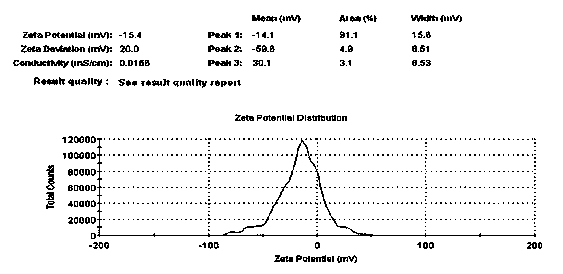
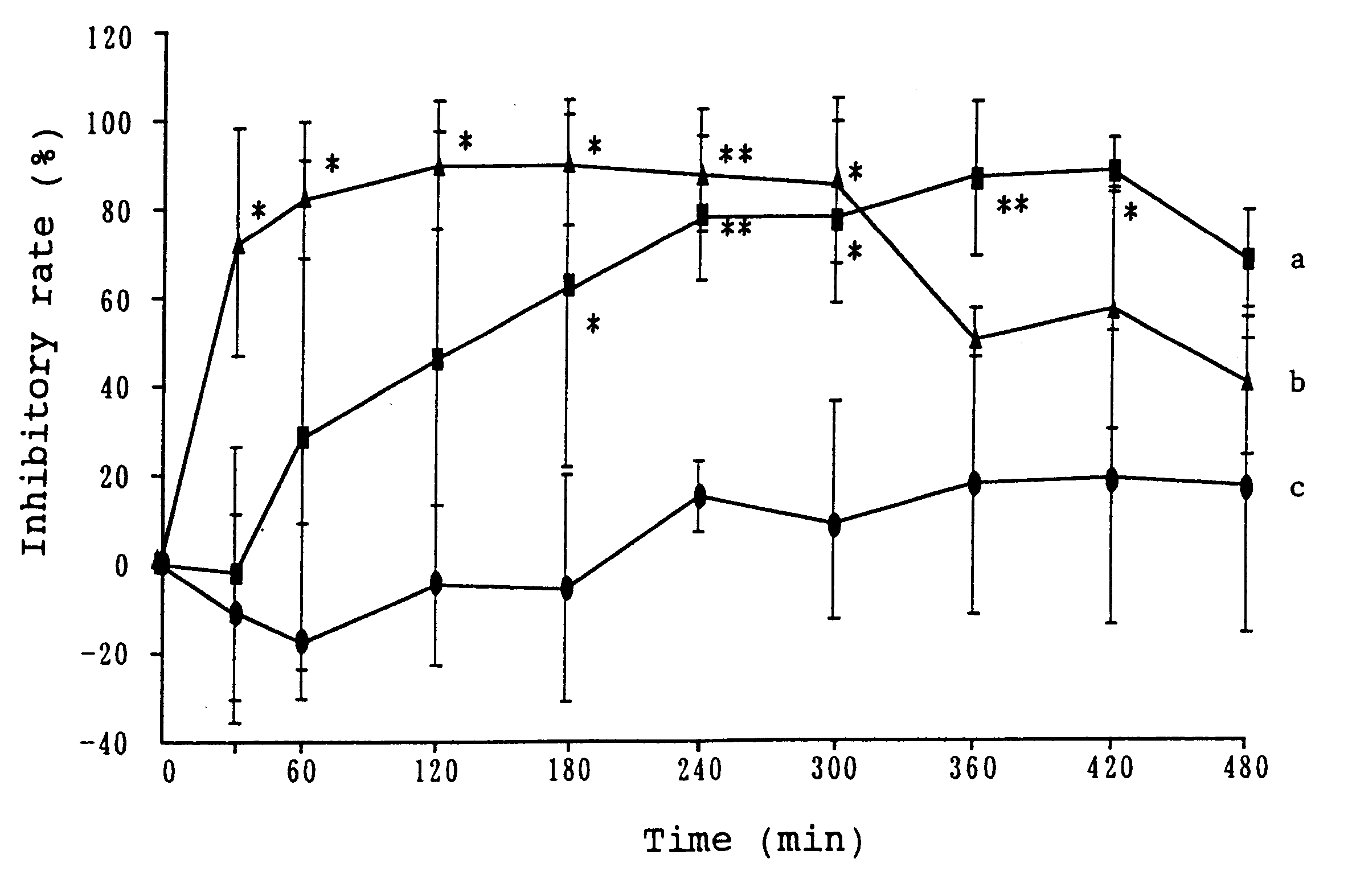
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
586 results about "Percutaneous absorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
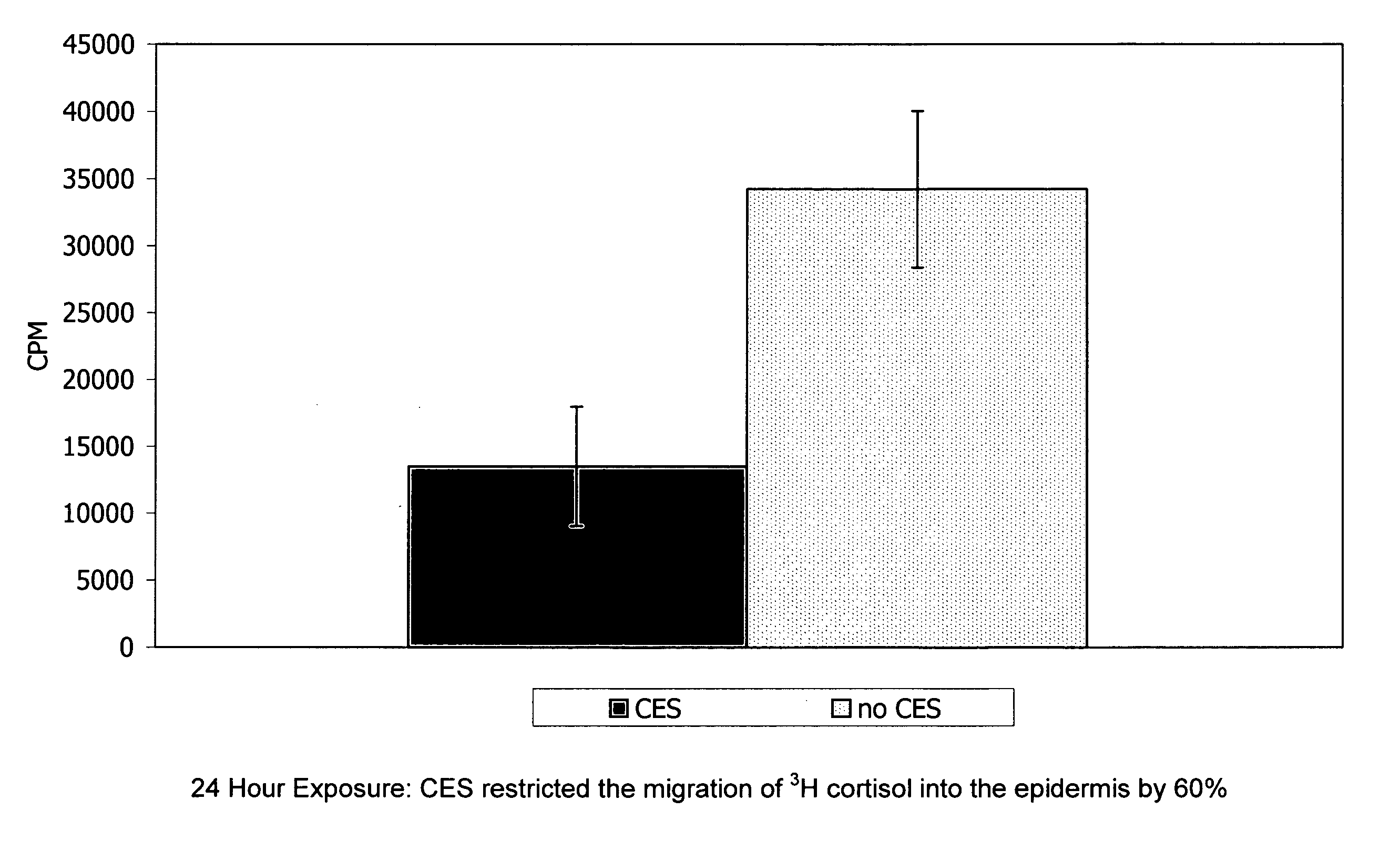
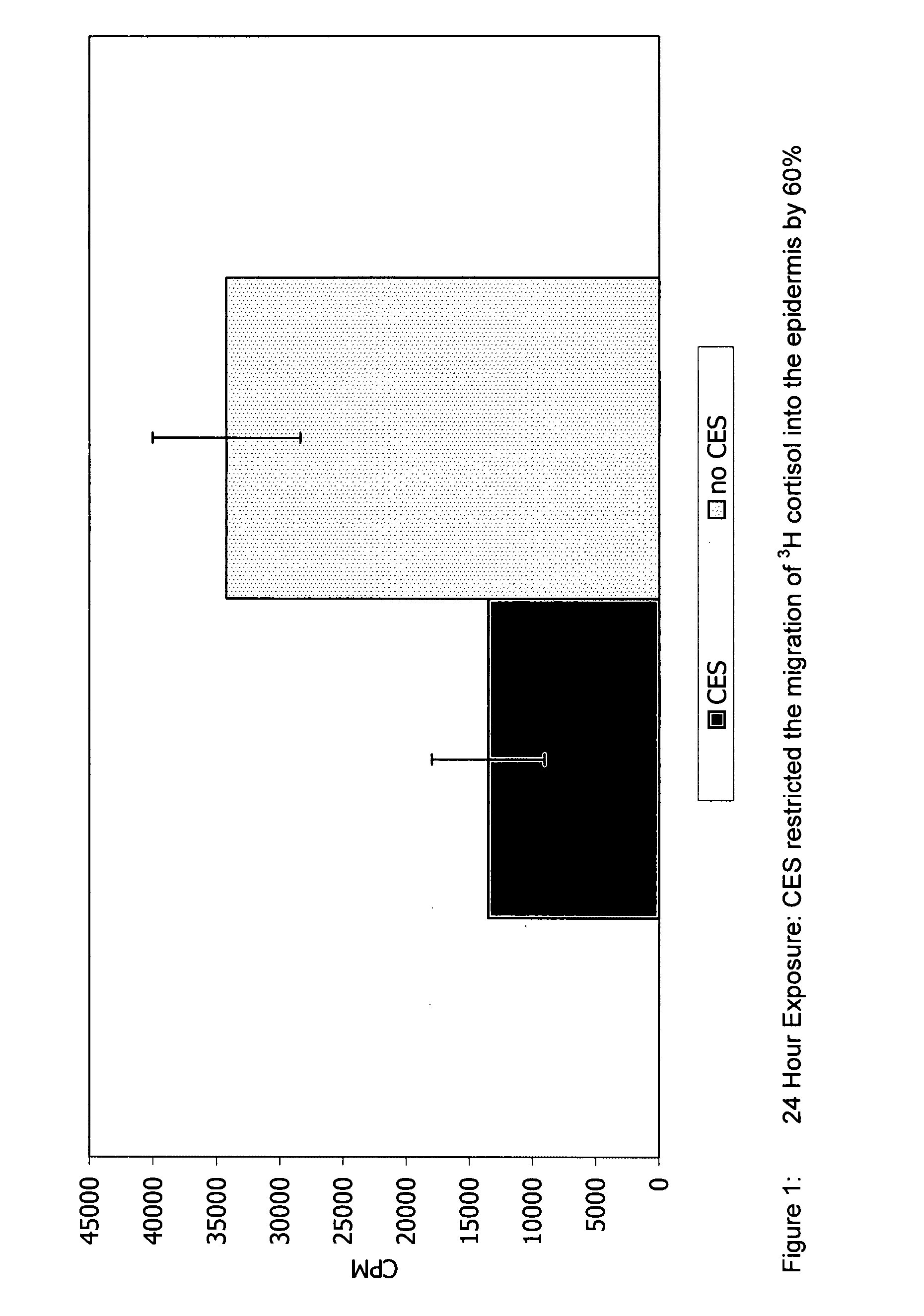
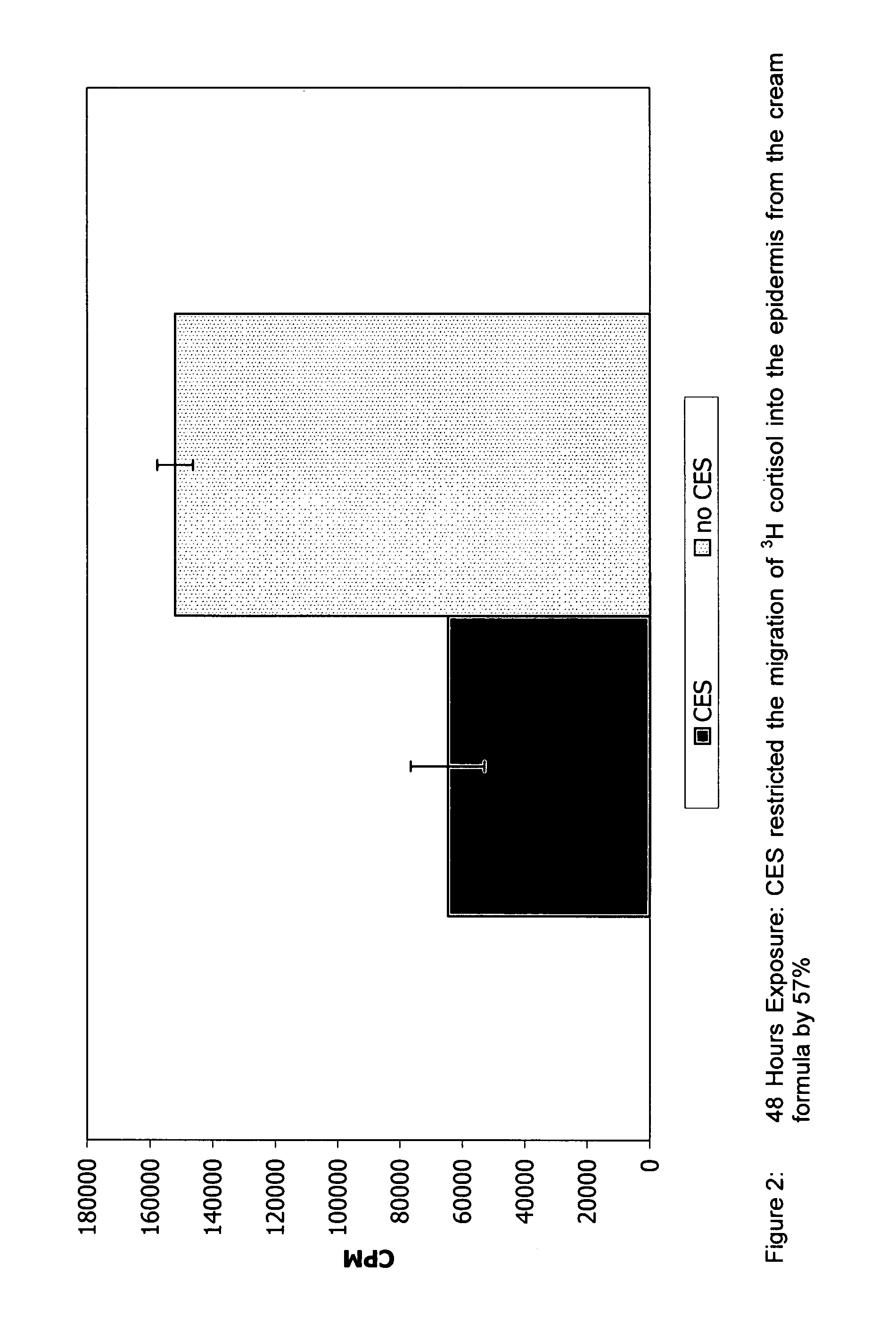
Preparations for percutaneous absorption
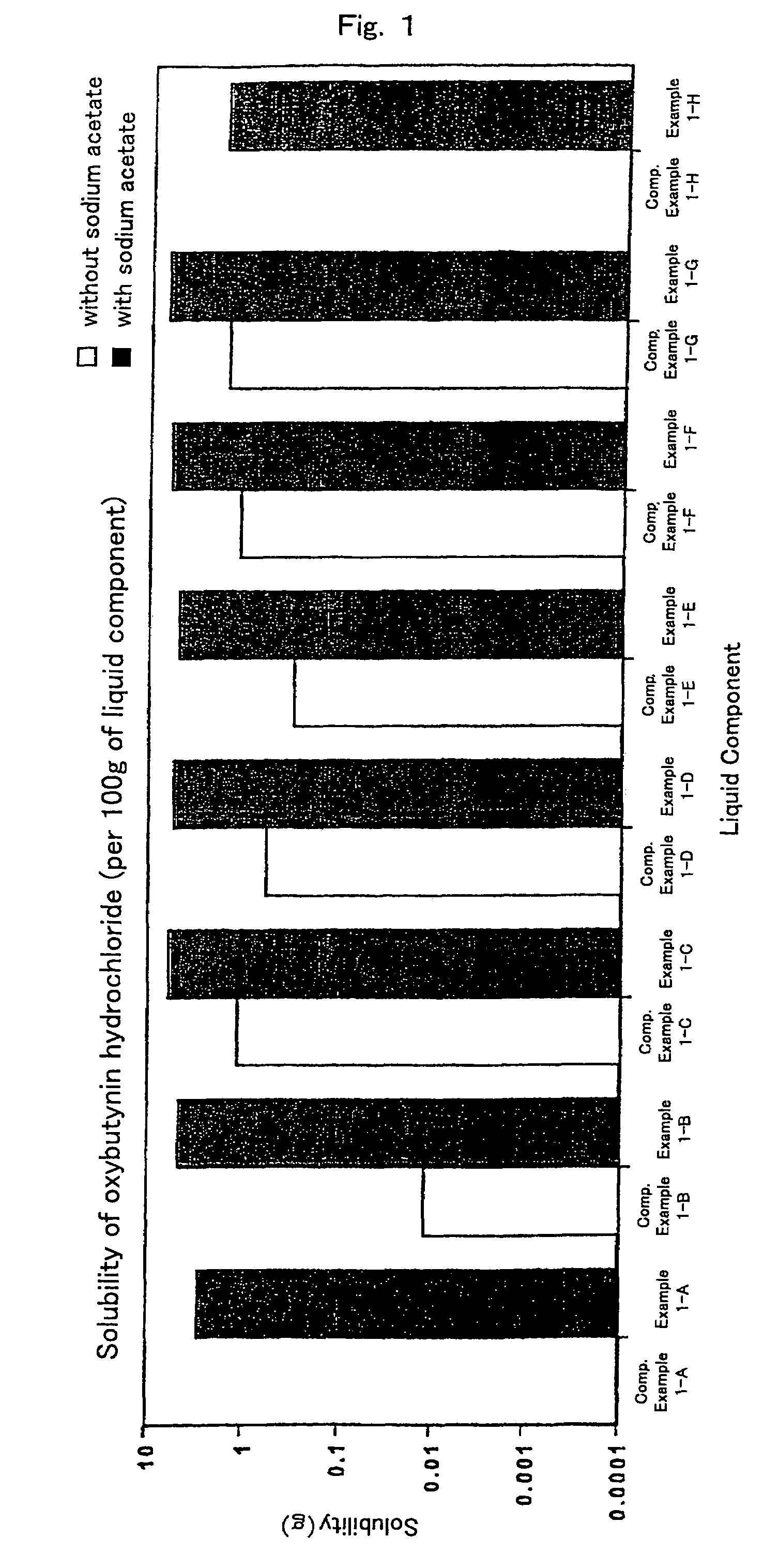
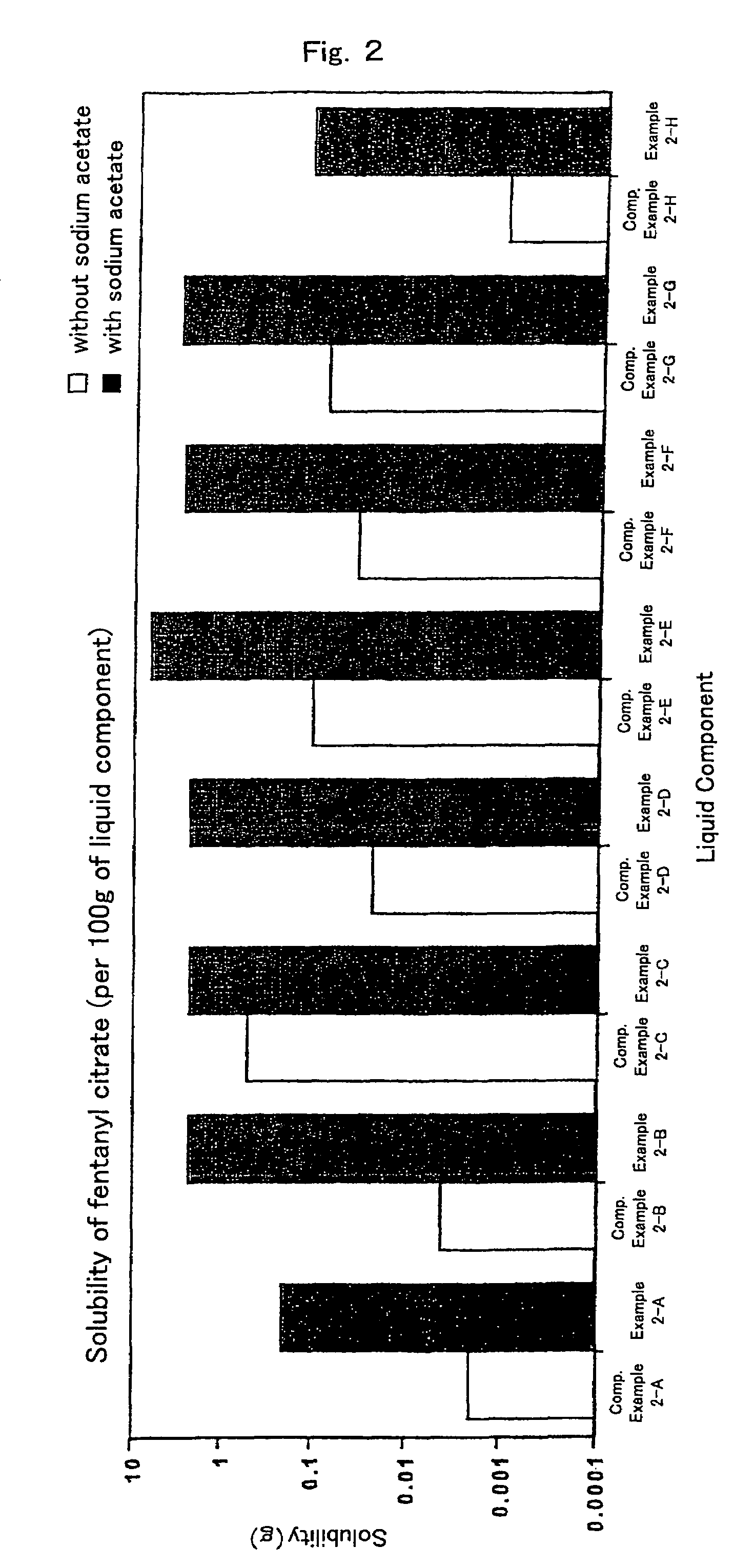
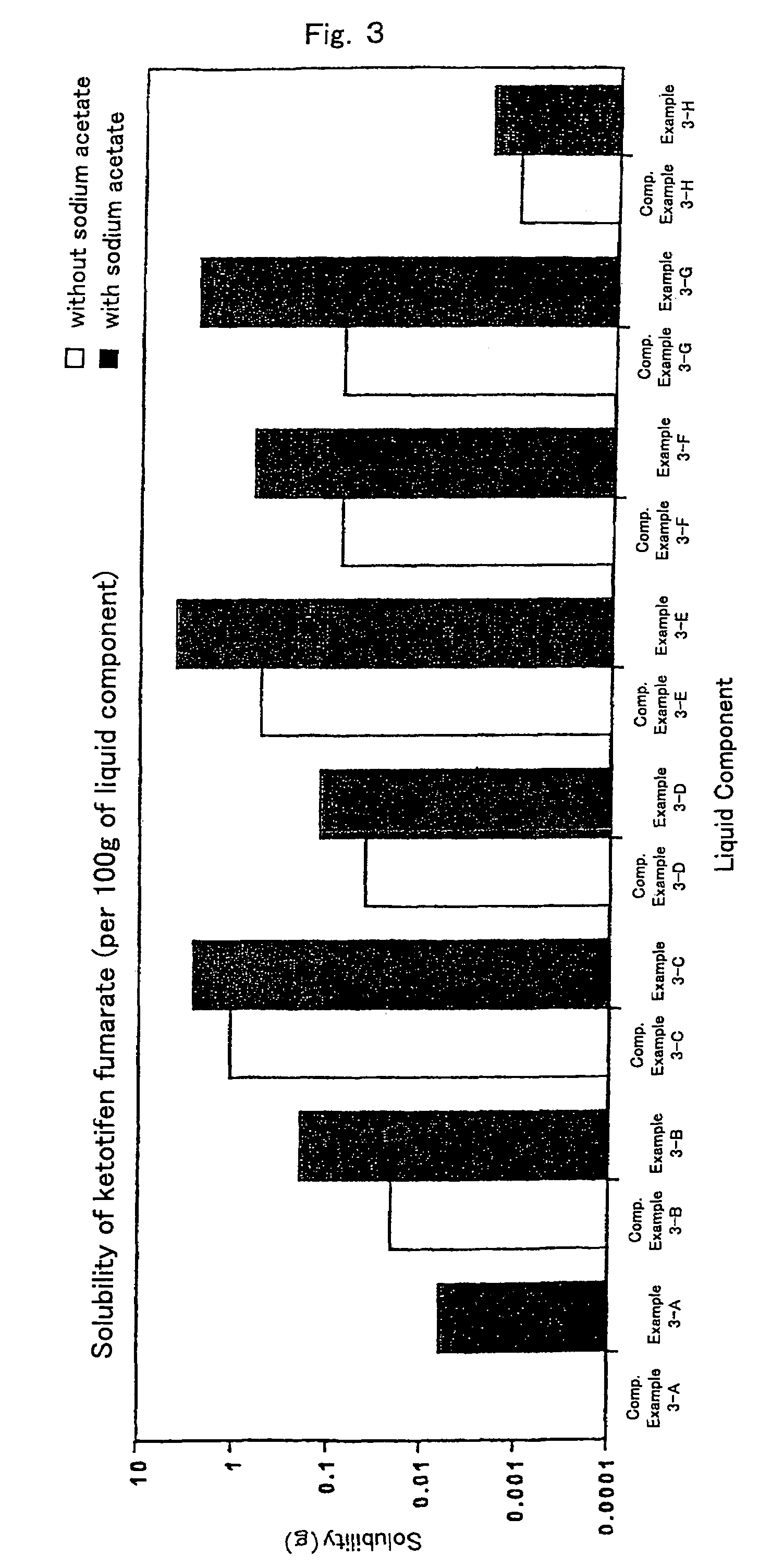
InactiveUS7504114B1Promote percutaneous absorptionSkin safeAntiinfectivesPharmaceutical non-active ingredientsOrganic acidSolubility
Preparations for percutaneous absorption comprising a basic drug or its salt dissolved in a liquid component and having an enhanced percutaneous absorbability and a safety to the skin, i.e., the administration site. The preparations for percutaneous absorption, preferably patches, contain a basic drug or its salt, an organic acid or its salt and a liquid component having a solubility parameter of from 7 to 13 (cal / cm3)1 / 2 and a have a very excellent skin permeability of the drug.
Owner:HISAMITSU PHARM CO INC
Micro-current Iontophoretic Percutaneous Absorptive Patch
A self-contained, conveniently disposable percutaneous absorptive patch of the present invention utilizes, in a novel ways, the existing percutaneous absorptive principles to delivering topical medications quickly, painlessly, and effectively through the skin barrier. The absorptive principles include the epidermal microcuts / micro-needles, permeation enhancers, and micro-current iontophoresis. Most importantly, the transdermal absorptive patch of the present invention introduces a novel incorporation of self-producing micro-electrical currents and micro-electrodes into the existing conventional iontophoretic principle.
