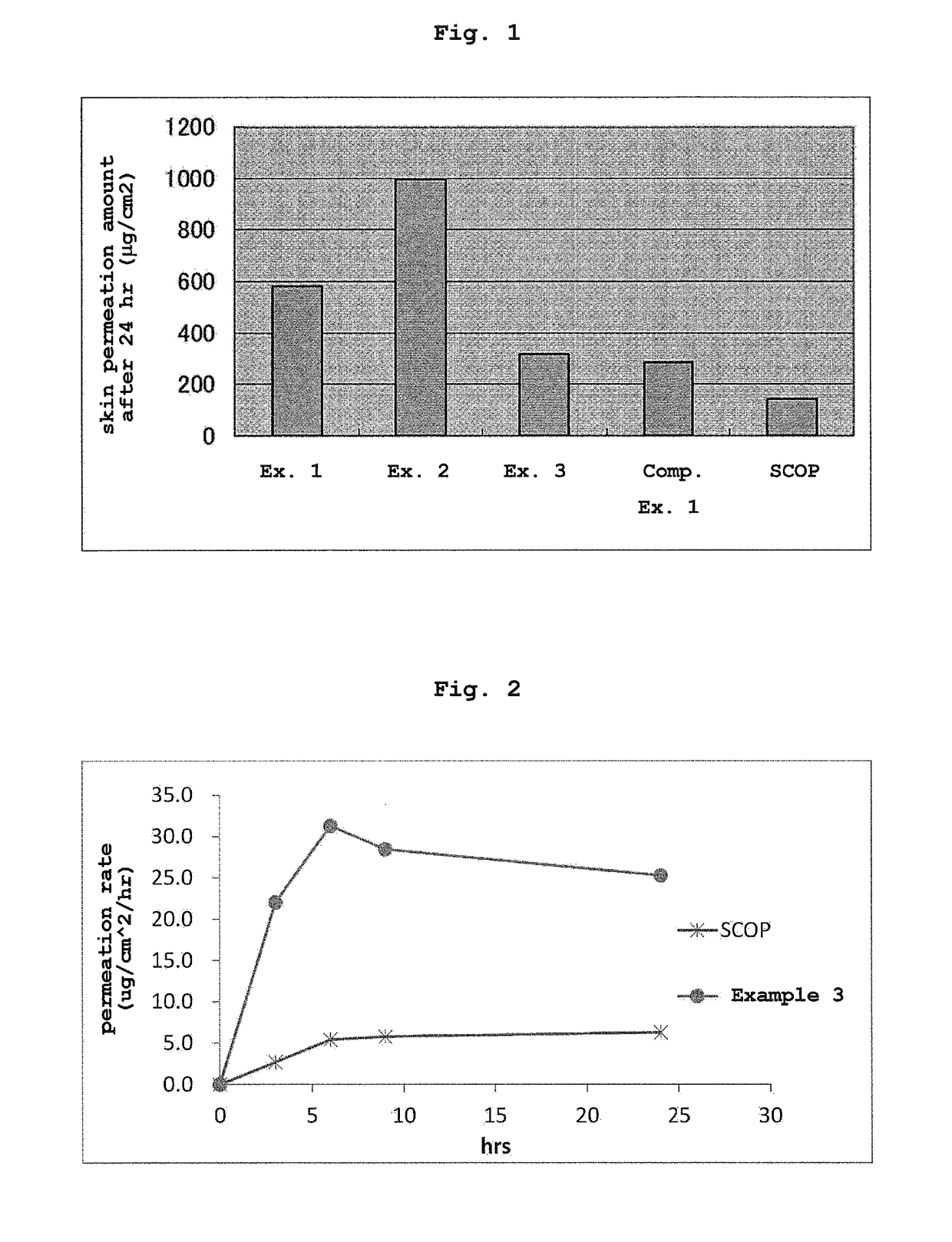
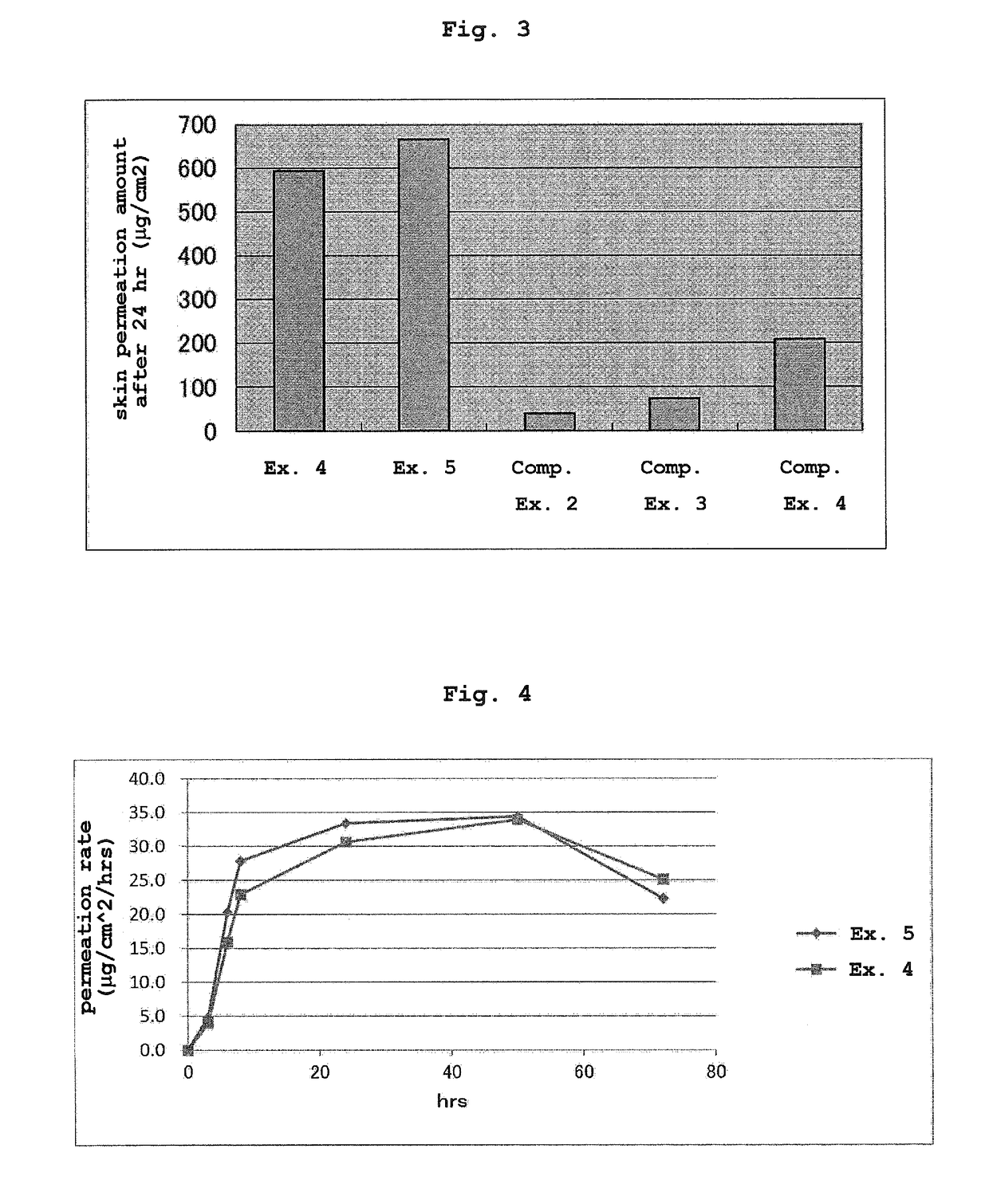
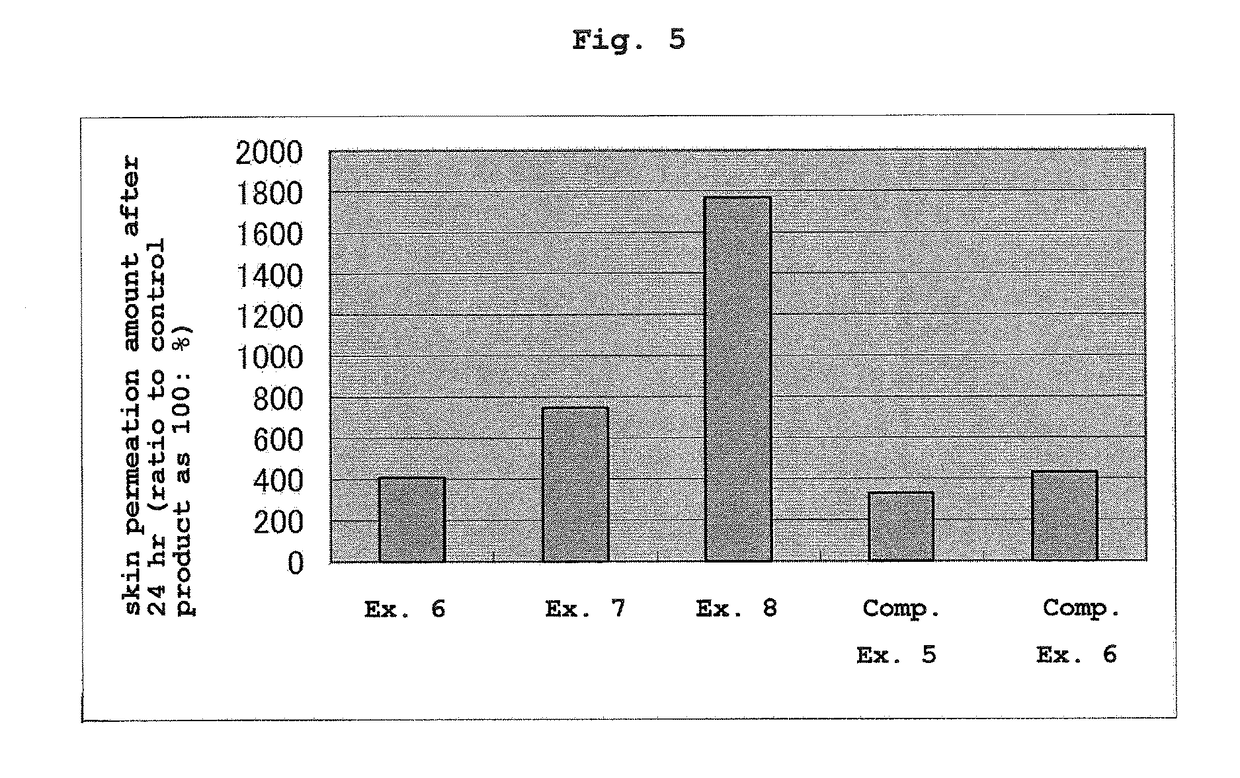
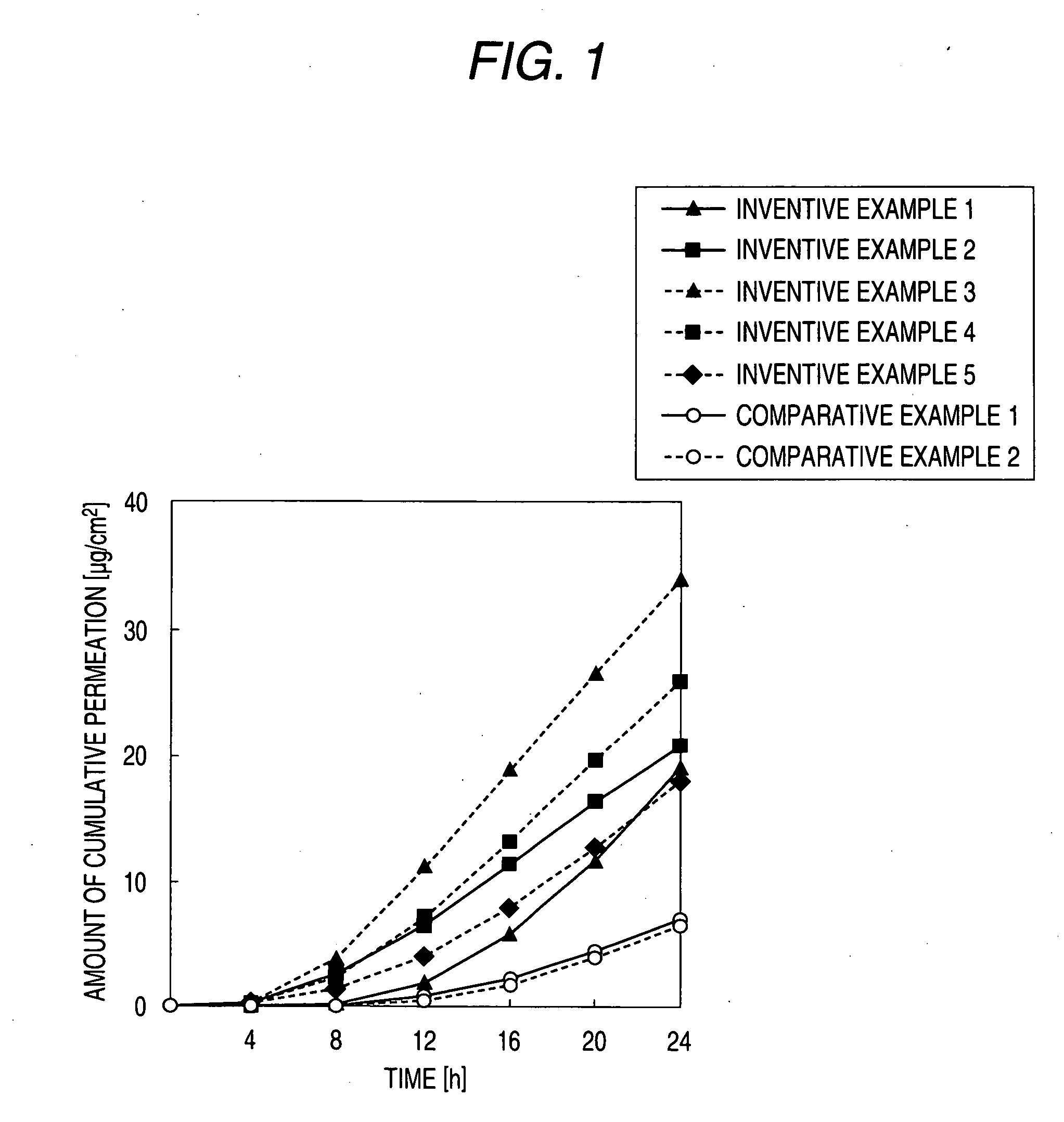
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "Improve drug solubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
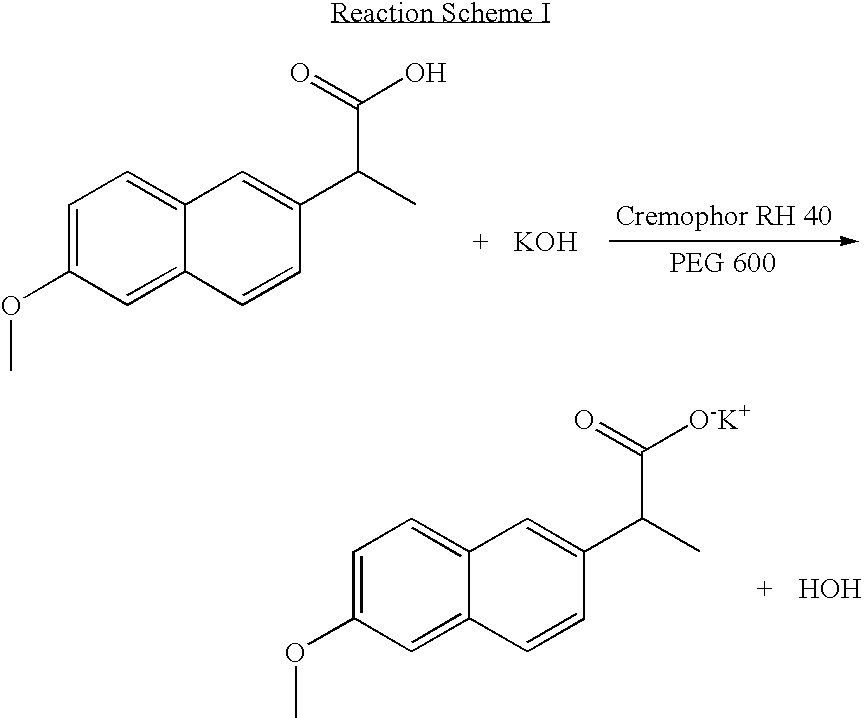
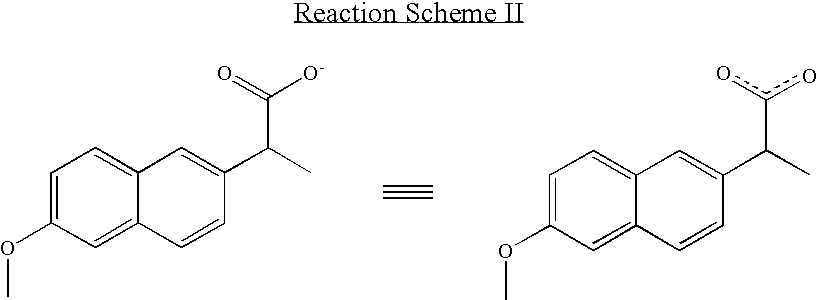
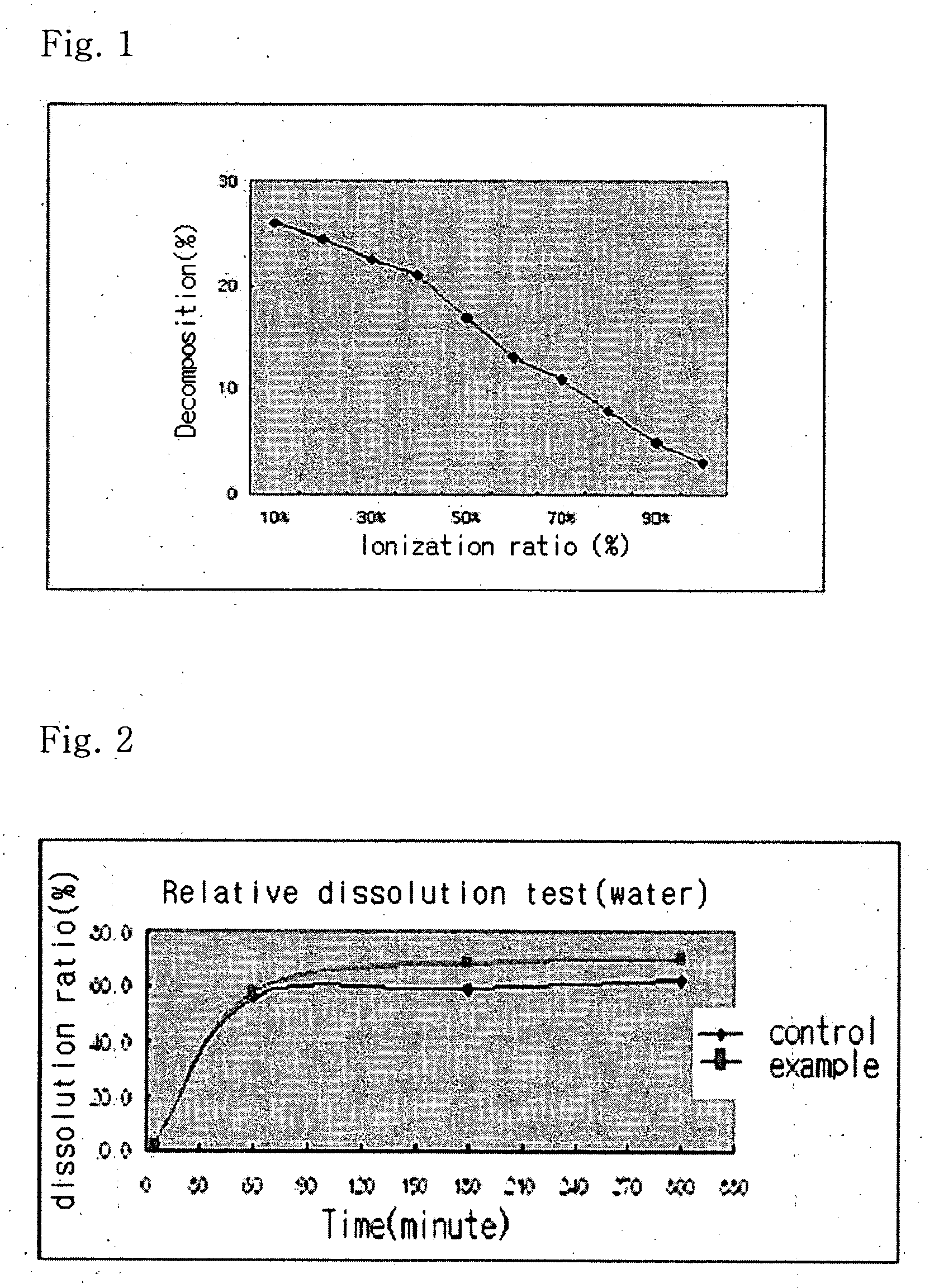
Solvent system of hardly soluble drug with improved dissolution rate
InactiveUS20040157928A1Good disintegrationPromote dissolutionBiocideAntipyreticDissolutionIonization
