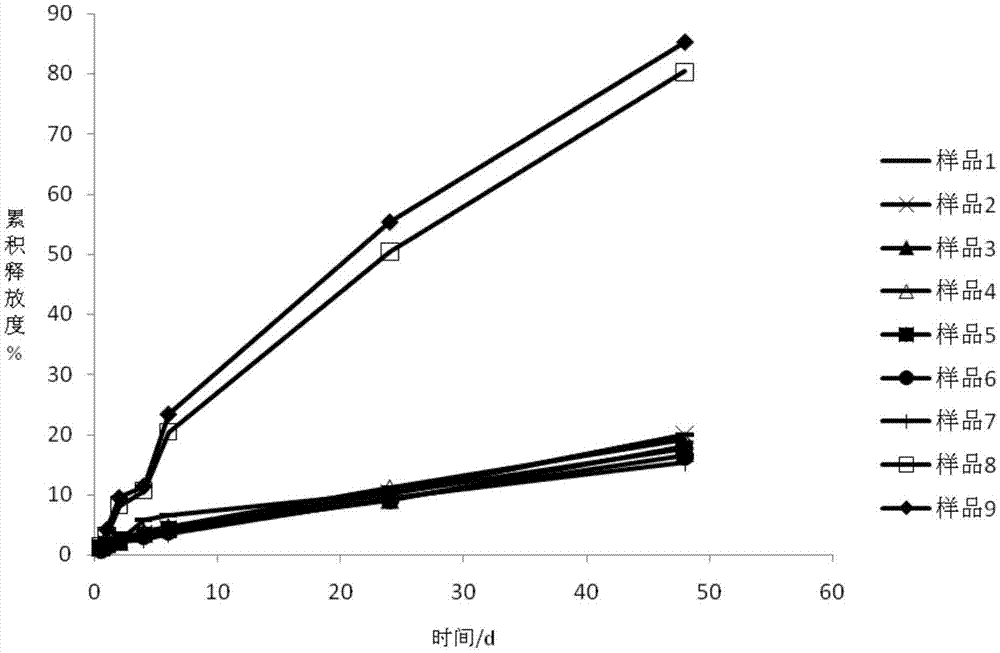
InactiveCN107982244ASimple preparation processLow costAntibacterial agentsOrganic active ingredientsP-dioxanoneBlood concentration
The invention relates to novel ceftriaxone sodium for injection and a preparation method thereof, and belongs to the technical field of medicines. The novel ceftriaxone sodium for injection consists of 1 part of ceftriaxone sodium, 0.30 to 0.33 part of ethyecellulose, 0.60 to 0.72 part of a stabilizer, 0.80 to 0.90 part of a surfactant, 70 to 80 parts of liquid paraffin and 0.27 to 0.30 part of ananti-sticking agent. The stabilizer p-dioxanone-lactide copolymer is a copolymer with a specific proportion, and plays an important role in preparation stability. According to the injection, the drugdelivery application twice every day becomes once administration through slow-release effect of a micro-capsule, the dosing interval is shortened, the injection is convenient to use, the biological availability is improved, the using amount of medicine is reduced, the curative effect is improved since the blood concentration is smooth, and the untoward effect is reduced. The novel ceftriaxone sodium for injection, provided by the invention, is simple in preparation technology and low in cost, is applicable for large-scale production, and is a product with very good market prospects.
Owner:石药集团中诺药业(石家庄)有限公司
Apremilast gel for injection to articular cavity and preparation method of apremilast gel
InactiveCN106924174AImprove disadvantagesReduce adverse reactionsOrganic active ingredientsAerosol deliveryArticular cavityAqueous solution
The invention mainly provides apremilast gel for injection to the articular cavity and a preparation method of the apremilast gel. According to the apremilast gel and the preparation method, polymer with the temperature-sensitive reverse gel property is adopted as a carrier material, and medicines are dispersed or dissolved in an aqueous solution of the carrier material. When the temperature is lower than the body temperature, a sample exists in the form of sol, and is injected into the body through a common syringe; when the temperature of the injected part rises to the body temperature, the sol is transformed into semi-solid gel, along with the slow dissolving of the carrier material, the medicines are released from the gel at the low rate, and the release of the medicines can last for several weeks to several months. The release ratio of the medicines can be realized by changing the variety, concentration, proportion and molecular weight of the gel material. The dosing interval of the gel is long, the preparation process is simple, the preparation process is mature, and the method is suitable for industrial mass production.
Owner:CHONGQING UNIV
Methods for administration of antibiotics
Owner:CUBIST PHARMA INC
Ambulatory medication on demand dispenser
ActiveUS9483621B2Drug and medicationsOral administration deviceComputer hardwareMedication Dispenser
Owner:ADVANCEN MOD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com