Owner:VOLT KEVIN
Percutaneous absorption preparations
InactiveUS20040258741A1Maintained relatively longConstant rateNervous disorderPharmaceutical non-active ingredientsSide effectPharmaceutical drug
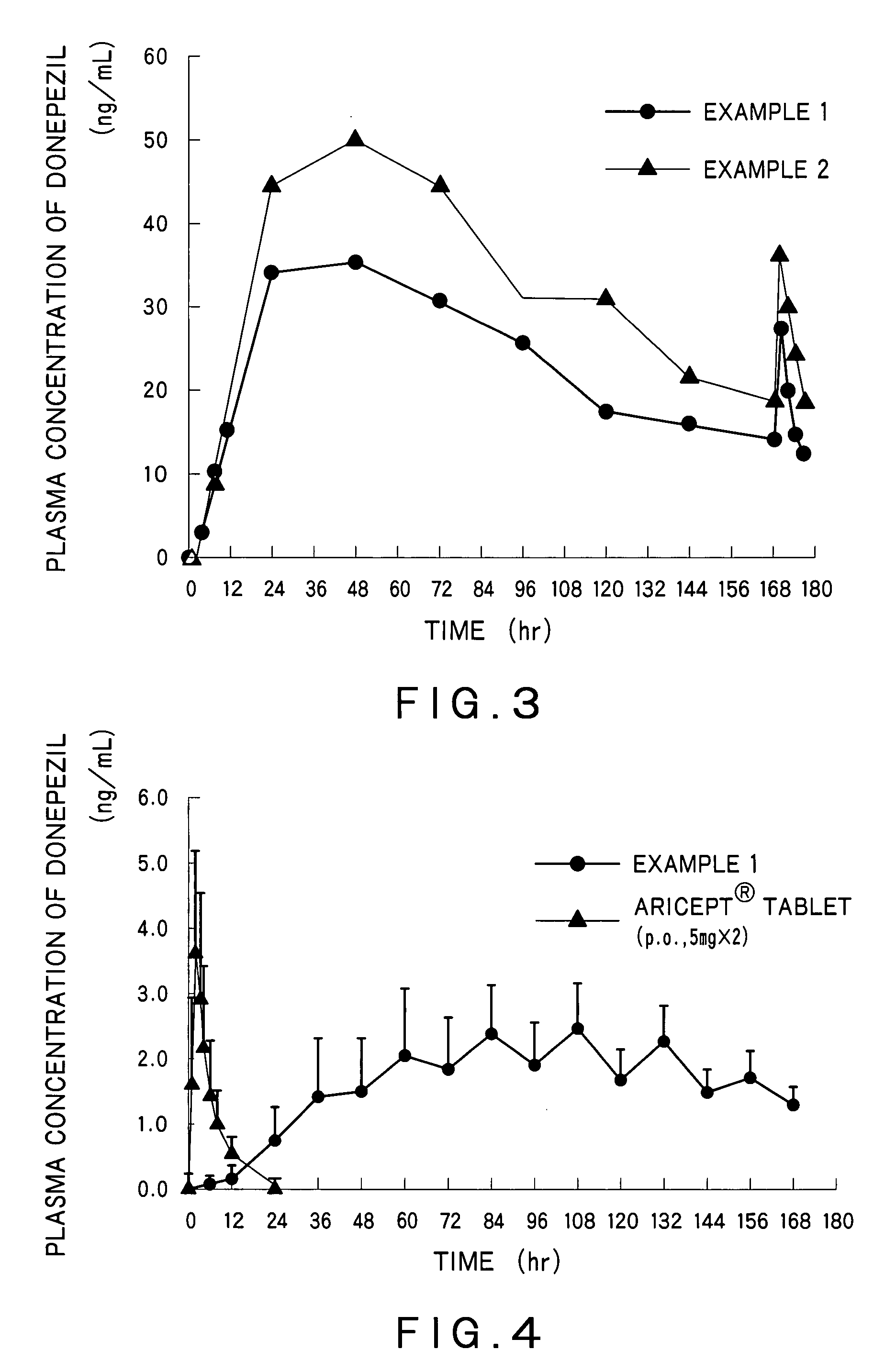
Percutaneous absorption preparations for treating dementia which contain an adhesive composition, characterized in that the adhesive composition contains the active ingredient in a dispersed state, the active ingredient is released at a pharmacologically effective rate and the skin permeation rate thereof is at least 1.2 mug / cm<2> / h. Thus, it is possible to provide percutaneous absorption preparations whereby the therapeutic effect can be sustained over a prolonged period of time without elevating the concentration of the active ingredient in the plasma to such a level as causing the expression of side effects in the administration of remedies for dementia.
Owner:HISAMITSU PHARM CO INC
Modification of percutaneous absorption of topically active materials
InactiveUS20080039405A1Cut skinExtended retention timeBiocideHydrocarbon active ingredientsAdditive ingredientRetention time
The present invention relates to methods of influencing the flux or surface retention time of a topically active pharmaceutical ingredient through skin and formulations relating thereto.
Owner:CRODA
Compositions and Methods for the Treatment of Inflammatory Dermatosis and Other Pathological Conditions of the Skin
InactiveUS20100080768A1Small sizeReduce appearanceOrganic active ingredientsAntipyreticActive agentSjogren's disease
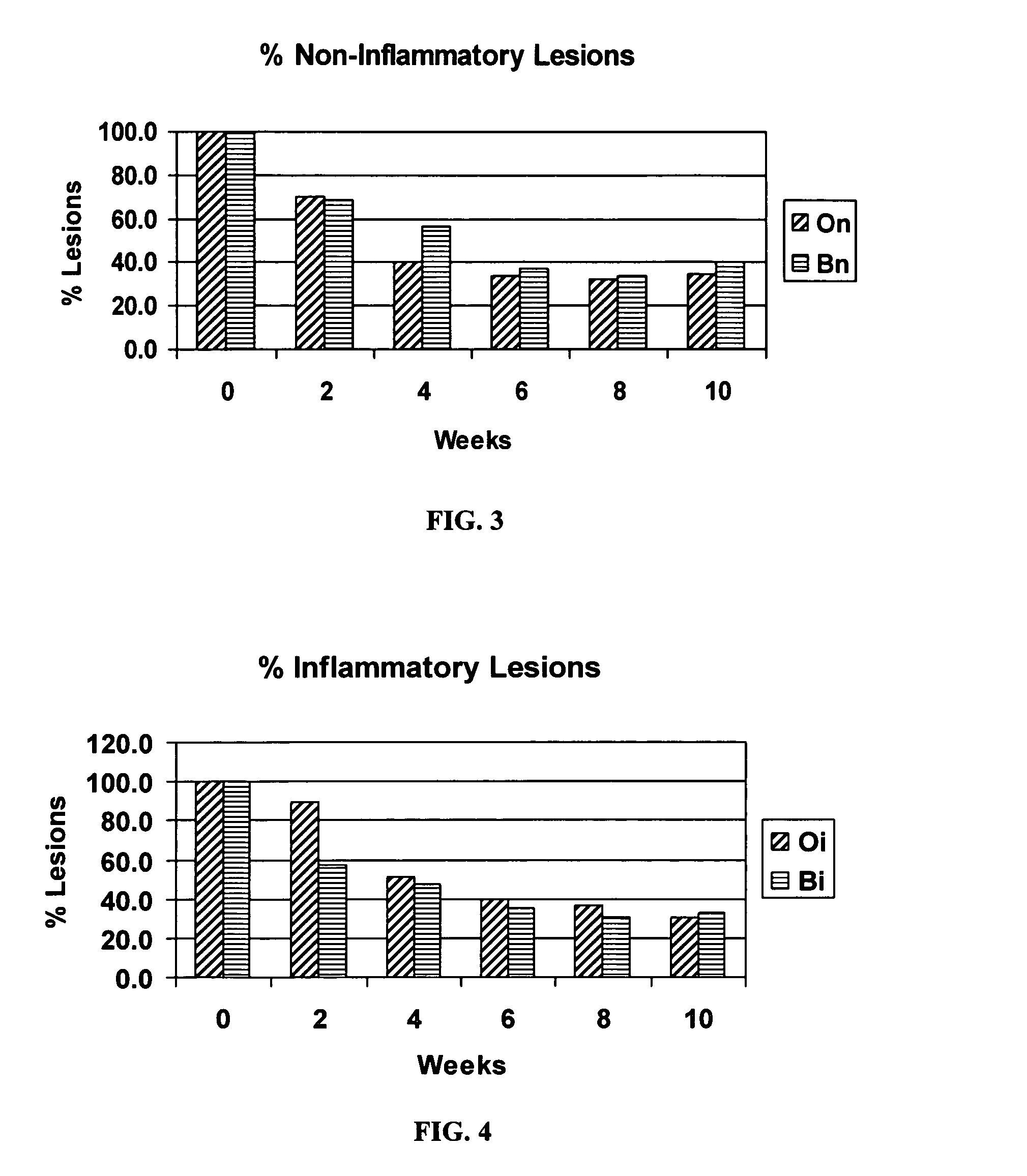
The present invention relates to a composition used as a vehicle for percutaneous absorption of Pharmaceutical and Cosmaceutical active agents that comprises Dimethiconol (hydroxyl-terminated polydimethydimethylsiloxane), dimethicone-350 (polydimethylsiloxane-350), cyclomethicone-5 nf (decamethylcyclopentasiloxane), alkymeth siloxane copolyol-lauryl peg / ppg 18 / 18 methicone (alkymethyl siloxane copolyol), cyclopentasiloxane and dimethicone Crosspolymer (silicone elastomer and decamethylcyclopentasiloxane), stearoxytrimethylsilane and stearyl alcohol (silicone wax), and deionized water. This composition serves several key applications: (1) it is a vehicle for percutaneous absorption of Pharmaceutical and Cosmaceutical active agents; (2) it acts as a method for utilizing other compositions in the treatment of inflammatory conditions of the skin including, but by no means limited to, atopic dermatitis (eczema), allergic contact dermatitis, seborrheic dermatitits, psoriasis, xerosis and atopia; (3) it is a treatment of inflammatory conditions of mucosae; (4) it relates to other compositions and methods for protecting and enhancing the barrier function of the skin.