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptance, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
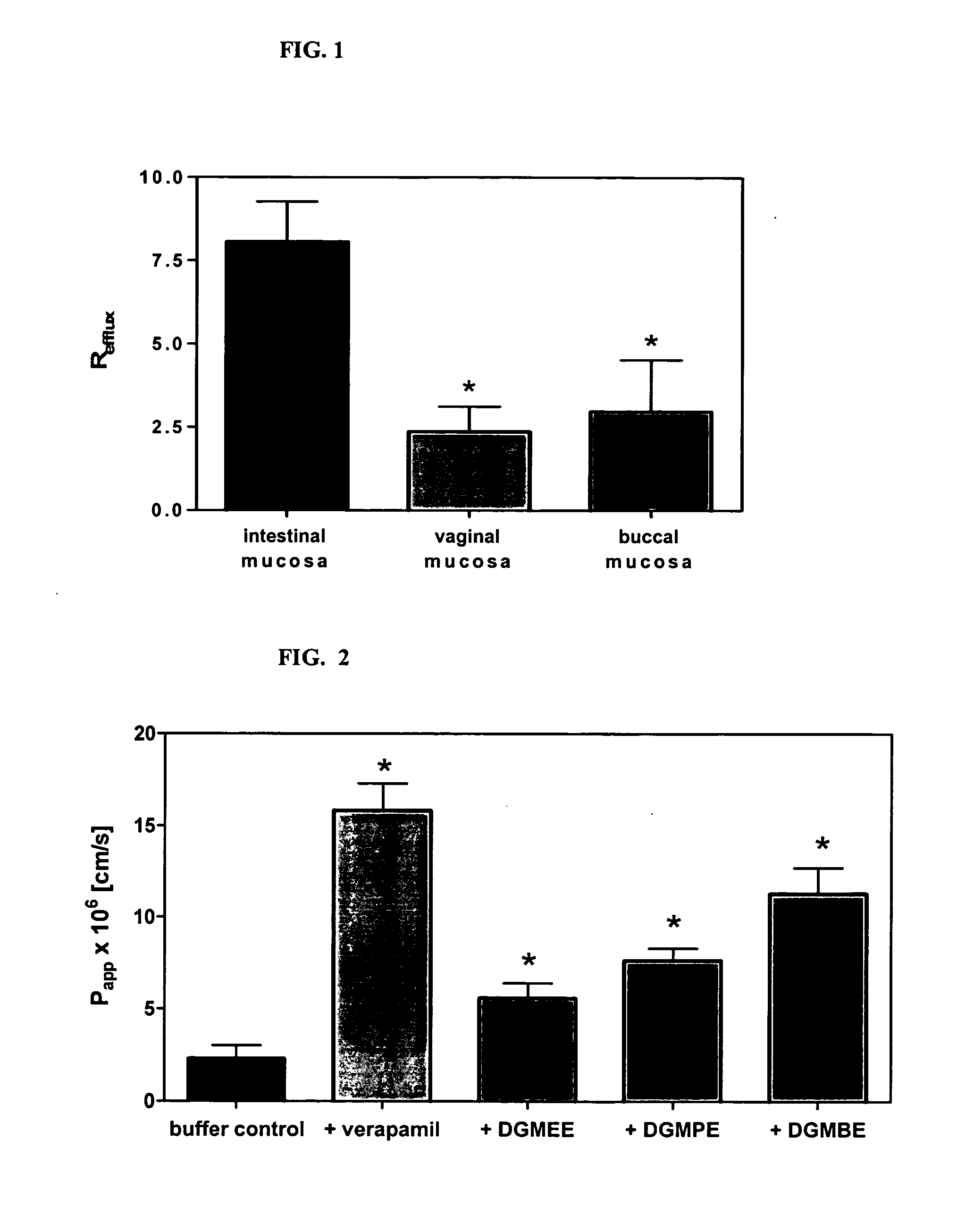
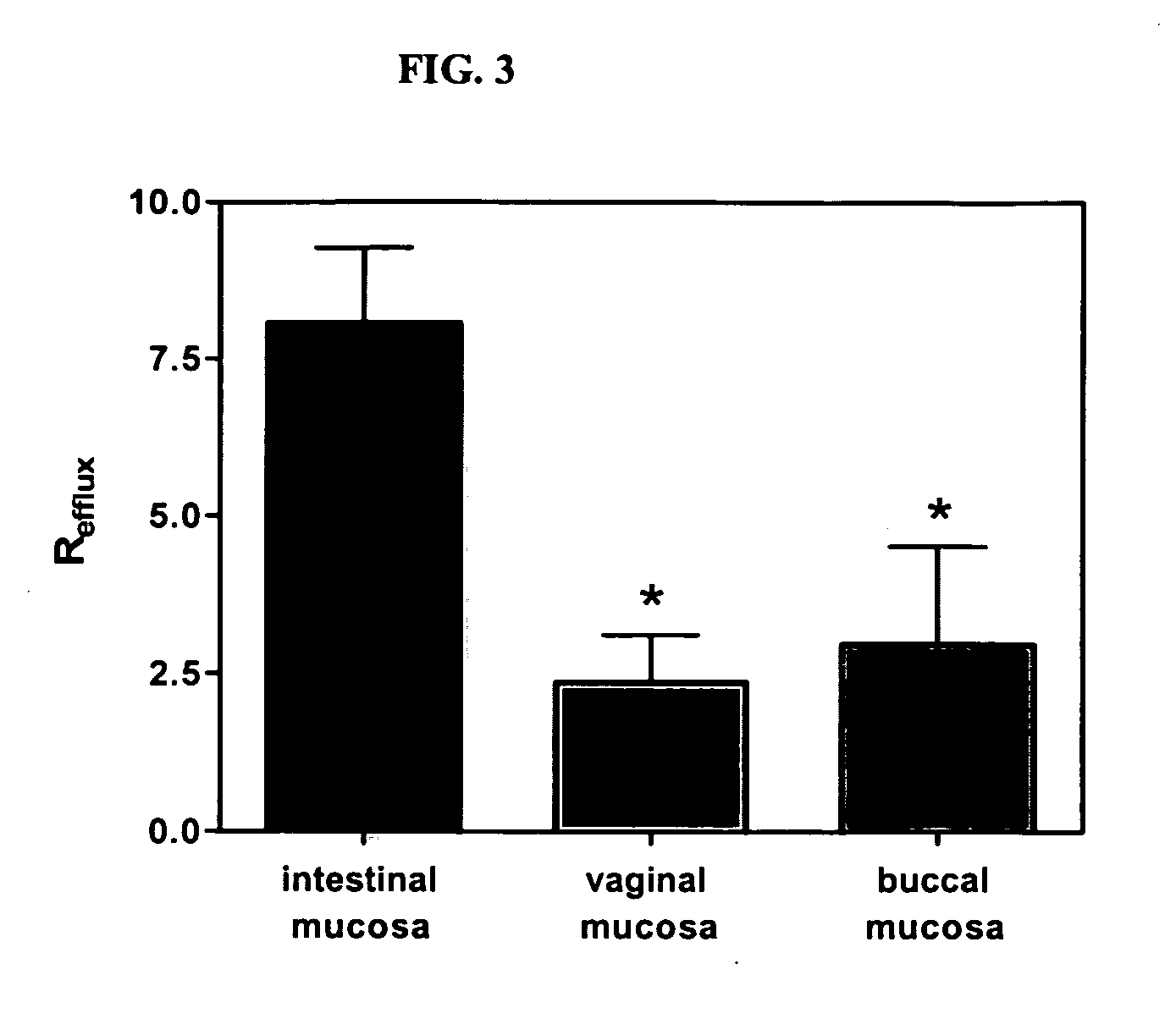
Method for augmentation of intraepithelial and systemic exposure of therapeutic agents having substrate activity for cytochrome P450 enzymes and membrane efflux systems following vaginal and oral cavity administration
InactiveUS20070036834A1Improve bioavailabilityImprove drug solubilityBiocidePowder deliveryWhole bodyMedicine
A vaginal or buccal delivery of therapeutic agents having a substrate affinity for metabolic cytochrome P-450 enzymes and membrane efflux transporter systems. A method for augmentation of systemic exposure to the therapeutic agents having a substrate affinity for cytochrome P-450 enzymes and membrane efflux transporter systems, by delivering said agents to the systemic circulation through vaginal or buccal mucosa.
Owner:FEMINA PHARMA +1
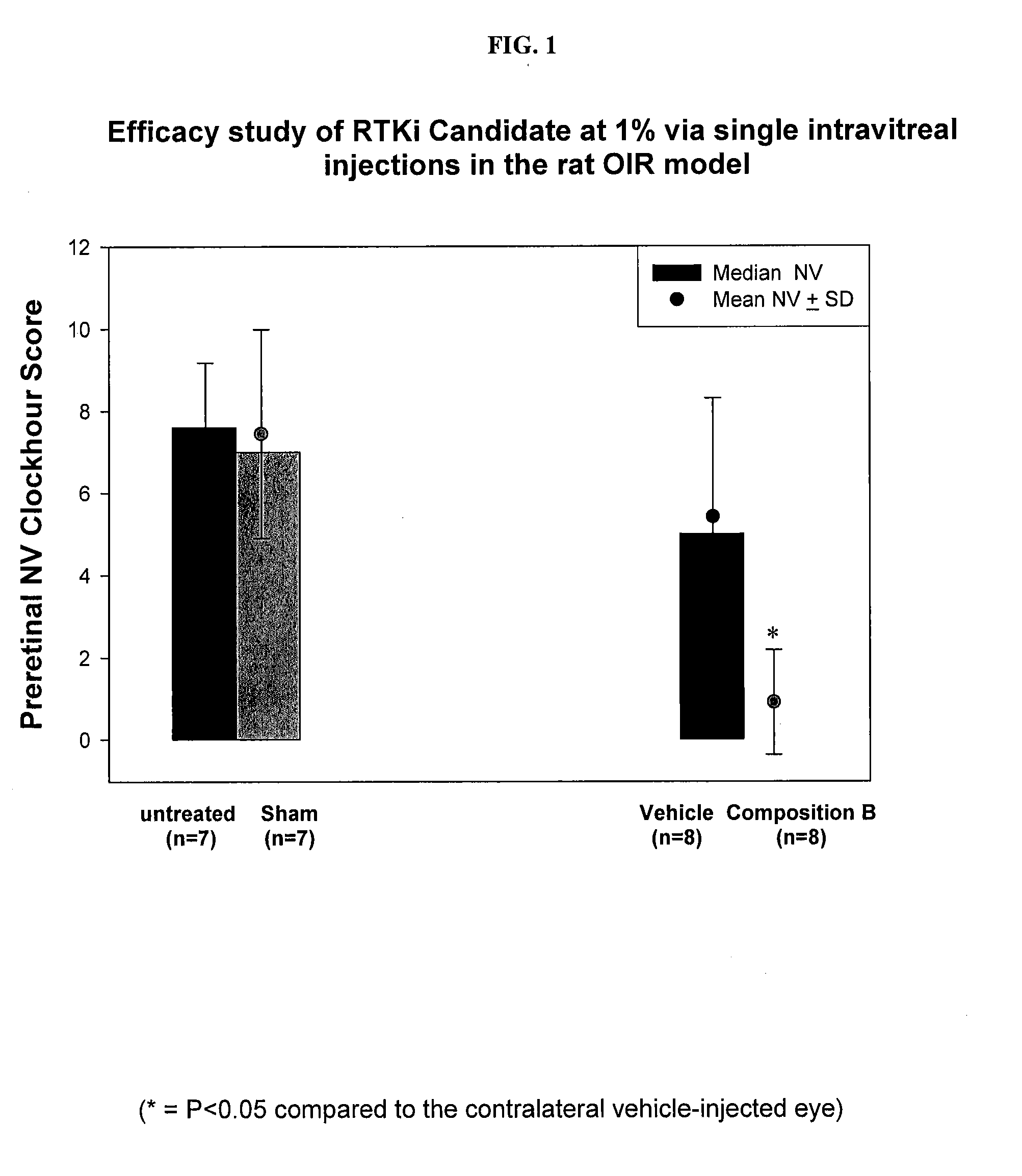
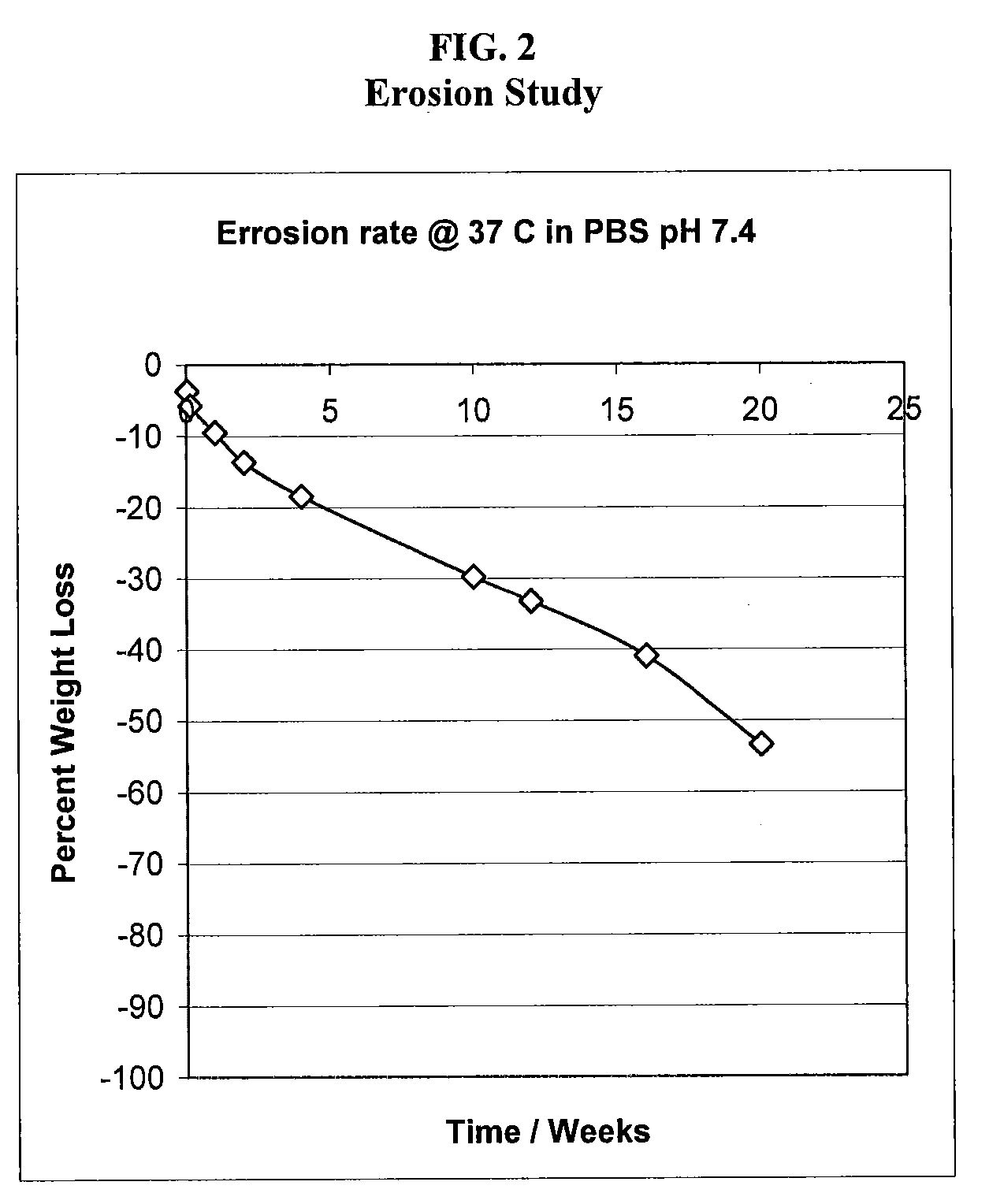
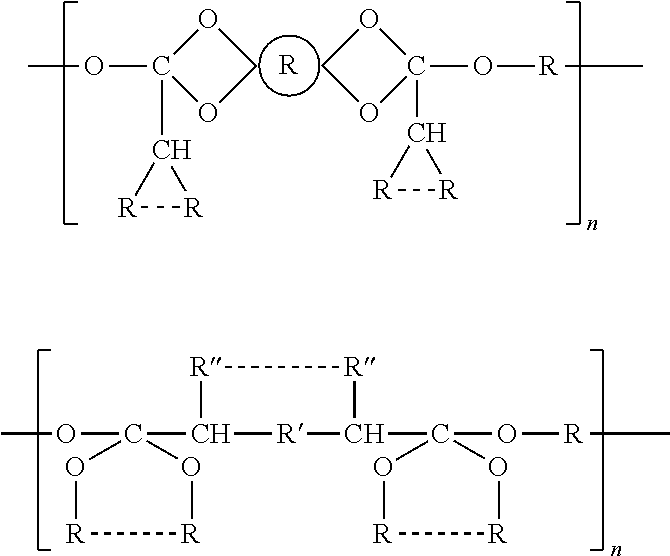
Water insoluble polymer matrix for drug delivery
InactiveUS8632809B2Improve solubilityImprove drug solubilityPowder deliverySenses disorderPolyesterActive agent