Owner:MCGRAW THOMAS L +3
Formulations for topical delivery of bioactive substances and methods for their use
ActiveUS7241456B2Efficient deliveryNon-irritating to the skinBiocideCosmetic preparationsHigh concentrationIrritation
The invention relates to topical delivery of bioactive agents. More particularly, the invention relates to anhydrous formulations for percutaneous absorption. The invention provides formulations that allow efficient topical delivery of high concentrations of bioactive substances for percutaneous absorption. The formulations according to the invention are generally non-irritating to the skin.
Owner:AUSTRALIAN IMPORTERS
Sorbefacients and preparations for percutaneous absorption containing the same
InactiveUS7153522B1Softness is littleSoftnessPowder deliveryOrganic active ingredientsMentholLuteal hormone
Percutaneous sorbefacients comprising hexylene glycol and 1-menthol, more particularly, percutaneous sorbefacients for female hormones or derivatives thereof; and preparations for percutaneous absorption which comprise a styrene / isoprene / styrene block copolymer and / or polyisobutylene, a softener and a tackifier as the base components, hormones, in particular, follicle hormone and / or luteal hormone as the drug component and hexylene glycol and 1-menthol as a sorbefacient.
Owner:HISAMITSU PHARM CO INC
Percutaneous absorption preparations of antidementia drugs
InactiveUS20070259028A1Good release performanceStable drug releasabilityBiocideNervous disorderDrug reservoirAlcohol
Disclosed is a percutaneous absorption preparation which enables the stable administration of an antidementia drug over a long period of time. More particularly, the percutaneous absorption preparation of the antidementia drug which is used as a plaster on skin comprises at least an adherent layer, an intermediate membrane, and a drug reservoir layer sequentially from the side which is plastered on skin, wherein the drug reservoir layer comprises at least an antidementia drug, an aminated polymer, a polyhydric alcohol, and one or more carboxylic acid esters, the intermediate membrane enables the controlled permeation of the antidementia drug into the side of skin, the adherent layer enables the plastering of the percutaneous absorption preparation on skin, and is permeable to the antidementia drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Percutaneous absorption preparation for treating ophthalmic disease, use thereof and method for migration of ophthalmic remedy into topical tissue in eye
InactiveUS20060036220A1Efficacy can be sustainedly developedCan be sustainedly developedSenses disorderAntipyreticDiseaseEyelid
A transdermal drug delivery system for treatment of ophthalmic diseases comprising a structure that a plaster layer containing a remedy for ophthalmic diseases is provided on a support, wherein the system is applied to a skin surface including a front surface of an eyelid to administer the remedy for ophthalmic diseases in the plaster layer to an ophthalmic topical tissue by percutaneous permeation substantially without being administered through a systemic blood flow. Use of the transdermal drug delivery system for treatment of ophthalmic diseases, comprising applying the transdermal drug delivery system to a skin surface including a front surface of an eyelid to transfer the remedy for ophthalmic diseases in the plaster layer to an ophthalmic topical tissue by percutaneous permeation substantially without being administered through a systemic blood flow, and a method for transferring the remedy for ophthalmic diseases to the ophthalmic topical tissue.
Owner:SENJU PHARMA CO LTD
Benzoyl peroxide compositions and methods of use
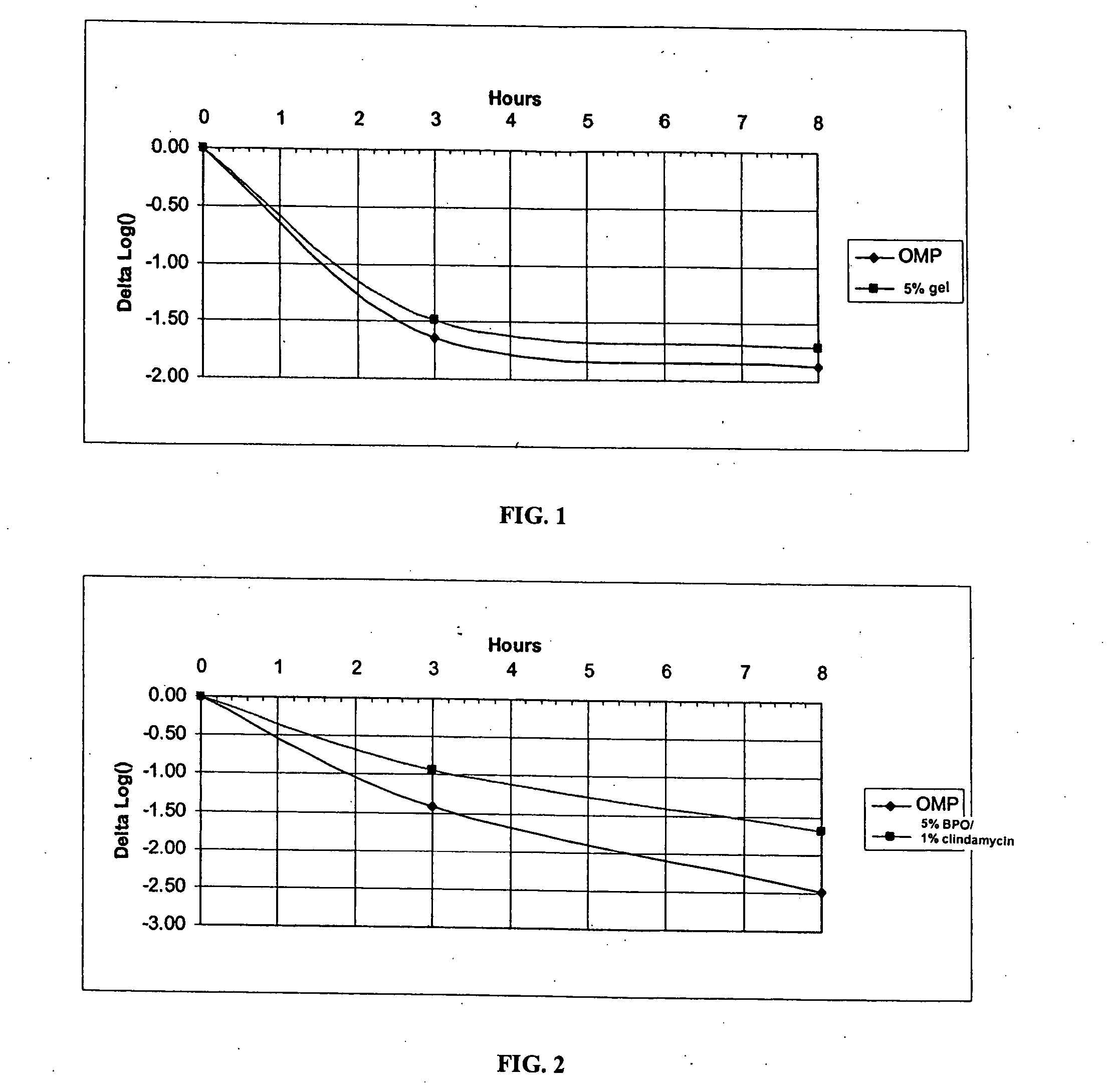
Solutions of benzoyl peroxide, in one or more solvents are provided that are suitable for direct topical application to the skin of a user or can be formulated into a product that is suitable for topical application to the skin of a user. In embodiments, the solutions include benzoyl peroxide and optionally additional active or inactive ingredients, and a solvent or mixture of solvents in which the benzoyl peroxide is prepared as a clear solution at 25° C. Methods of using the solutions are also described. Product formulations such as emulsion are also described. The solutions and product formulations can increase the efficacy and percutaneous absorption of benzoyl peroxide.