Disclosed is a pharmaceutical composition comprising (a) a bioerodible water insoluble polymer matrix comprising a polyester polymer, wherein the polymer matrix has a melting point of less than 60° C. and (b) an active agent dispersed within the polymer matrix, wherein the composition is formulated to controllably release the active agent for a pre -determined period of time to a target site. Also disclosed are methods of treating a disease or condition with the disclosed compositions.
Owner:ALCON RES LTD
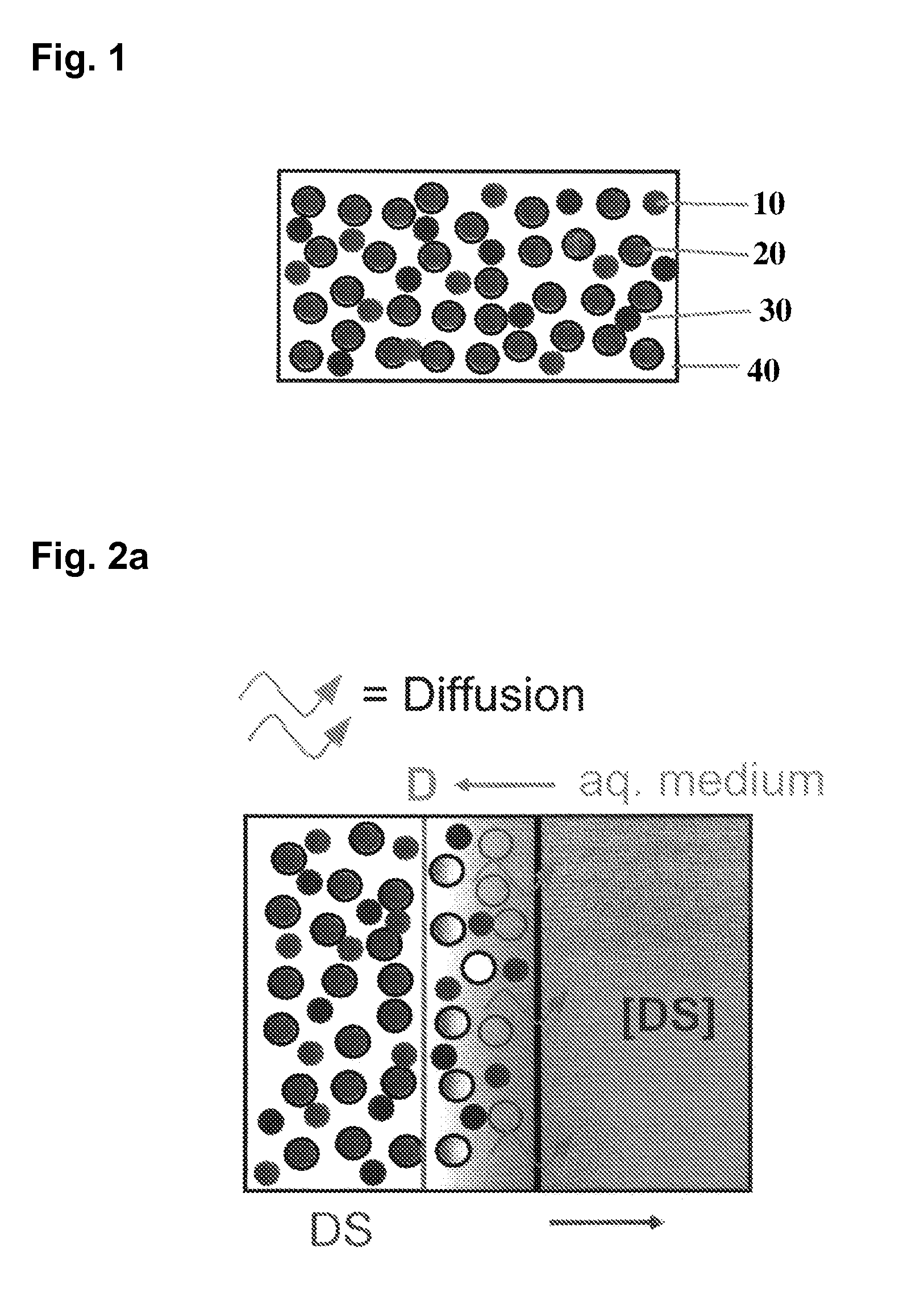
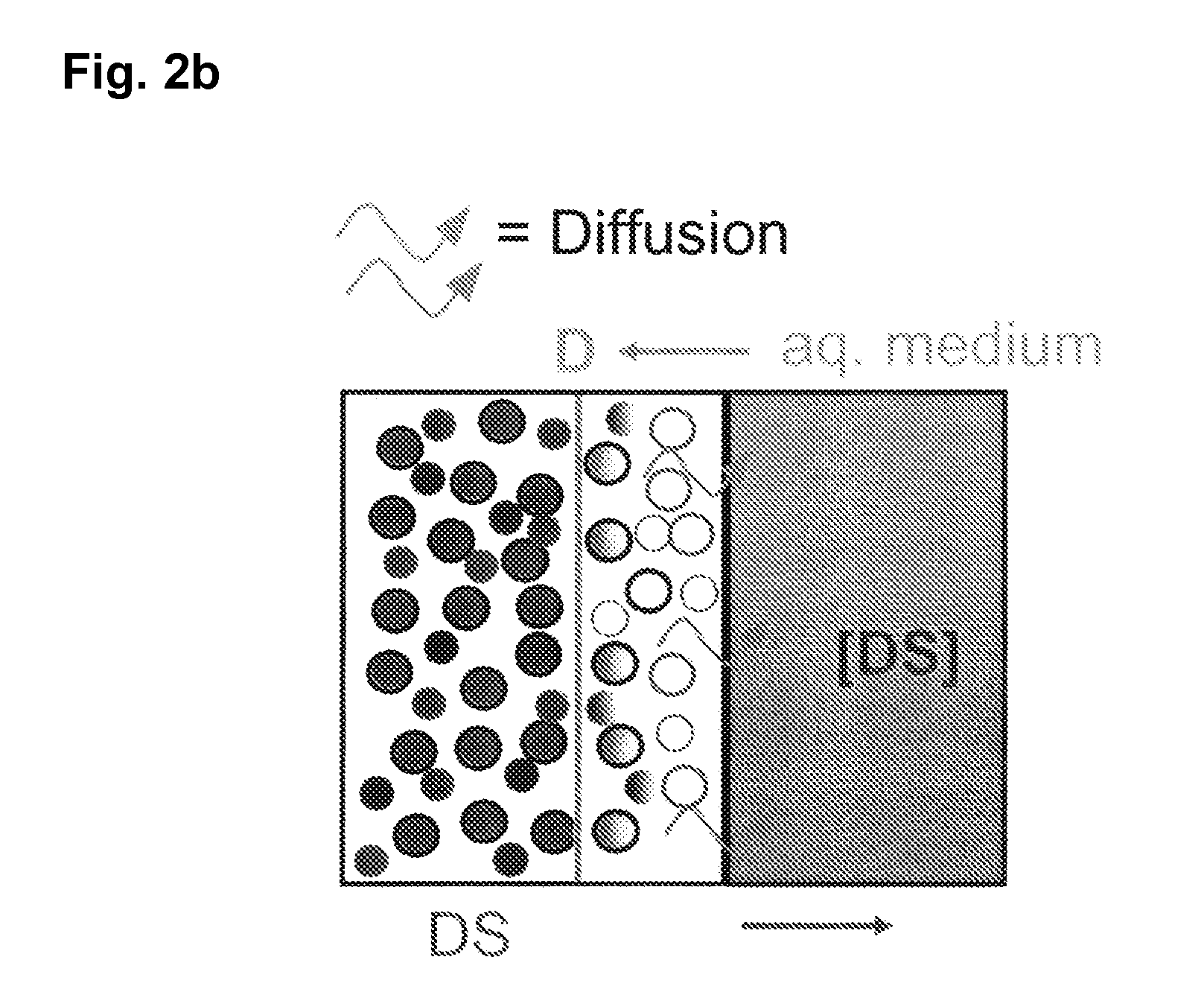