Owner:JR CHEM LLC
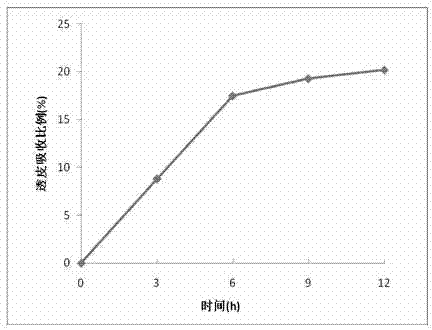
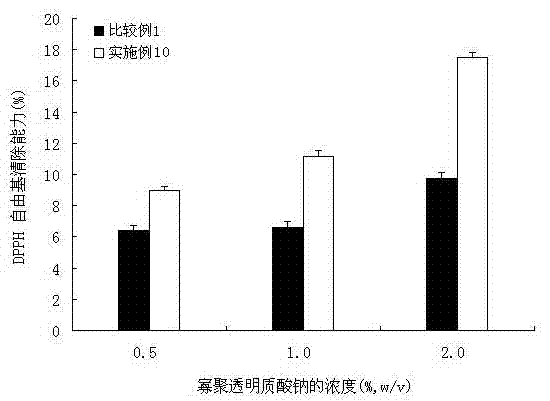
Method for preparing oligomeric hyalurate by digestion method, and oligomeric hyalurate and application thereof
ActiveCN102876748ALow costImprove stabilityMicroorganism based processesFermentationAntioxidant capacityKetone
The invention discloses a method for preparing oligomeric hyalurate by a digestion method, and the oligomeric hyalurate and an application thereof. Bacillus hyaluronidase obtained through fermental cultivation of Bacillus sp. A50 CGMCC NO.5744 is used for degrading hyaluronic acid or a salt thereof, and the method comprises the steps of preparing the hyaluronic acid or the salt thereof, enzymolysis, inactivation, filtering, settling, dewatering and drying. According to the method, the hyaluronidase produced by the Bacillus is used for degrading the hyaluronic acid or the salt thereof, and the oligomeric hyalurate is prepared through dezymotizing, alcohol or ketone settling and dewatering and drying. The method is simple to operate and mild in condition, has no destroy on the product structure and no environmental pollution, the hyaluronidase for fermentation is low in cost and is suitable for large-scale industrial production, the prepared oligomeric hyalurate has the advantages of good percutaneous absorption capability, high purity, no cytotoxicity, strong oxidation resistance and the like, and can be used in fields such as cosmetics, foods and medicines.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Tranexamic acid skin externally applied nano-preparation, as well as preparation method and use thereof
InactiveCN103565743AIncrease percutaneous permeabilityGood curative effectPeptide/protein ingredientsEmulsion deliveryUse medicationMedicine
The invention relates to a tranexamic acid skin externally applied nano-preparation, as well as a preparation method and a use thereof. The nano-preparation is prepared from tranexamic acid, medicated oil, an emulsifier, pharmaceutical additives, a thickener and pure water. The tranexamic acid skin externally applied nano-preparation provided by the invention has good physiological skin compatibility and can effectively promote the percutaneous absorption of tranexamic acid, improve the effect of preventing or treating pigmentation of the tranexamic acid and avoid toxicity and side effects, which are caused by systemic administration of the tranexamic acid.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY +1
Percutaneous absorption preparations of antidementia drugs
InactiveUS7858114B2Good release performanceStable drug releasabilityBiocideNervous disorderDrug reservoirAlcohol
Disclosed is a percutaneous absorption preparation which enables the stable administration of an antidementia drug over a long period of time. More particularly, the percutaneous absorption preparation of the antidementia drug which is used as a plaster on skin comprises at least an adherent layer, an intermediate membrane, and a drug reservoir layer sequentially from the side which is plastered on skin, wherein the drug reservoir layer comprises at least an antidementia drug, an aminated polymer, a polyhydric alcohol, and one or more carboxylic acid esters, the intermediate membrane enables the controlled permeation of the antidementia drug into the side of skin, the adherent layer enables the plastering of the percutaneous absorption preparation on skin, and is permeable to the antidementia drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Ginseng-based whitening and anti-aging nanoemulsion essence and preparation method thereof
InactiveCN103462846AEasy to prepareGood repeatabilityCosmetic preparationsToilet preparationsBiotechnologyFruit juice