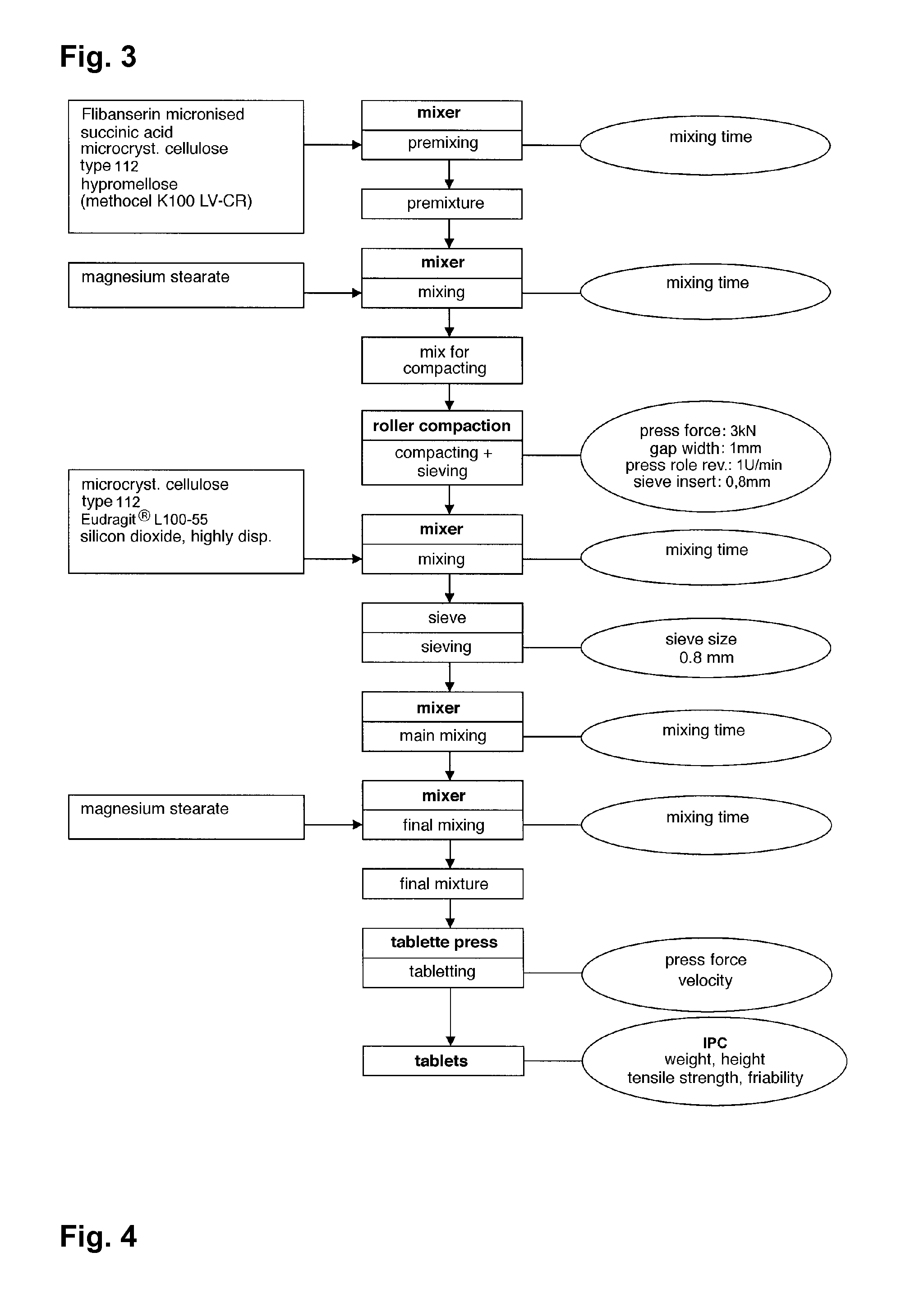
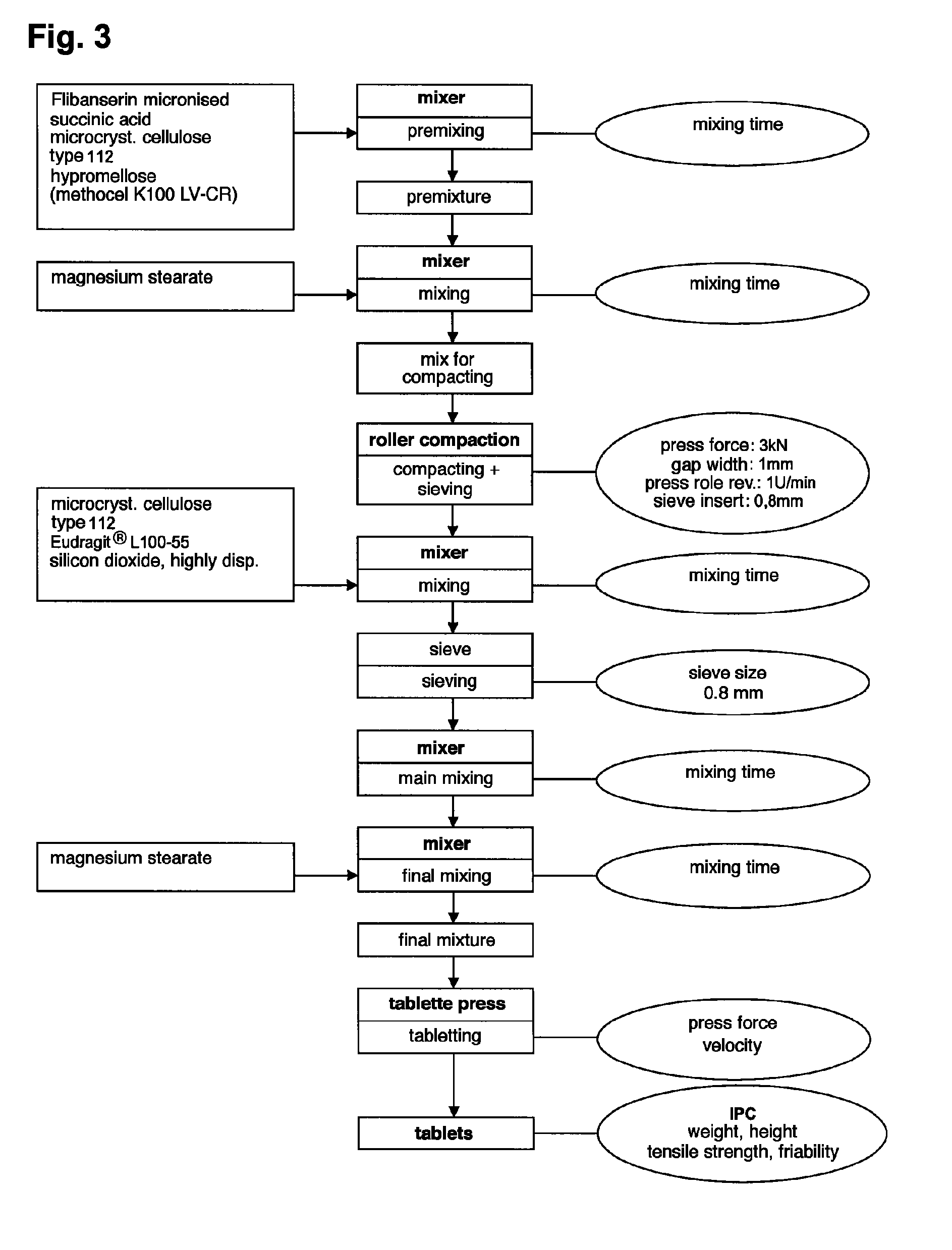
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
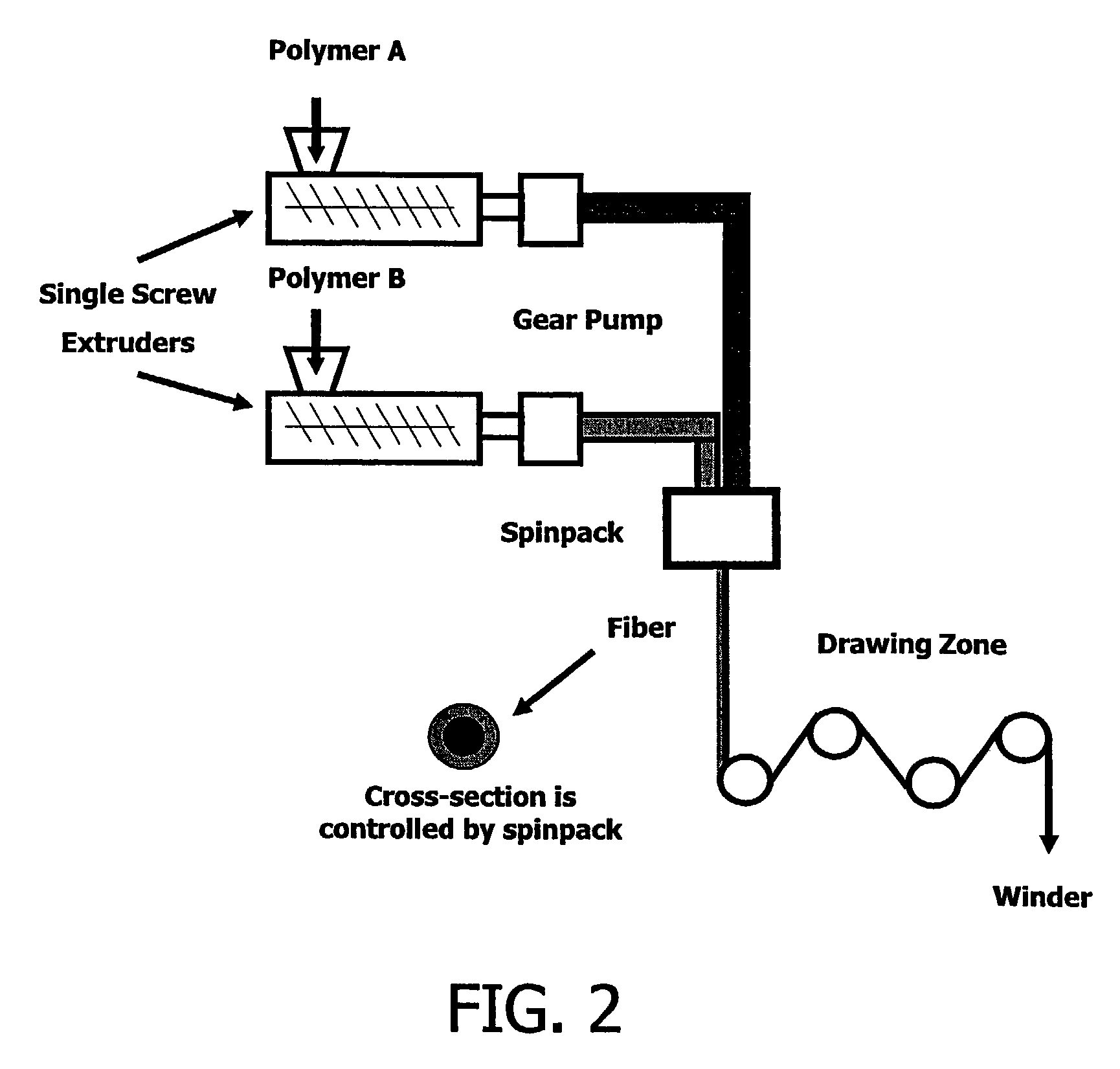
Fiber-based nano drug delivery systems (NDDS)
InactiveUS7491407B2High dissolution rateIncrease surface areaSynthetic resin layered productsWoven fabricsFiberDrug compound
A drug delivery system in the form of homo-component, bi-component or multi-component fibers wherein one of more of the components comprise a drug compounded with a polymer carrier. These fibers are packed to form a tablet directly, or are chopped and placed in a capsule.