The invention relates to the technical field of pharmaceutical nanoemulsion drug delivery systems and transdermal absorption, in particular relates to ginseng-containing whitening and anti-aging nanoemulsion essence and a preparation method thereof, and belongs to a new pharmaceutical technology and the field of study on new formulations applied to cosmetics. The nanoemulsion essence is characterized in that a natural or food-grade emulsifier and an oil phase are selected, wherein double distilled water containing fruit juice, vegetable juice or honey is used as an oil phase. The nanoemulsion essence comprises the following components in percent: 10.30 to 80.60% of emulsifier / co-emulsifier, 1.0 to 40.80% of oil phase, 0.10 to 14.0% of ginseng, and the balance of the water phase. The confocal microscopy result shows that the transdermal absorption mechanism of the essence is the mechanism of majorly dispersing among cells of cutaneous appendages at the beginning and then dispersing and penetrating through cytomembrane. The nanoemulsion essence has high absorption, is innocuous, unpoisonous and pollution-free, does not damage the skin, and can completely realize the green, health, whitening, anti-aging and beautifying makeup concept.
Owner:JILIN UNIV
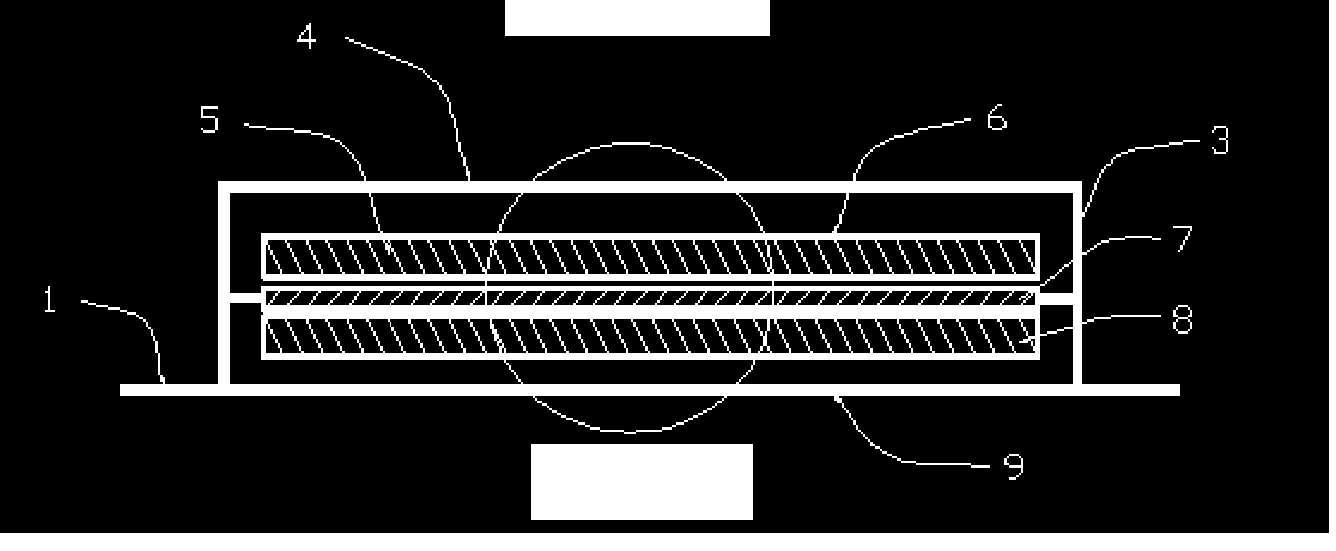
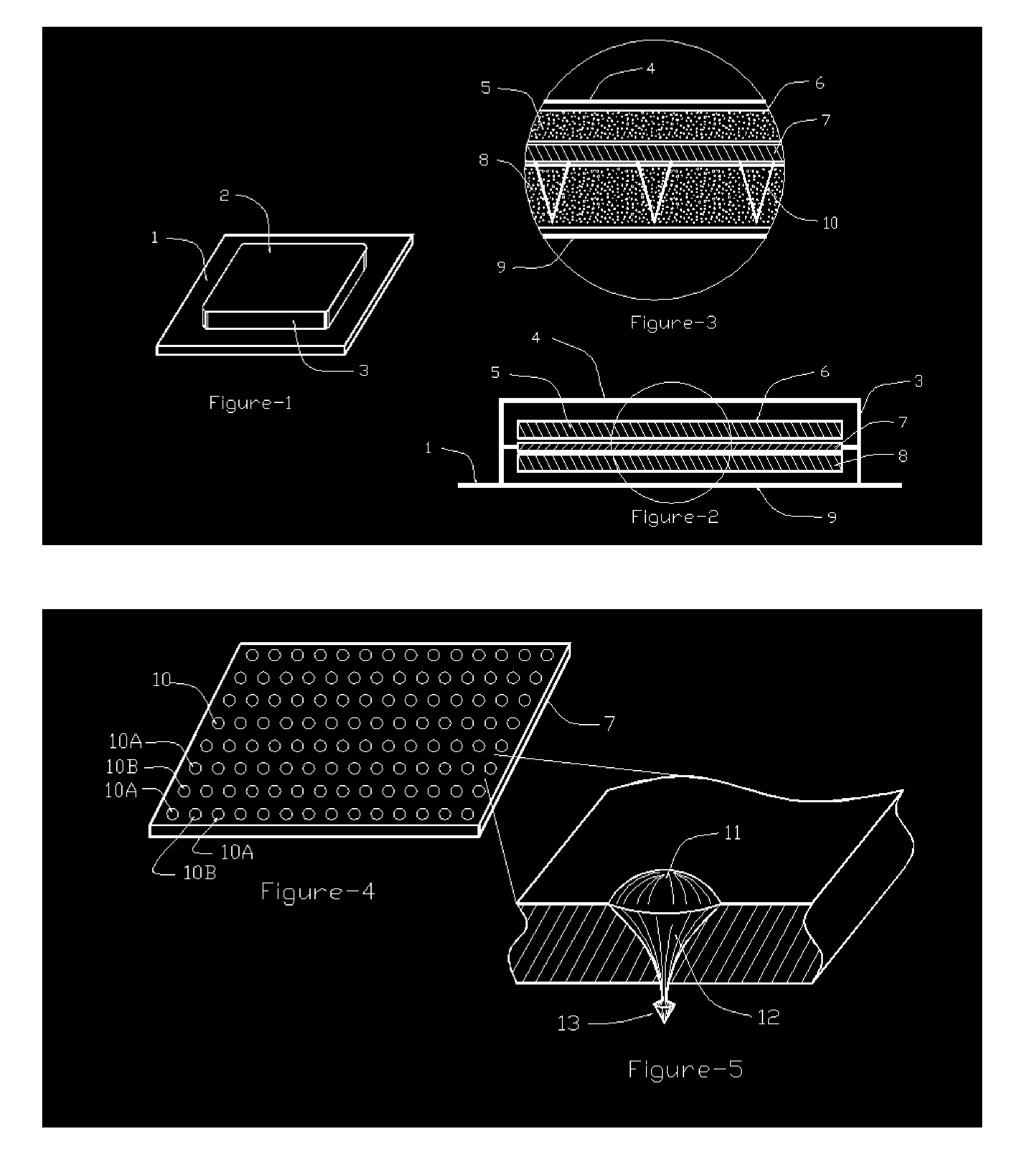
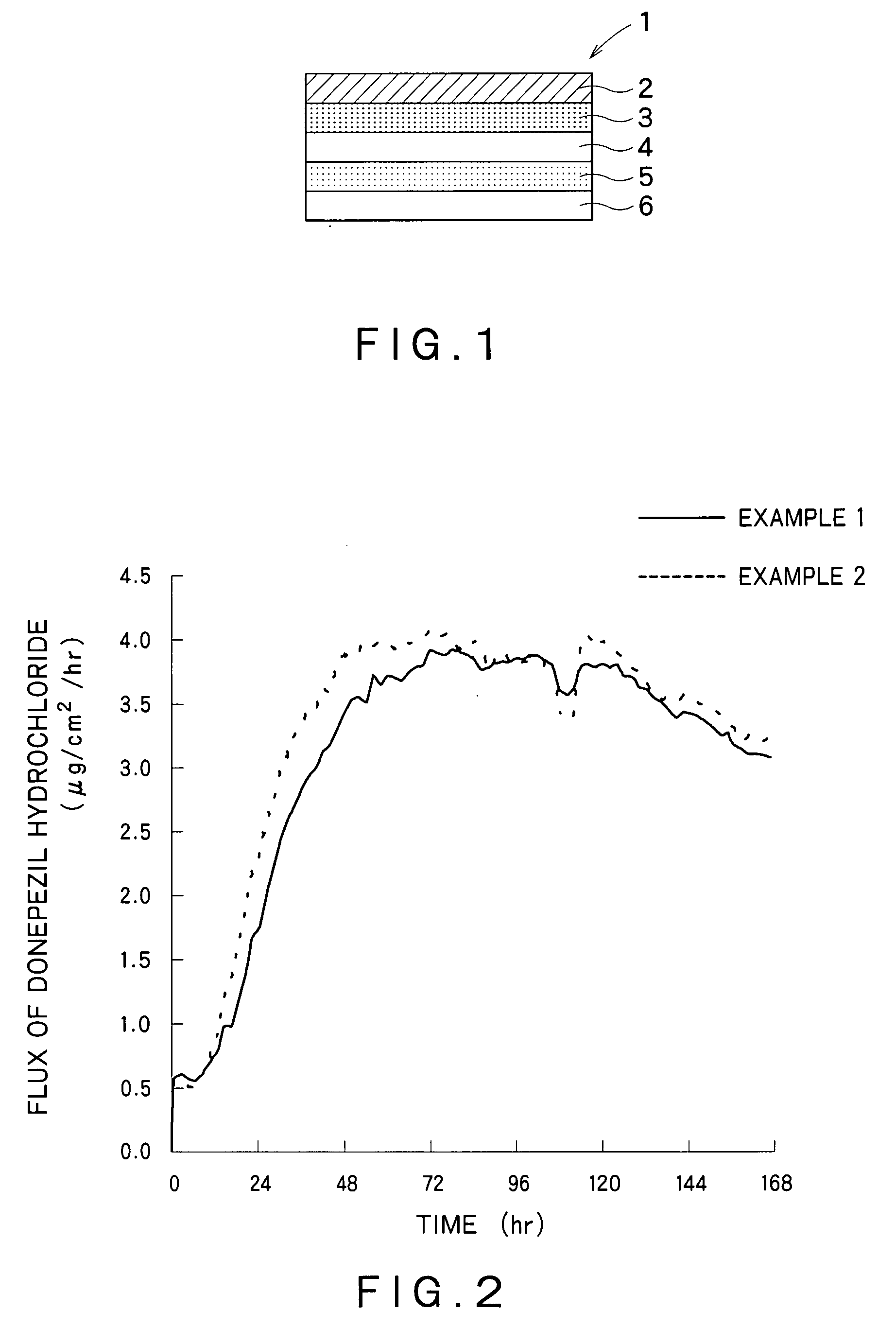
Patch
InactiveUS6211425B1Improve long-term stabilitySmooth releaseOrganic active ingredientsThin material handlingTransdermal patchDisease
A transdermal patch containing a drug comprising formoterol and / or a salt, a solvent for the drug, a pressure-sensitive adhesive comprising an ethylene / vinyl acetate copolymer resin and at least one of a filler and a plasticizer. The patch is substantially water-free. The patch serves to promote percutaneous absorption of the formoterol and / or a salt thereof and allow the efficacy of the drug to stably persist for a long period of time. The patch is thus effective for diseases such as asthma, which can be cured or prevented by exciting beta-receptors.
Owner:SAITAMA DAIICHI SEIYAKU
Percutaneous absorption type plaster
ActiveUS20050129748A1Improve balanceSufficient solubilityBiocideNervous disorderTransdermal patchPetroleum resin
A transdermal patch comprising a backing 2 and an adhesive layer 3 laminated on the backing 2, wherein the adhesive layer 3 comprises a rosin-based resin and petroleum resin as a tackifier, the total compounding amount of the rosin-based resin and the petroleum resin is in a range of 15% by mass to 50% by mass, and compounding amount of the petroleum resin is ⅓ times by mass to 4 times by mass as that of the rosin-based resin.
Owner:HISAMITSU PHARM CO INC
Percutaneous absorption agent containing Ailamode, preparation method and medical uses thereof
InactiveCN101401783AAvoid damageAvoid first pass reactionOrganic active ingredientsAntipyreticPercutaneous absorptionIguratimod
The invention relates to a transdermal absorbent containing Iguratimod, which comprises gellies, ointment, and cream, wherein the mass percentage content of the Iguratimod in the transdermal absorbent is between 0.1 and 10 percent, and preferably the mass percentage content of the Iguratimod in the transdermal absorbent is between 0.4 and 3 percent; and the invention also discloses requirements of the transdermal absorbent on the selections of matrix and a transdermal enhancer. Furthermore, the invention also provides a method for preparing the transdermal absorbent containing the Iguratimod and pharmaceutical application thereof.
Owner:杨喜鸿
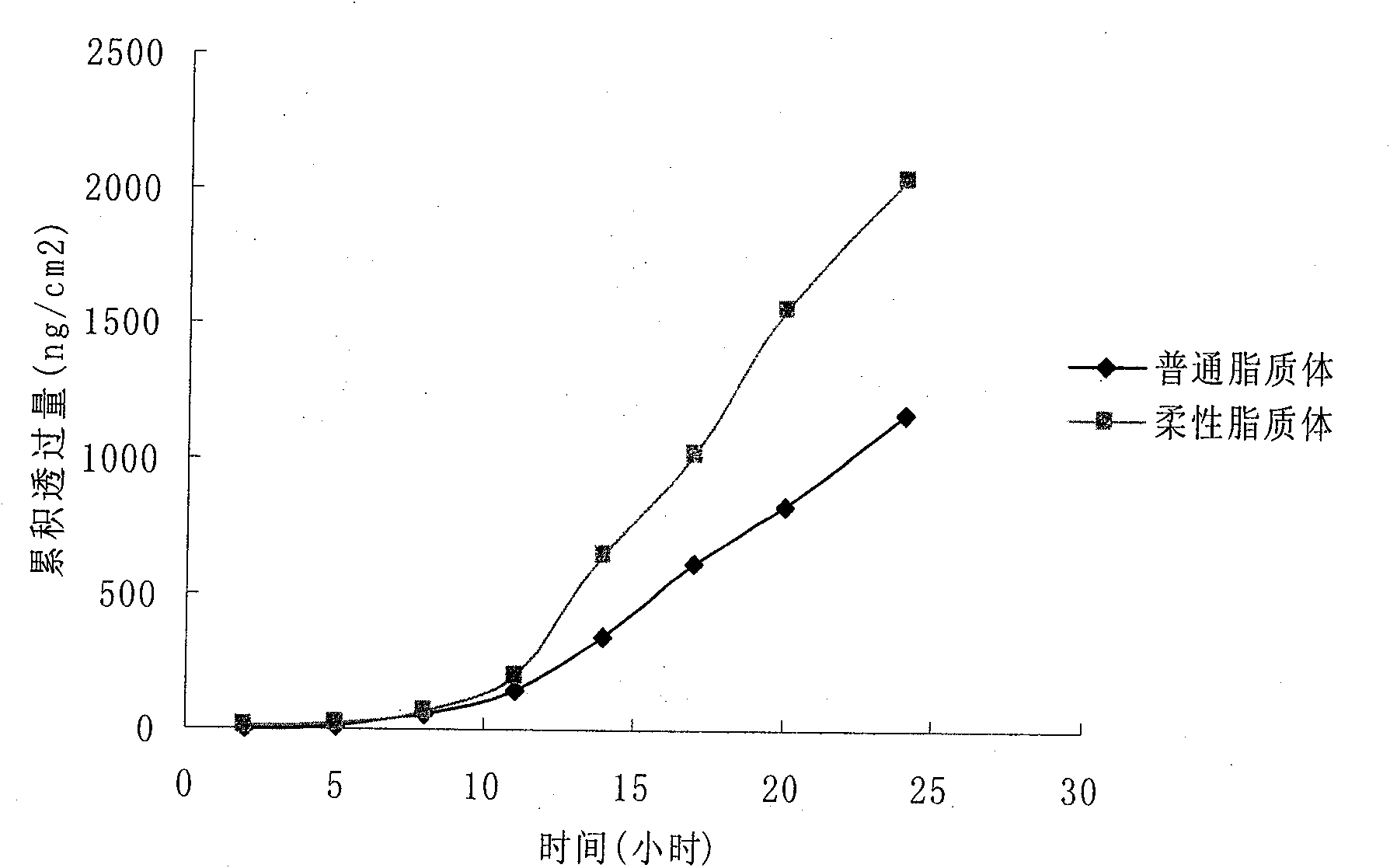
Resveratrol flexible liposome and preparation method thereof
InactiveCN101874763AImprove hydrophilicityEfficient penetrationCosmetic preparationsHydroxy compound active ingredientsActive componentMedicine
The invention discloses a resveratrol flexible liposome which is characterized by comprising the following components in mass percent (in terms of the weight of the liposome): 0.1-10% of resveratrol, 0.5-10% of phospholipid and 0.1-30% of flexible agent. In addition, the invention also discloses a preparation method of the resveratrol flexible liposome. The resveratrol flexible liposome of the invention has high flexibility and penetrability and can greatly improve the percutaneous absorption of active components.