Owner:NORTH CAROLINA STATE UNIV
Transdermal delivery system for water insoluble drugs
InactiveUS7395111B2Minimal irritationMinimal sensitizationOrganic active ingredientsBiocideWater insolubleWater insoluble drug
Owner:SYNERON MEDICAL LTD
Parenteral and oral formulations of benzimidazoles
ActiveUS20090048322A1Good treatment effectImprove drug solubilityOrganic active ingredientsBiocideBenzimidazole derivativeMebendazole
Provided herein are drug delivery systems, such as self-nanoemulsifying drug delivery systems, self-emulsifying drug delivery systems and parenteral microemulsion formulations, suitable for parenteral or oral delivery to a subject. The drug delivery systems may comprise a benzimidazole derivative, e.g., mebendazole, an oil, a surfactant, a cosurfactant and a dipolar aprotic solvent in a microemulsion formulation. Also provided are methods for improving the bioavailability of a benzimidazole derivative during treatment of a pathophysiological condition by using a formulation combining a particular emulsion droplet diameter and ratio of the surfactant:cosurfactant therein, for increasing concentration and retention of a benzimidazole derivative in the lung via a parenterally administerable microemulsion with droplet size of about 35 nm to less than 100 nm and for defining hemolytically safe microemulsions of a benzimidazole derivative during a therapeutic treatment via a parenterally administerable microemulsion with a surfactant:cosurfactant content by weight of about 6% to 48%.
Owner:UNIV HOUSTON SYST
Transdermal delivery of medicaments with combinations of cetylated fatty fatty ester penetrant complexes
InactiveUS20110065627A1Facilitate more efficacious permeationReduce resistanceCosmetic preparationsBiocideWrinkle skinAntioxidant
This invention describes a topical delivery mechanism that contains a mixture of cetylated fatty esters that act as transdermal carriers of desired therapeutic molecules. The proposed cetyl fatty ester penetrant-complex (Base CFEP-complex) contains specific cetyl fatty esters, polar solvents, a carrier base (gel, cream, lotion, patch or stick gel), antioxidants and the desired pharmaceutical, cosmetic or antigenic response eliciting molecules that are efficaciously delivered by selectively varying component ratios in the complex. The invention proposes the use of transdermal delivery of medications such as those used in treatment of urinary incontinence, testosterone deficiency, arthritic and joint pain and other pains such as pain in the neck, lower back, back, knees, headaches, and other types of inflammatory pains, peripheral neuropathic pain, pain associated with repetitive strain injuries such as myofacial pain, rapid treatment of epileptic seizures, soluble antigens in the immuno-therapeutic treatment of allergies, actives in the treatment of foot cracks and elbow cracks, actives in the treatment of facial and other wrinkles in the form of anti-aging creams and gels and other topically delivered therapies.
Owner:CYMBIOTICS
Spray dried formulation
ActiveUS20100029667A1Efficiently formedImprove drug solubilityBiocideOrganic chemistryOrganosolvSpray dried
Pharmaceutical compositions comprising a poorly water soluble ionizable drug, a cationic species and a dispersion polymer are disclosed, together with a process for forming the compositions. The neutral form of the drug has (i) a solubility of less than 1 mg / ml, in aqueous solution at a pH between 6 and 7, (ii) a solubility of less than 20 mg / mL in a volatile organic solvent, and (iii) an acidic pKa value of greater than 5. At least 90 wt % of the drug in the solid dispersion being in a non-crystalline form. The drug, the cationic species, and the dispersion polymer constitute at least 80 wt % of the solid dispersion.
Owner:LONZA BEND INC
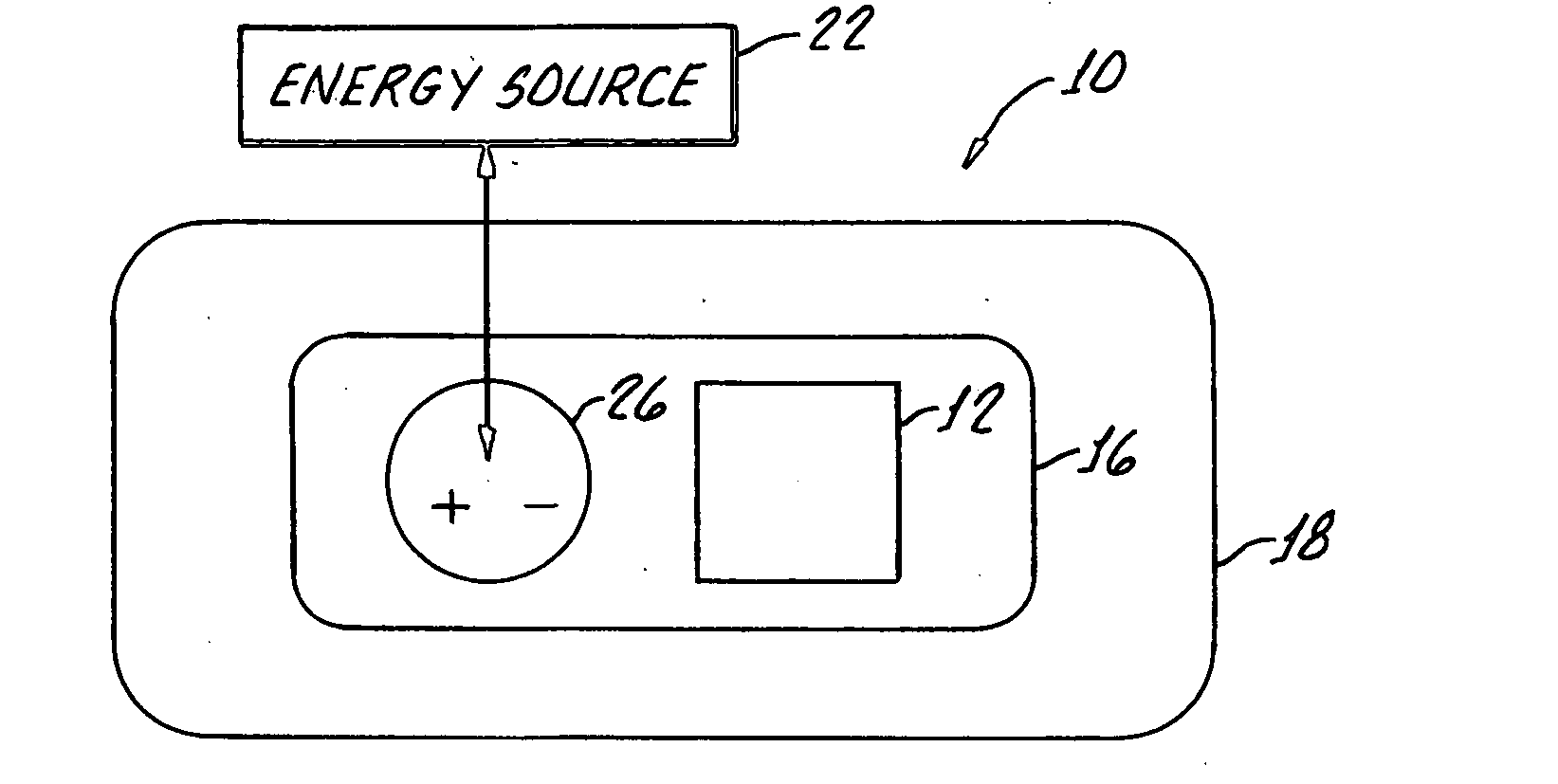
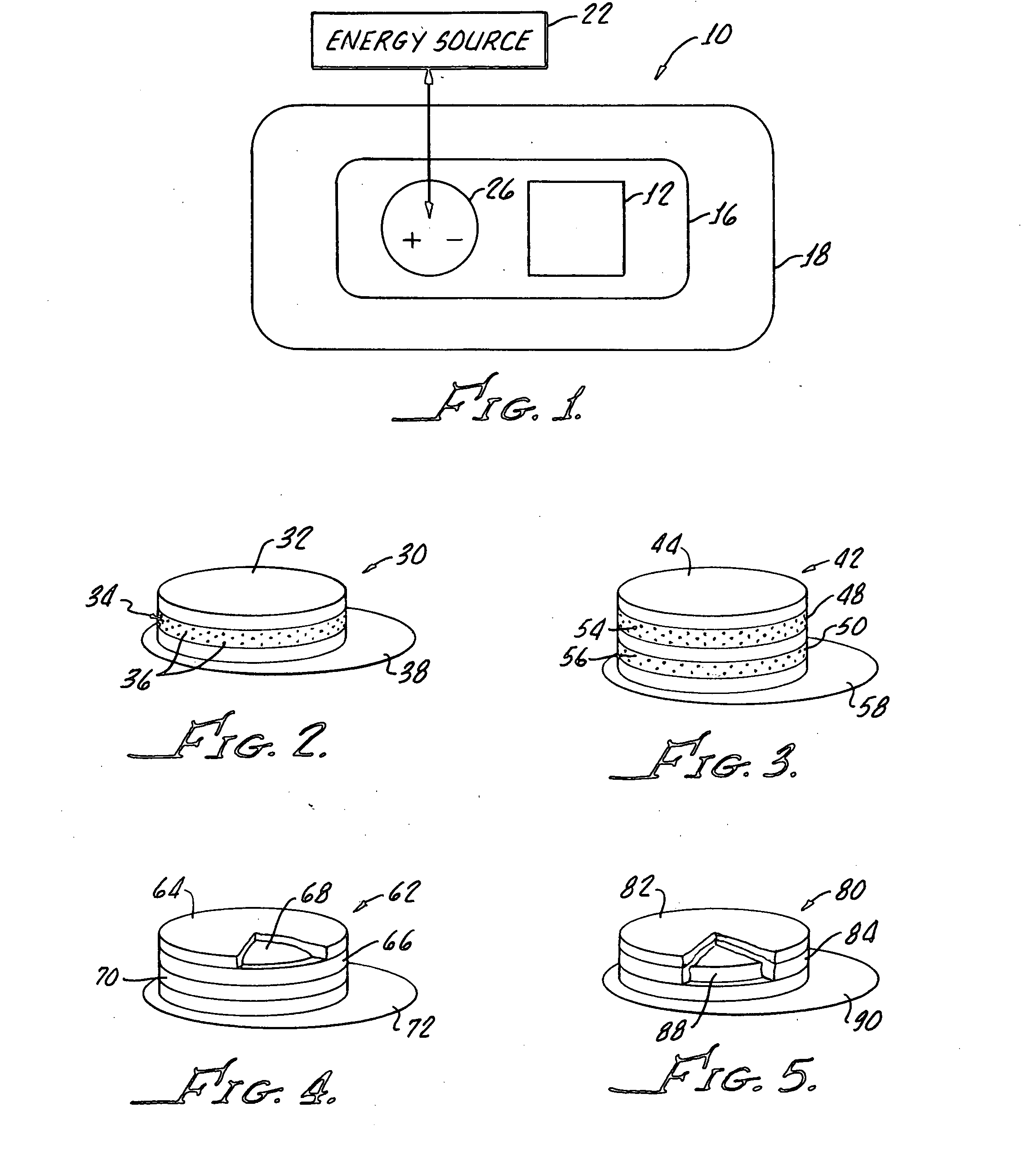
Transdermal drug delivery system
A transdermal drug delivery system includes a drug source, an adhesive for applying the system to a skin surface, a penetration enhancer for enhancing penetration of the drug into the skin surface, an energy source and a contact for conveying energy from said energy source through said adhesive and the penetrating enhancer.
Owner:ALLERGAN INC
Adhesive preparations
InactiveUS20070184097A1Improve drug solubilityImprove skinSynthetic polymeric active ingredientsAmine active ingredientsOrganic acidPercutaneous absorption
Adhesive preparations with improved percutaneous absorption of physiologically active substances, in particular, matrix adhesive preparations containing a base drug salt and an organic acid salt having an mean diameter of from 0.1 to 100 μm are provided.
Owner:HISAMITSU PHARM CO INC
Parenteral and oral formulations of benzimidazoles
ActiveUS20050038096A1Good treatment effectImprove drug solubilityBiocideDispersion deliveryBenzimidazole derivativePolyol
Pharmaceutical compositions of a benzimidazole or a benzimidazole derivative are disclosed. For example, in certain embodiments the pharmaceutical compositions include a benzimidazole, a polyol, and a dipolar aprotic solvent. In other embodiments, pharmaceutical compositions include a benzimidazole, an oil, a dipolar aproptic solvent, and a surfactant. In certain embodiments, the benzimidazole is mebendezole. The pharmaceutical compositions are formulated for delivery to a subject by any means, and include formulations for oral and parenteral delivery.
Owner:UNIV HOUSTON SYST
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS8545886B2Reduce solubilitySufficiently slow releasePowder deliveryNervous disorderExtended release tabletsOral medication
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting ofa) flibanserin or a pharmaceutically acceptable derivative thereof as active substance;b) one or more pharmaceutically acceptable pH-dependent polymers;c) one or more pharmaceutically acceptable pH-independent polymers;d) one or more pharmaceutically acceptable acids; ande) optionally one or more additives.The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
Parenteral and oral formulations of benzimidazoles
ActiveUS7419996B2Good treatment effectImprove drug solubilityBiocideDispersion deliveryBenzimidazole derivativeMebendazole
Pharmaceutical compositions of a benzimidazole or a benzimidazole derivative are disclosed. For example, in certain embodiments the pharmaceutical compositions include a benzimidazole, PEG 400, and a dipolar aprotic solvent. In other embodiments, pharmaceutical compositions include a benzimidazole, an oil, a dipolar aproptic solvent, and a surfactant. In certain embodiments, the benzimidazole is mebendezole. The pharmaceutical compositions are formulated for delivery to a subject by any means, and include formulations for oral and parenteral delivery.
Owner:UNIV HOUSTON SYST
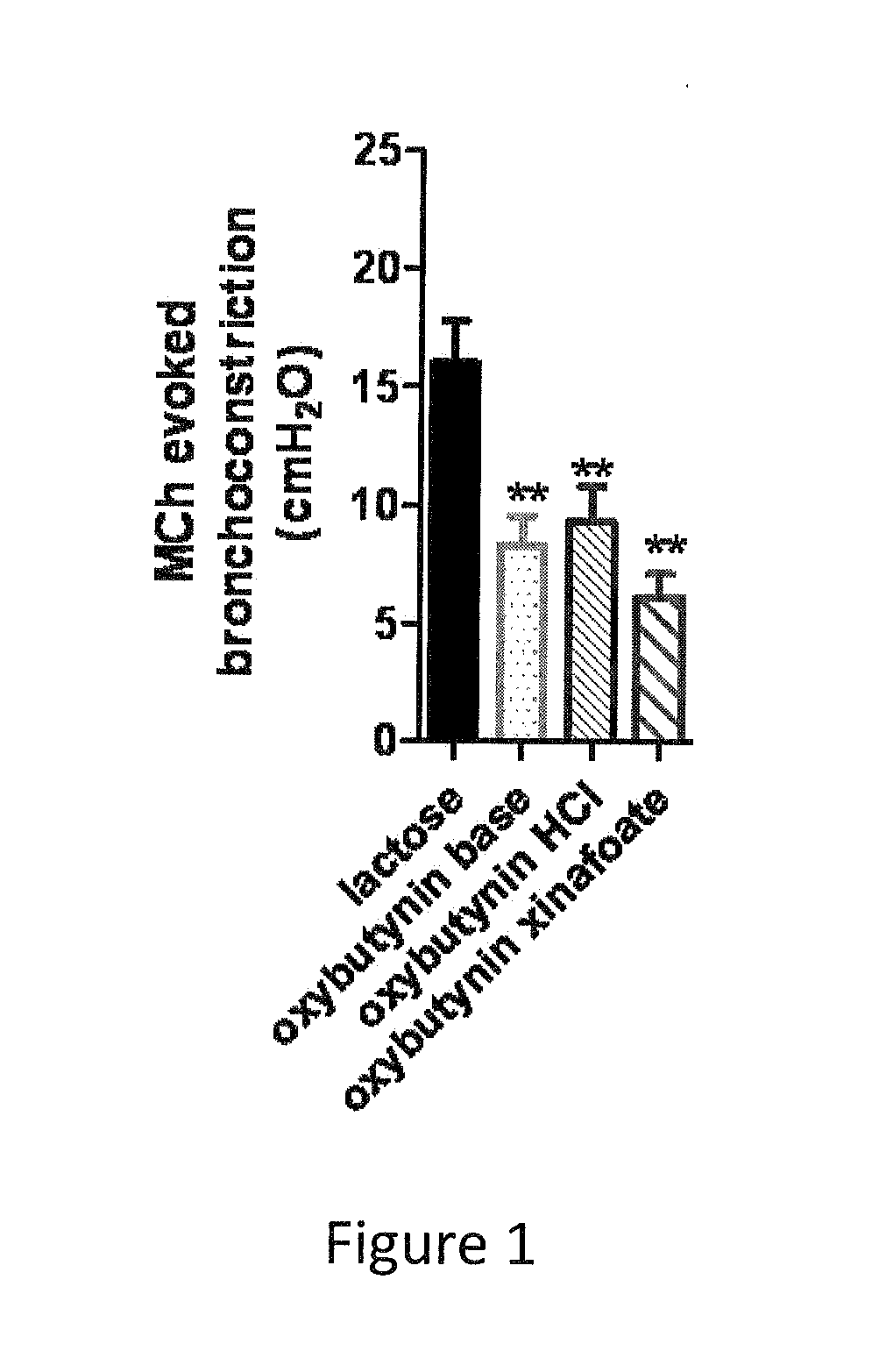
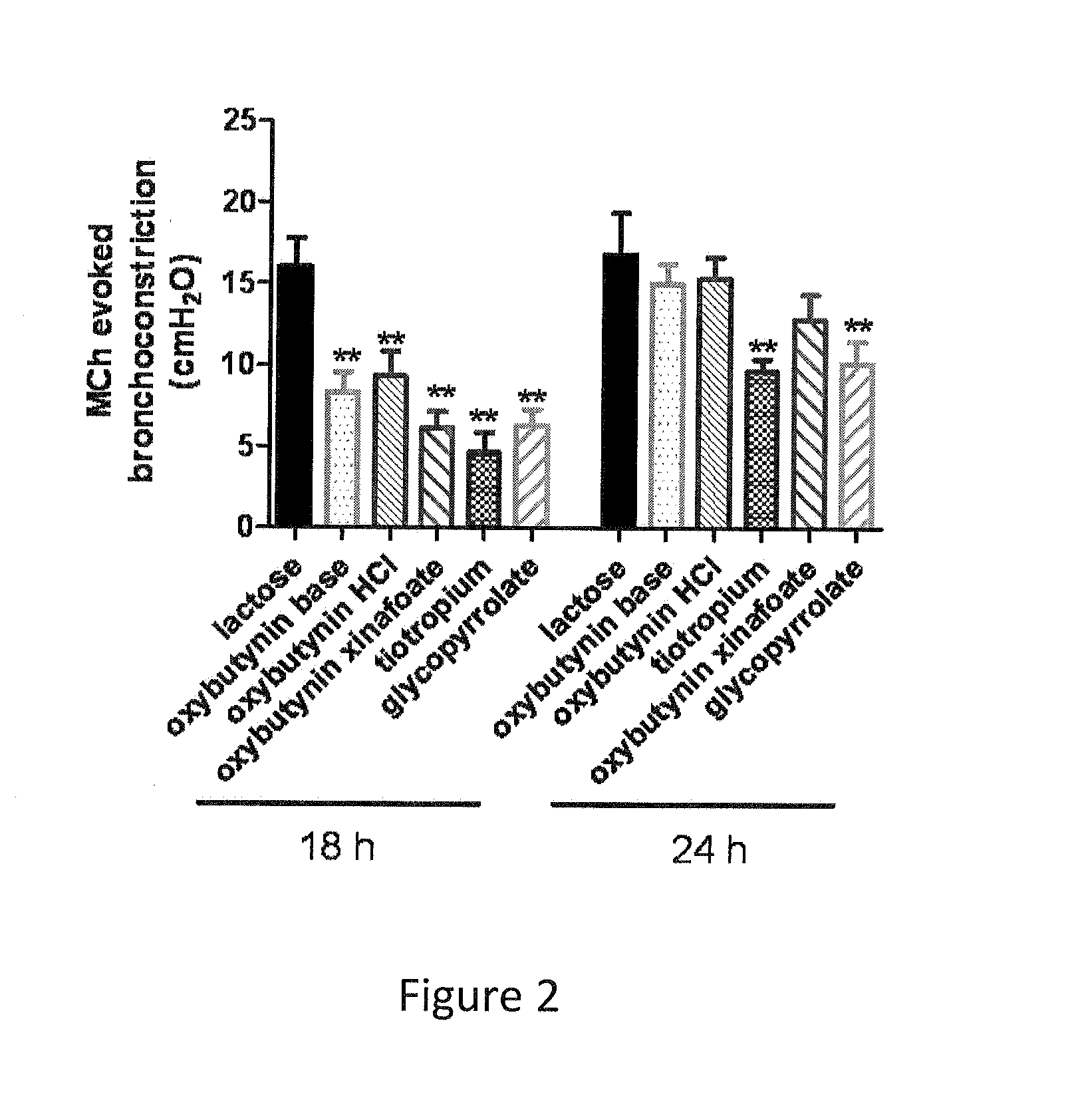
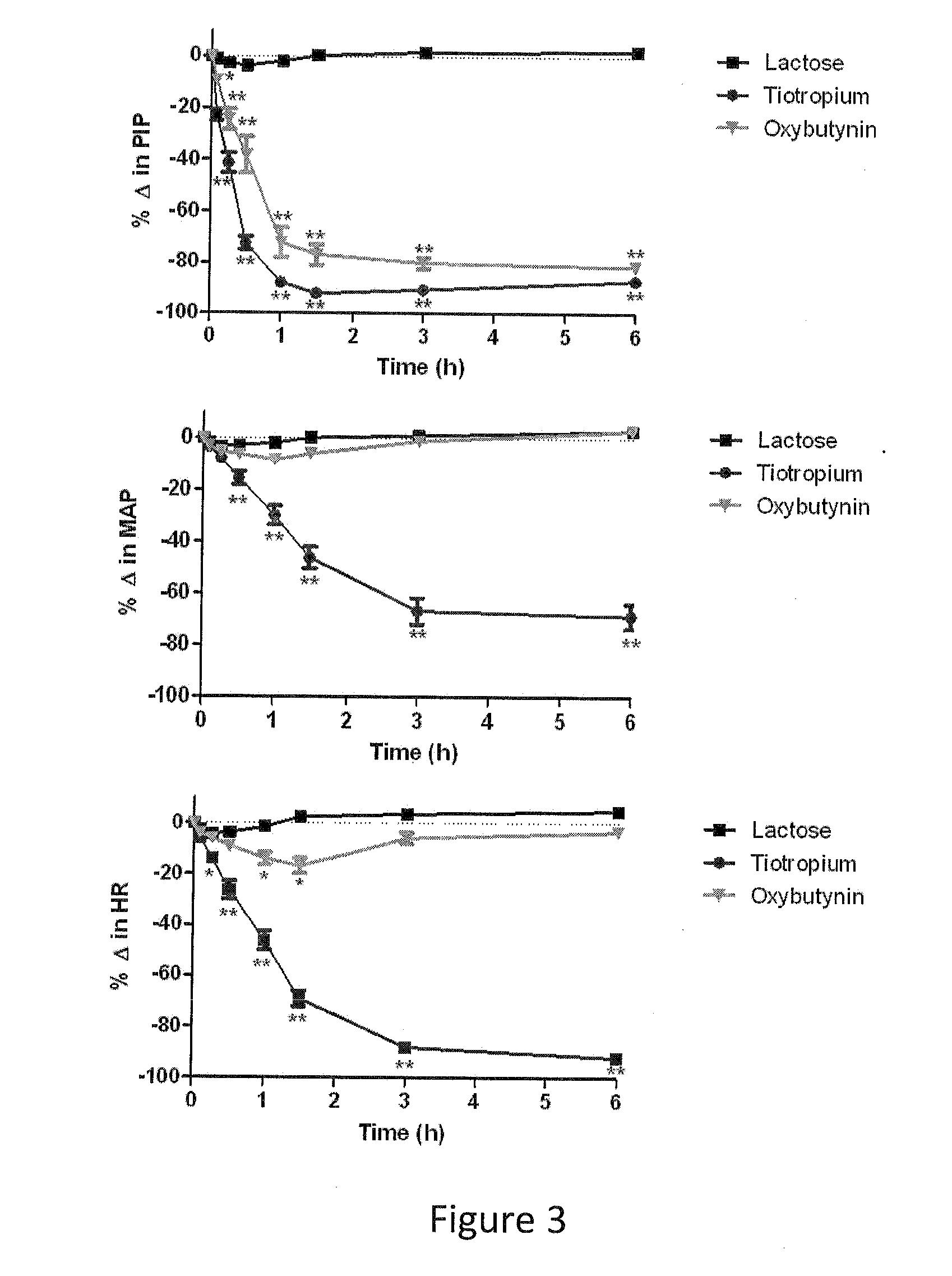
Methods and compositions for administration of oxybutynin
The present invention is directed to methods and compositions for treating pulmonary disease comprising delivering directly to a patient's lungs a therapeutically effective amount of oxybutynin in combination with one or more pharmaceutically effective agents. Oxybutynin may be selected from the group consisting of, but not limited to, a xinafoate salt, a palmitate salt, a pamoic salt, a resonate salt, a laurate salt and other salts. The pharmaceutically effective agents comprise bronchodilators, antiinflammatories, corticosteroids, corticosteroid reversal agent or alveolar growth agents or other agents selected from proteinase or protease inhibitors.