Owner:SHANGHAI JAHWA UNITED
Flexible nanoliposomes with charges for cosmetics and preparation method thereof
ActiveCN102397168AProlong the action timeGive full play to the effect of skin care and nourishing skinCosmetic preparationsToilet preparationsCholesterolFreeze-drying
The invention relates to flexible nanoliposomes with charges for cosmetics and a preparation method thereof. The flexible nanoliposomes with the charges for the cosmetics comprise the following components in part by weight: 1-1000 parts of neutral phospholipid, 0.5-500 parts of phospholipid with the charges, 1-1000 parts of cholesterol, 0.5-500 parts of surface active agent, 0.1-20 parts of hyaluronic acid, 0.5-250 parts of cosmetic active ingredients and 100-8000 parts of freeze drying protective agent. The flexible nanoliposomes with the charges for the cosmetics can obviously improve the stabilities of the cosmetic active ingredients, promote the percutaneous absorption and the interaction efficiency of the active ingredients, and also improve the retention and action time of the active ingredients on the skin surface and the skin deep layer; and the flexible nanoliposomes with the charges for the powdery cosmetic active ingredients are also benefit to the use, transportation and storage. The preparation method of the flexible nanoliposomes with the charges can adopt a mechanized method, so the stability of the product technology and quality is good, and the repeatability is high.
Owner:江妍
Tape material for transcutaneous absorption
A preparation for transdermal absorption is disclosed which is suited for alleviating lasting pains caused by herpes zoster or postherpetic neuralgia and is practical and more improved in drug efficacy, safety and application characteristics. This tape preparation for transdermal absorption is obtained by causing an adhesive mass prepared by incorporating 1–30 parts by weight of a local anesthetic as an active ingredient in 100 parts by weight of a nonaqueous adhesive mass base comprising 5–50% by weight of a styrene-isoprene-styrene block copolymer, 1–60% by weight of an alicyclic saturated hydrocarbon resin, 5–60% by weight of liquid paraffin and 1–30% by weight of butyl rubber to be supported on a backing.
Owner:YUTOKU PHARMA IND +1
Percutaneous absorption system and percutaneous absorption method
Owner:LINTEC CORP +1
Preparation technology of micro emulsion containing artemisic methyl ether (or artemisic ethyl ether or artemisic succinate)
InactiveCN1650854AImprove or increase solubilityImprove or increase effectivenessAntiparasitic agentsEmulsion deliveryEmulsionAdditive ingredient
An artemether microemulsion as novel antimalaria contains artemether, arteether or artesunate, emulsifier, emulsifying aid and plant oil. It can be applied by intravenous injection, oral taking, percutaneous absorption, and spray. Its preparing process is also disclosed.
Owner:广州中生生物技术有限公司
Phentolamine external-applied preparation and its preparation method
InactiveCN101143127AGuaranteed validityImprove transdermal drug absorptionOrganic active ingredientsSexual disorderWestern medicineSide effect
The invention belongs to the western medicine preparation field and concretely relates to an efficient and quick effective external preparation of phentolamine which considers the volatile oil of natural plant and the extracted finished product of the volatile oil of the natural plant as a percutaneous sorbefacient and the preparation method of the external preparation. The invention selects the percutaneous sorbefacient of the natural volatile oil to prepare an external percutaneous preparation of the phentolamine of the invention by considering the phentolamine as the only curative active matter of the preparation and adding the necessary medicinal auxiliary materials. The animal experiment testifies that the invention can keep the drug usability and synchronously reduce the medicament dosage greatly and reduce the side effect to the whole body and the use cost. Compared with the prior art, the preparation can improve the percutaneous absorption of the drug dosage of the phentolamine very obviously and shortens the latent period of effecting, and the preparation has good medicament safety and can provide the patient with more satisfying curative effect.
Owner:FUDAN UNIV
Medical adhesive composition, medical adhesive tape using the same and tape preparation for percutaneous absorption
The present invention provides a medical adhesive tape and a tape preparation for percutaneous absorption, both having an adhesive layer that resists glue remainder and stickiness upon peeling off of the tape even after adhesion to the human skin for a long time, and a medical adhesive composition to be used for such tapes. The medical adhesive composition of the present invention includes, as essential elements, (A) a copolymer containing (a) alkyl (meth)acrylate, wherein the alkyl moiety has 4 to 18 carbon atoms, in a proportion of not less than 50 wt % of the copolymer and (b) a carboxyl group-containing vinyl compound in a proportion of 0.1 wt %–10 wt % of the copolymer, (B) an alkoxide or a chelate compound of at least a metal selected from titanium, zirconium, zinc and aluminum, and (C) a polyol compound in a proportion of 0.2 wt %–5 wt % of the aforementioned medical adhesive composition. The medical adhesive tape of the present invention and tape preparation for percutaneous absorption are characterized in that the adhesive layer contains the above-mentioned medical adhesive composition of the present invention.
Owner:NITTO DENKO CORP
System for improved percutaneous absorption of skin benefiting agents
InactiveUS20060251598A1Cosmetic preparationsHair removalPercutaneous absorptionBULK ACTIVE INGREDIENT
Stable topical compositions containing active ingredients that are prone to oxidation, and methods for improving the percutaneous absorption of an active ingredient are provided.
Owner:OMP
Adhesive preparations
InactiveUS20070184097A1Improve drug solubilityImprove skinSynthetic polymeric active ingredientsAmine active ingredientsOrganic acidPercutaneous absorption
Adhesive preparations with improved percutaneous absorption of physiologically active substances, in particular, matrix adhesive preparations containing a base drug salt and an organic acid salt having an mean diameter of from 0.1 to 100 μm are provided.
Owner:HISAMITSU PHARM CO INC
Process for the preparation of pharmaceutical compositions for topical delivery of cyclooxygenase-2-enzyme inhibitors
InactiveUS20050049291A1Enhanced percutaneous bioavailabilityGood effectBiocidePowder deliveryCyclooxygenasePercutaneous absorption
A present invention relates to a pharmaceutical composition for topical delivery comprising a pharmaceutically effective amount of drug(s), that acts selectively as a cyclooxygenase-2 enzyme inhibitor. The composition provides better percutaneous absorption and enhanced efficacy.
Owner:RANBAXY LAB LTD
Percutaneous absorption-type pharmaceutical preparation
InactiveUS20060292210A1Reduce forceIncrease forceBiocideOrganic active ingredientsMetal chlorideAdhesive
A stable percutaneous absorption-type pharmaceutical preparation for percutaneous administration of selegiline or selegiline hydrochloride, which does not suffer a decrease in the cohesive force of the adhesive layer therein even in the presence of sweat components due to perspiration during wear and which is free from cohesive failure and resultant adhesive remaining when stripped off, is provided. A percutaneous absorption-type pharmaceutical preparation which comprises: a support; and an adhesive layer containing a metal chloride, an adhesive and at least one of (−)-(R)—N,α-dimethyl-N-2-propynylphenethylamine and its hydrochloride, wherein the adhesive layer is subjected to a crosslinking treatment.
Owner:NITTO DENKO CORP +1
Formulations for topical delivery of bioactive substances and methods for their use
ActiveUS20070071711A1Efficient deliveryNon-irritating to the skinPowder deliveryCosmetic preparationsHigh concentrationPercutaneous absorption
The invention relates to topical delivery of bioactive agents. More particularly, the invention relates to anhydrous formulations for percutaneous absorption. The invention provides formulations that allow efficient topical delivery of high concentrations of bioactive substances for percutaneous absorption. The formulations according to the invention are generally non-irritating to the skin.
Owner:CELLMEDICS
External preparation for skin
InactiveUS20100021405A1Good formulation stabilityEffective penetrationCosmetic preparationsBiocideHigh concentrationPreparing skin
The present invention provides an external preparation for skin comprising a phospholipid having an iodine value of 80 to 110, ethanol in an amount of 55 to 83 wt % and water in an amount of 15 to 43 wt %, which is improved in it's preparation stability by suppressing an increase in an acid value of phospholipid. The present invention also provides an external preparation for skin which is improved in percutaneous absorption of medically effective ingredient(s). The present invention provides further a method for suppressing an increase in an acid value of phospholipid by comprising phospholipid having high iodine value and high concentration of ethanol and water, as well as a method for improving percutaneous absorption of medically effective ingredient(s) in external preparation for skin.
Owner:ROHTO PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com