Owner:MICRODOSE THERAPEUTX INC
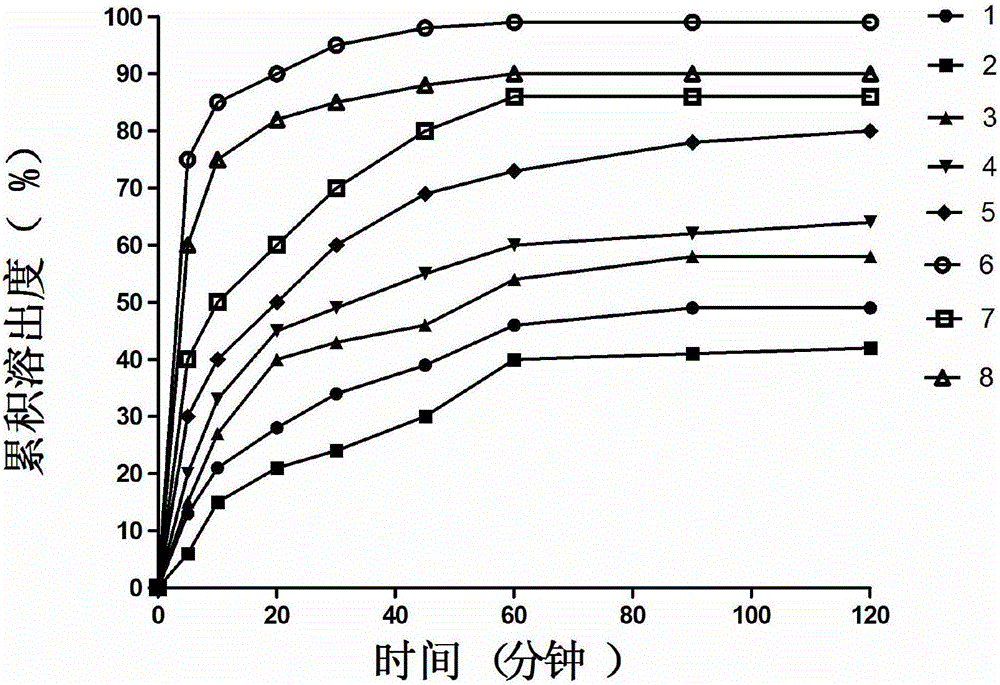
Solid dispersion as well as preparation method and application thereof
ActiveCN106420633AImprove solubilityImprove bioavailabilityPharmaceutical non-active ingredientsGranular deliveryAlpha-TocopherolPolyethylene glycol
The invention relates to solid dispersion as well as a preparation method and an application thereof. The solid dispersion is prepared from indissolvable drugs, a surfactant and a water-soluble polymer material with a spray drying method after mixing and heating dissolution, wherein the surfactant is selected from at least one of sodium dodecyl sulfate, poloxamer, tween, alpha-tocopherol, succinate, polyethylene glycol, sodium cholate and polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer; the water-soluble polymer material is selected from at least one of povidone, copovidone, hydroxypropyl methylcellulose and polyethylene glycol. An organic solvent is not required when the solid dispersion is prepared with the spray drying method, and the problem of organic solvent residues is solved. By means of the solid dispersion, the dissolvability of the indissolvable drugs is increased, the dissolution speed and the dissolubility are remarkably increased, and the bioavailability of the indissolvable drugs is improved.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Patches containing buprenorphine hydrochloride
InactiveUS20020182247A1Improve breathabilitySafety and economy can be providedPowder deliveryOrganic active ingredientsDrugPolymer chemistry
A patch comprising an adhesive layer formed on one surface of a flexible support, wherein said adhesive layer containing a drug, an absorption enhancer and an adhesive comprising; (i) said drug is buprenorphine hydrochloride and / or buprenorphine, and (ii) said absorption enhancer is a mixture of polyoxyethylene sorbitan mono fatty acid ester having 6 to 20 of oxyethylene units and 12 to 18 of carbon number of fatty acid ester, and at least one selected from the group consisting of liquid higher fatty acid ester, 60 to 180 of molecular weight of liquid poly hydric alcohol, lactic acid and triacetin, and (iii) said adhesive is an acrylic-based adhesive.
Owner:TEIJIN LTD
Drug sustained release agent based on oleanolic acid and a preparation method thereof
InactiveUS20160144040A1Avoid fluctuationHigh biological activityBiocideAntipyreticLong actingSolubility
The present invention relates to the technical field of oleanolic acid drugs and provides a drug sustained release agent based on oleanolic acid and a preparation method thereof. The drug sustained release agent based on the oleanolic acid is applied to drugs with the oleanolic acid as a main drug component and is prepared from the components including a drug carrier, a hydrophilic gel material, a erodible matrix material and an insoluble matrix material, wherein the drug carrier is β-cyclodextrin-chitosan composites, wherein the oleanolic acid is from a plant raw material, and a host-guest inclusion complex is composed of the main drug component and the drug carrier according to the mass ratio of 0.1:0.1-0.1:5. A preparation method comprises the following steps: preparing the inclusion complex, mixing auxiliaries, carrying out compression moulding and the like. The drug sustained release agent based on oleanolic acid has the characteristics of stable drug concentration, high biological activity, good drug solubility and long acting effect.
Owner:SCHOOL OF MEDICINE JIAYING UNIV
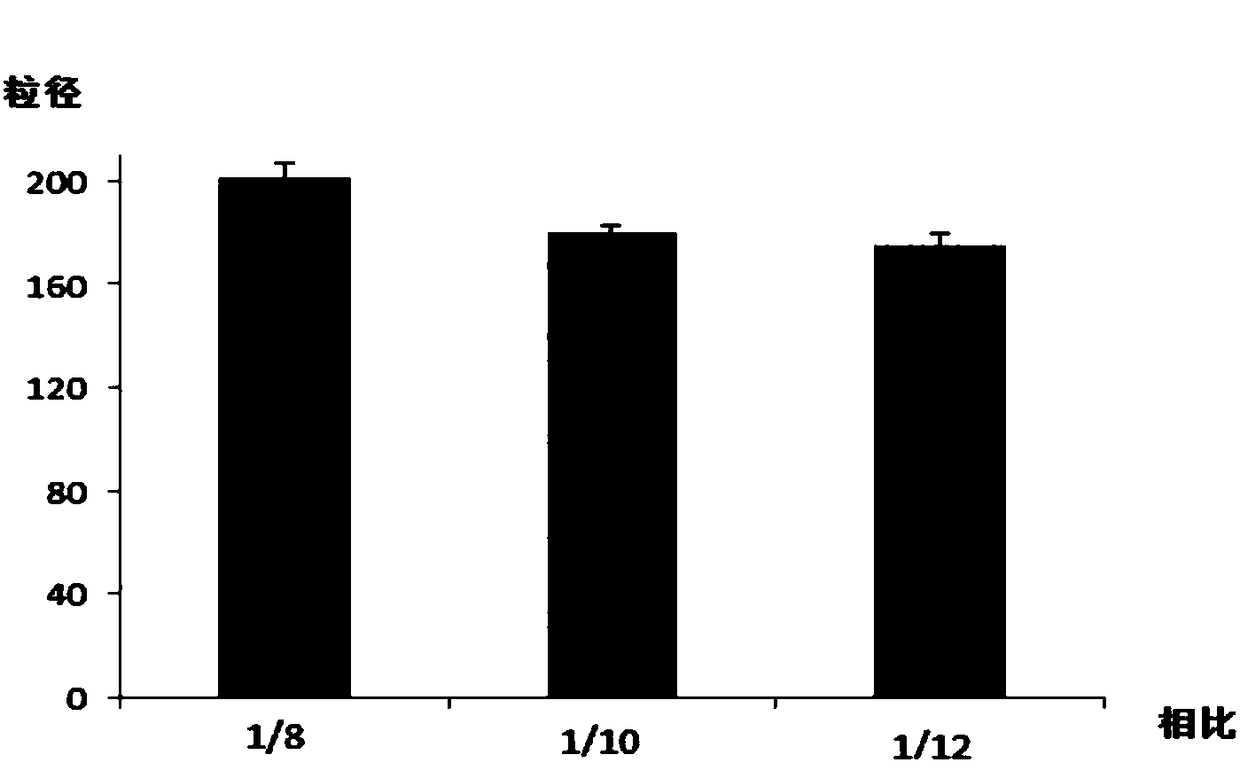
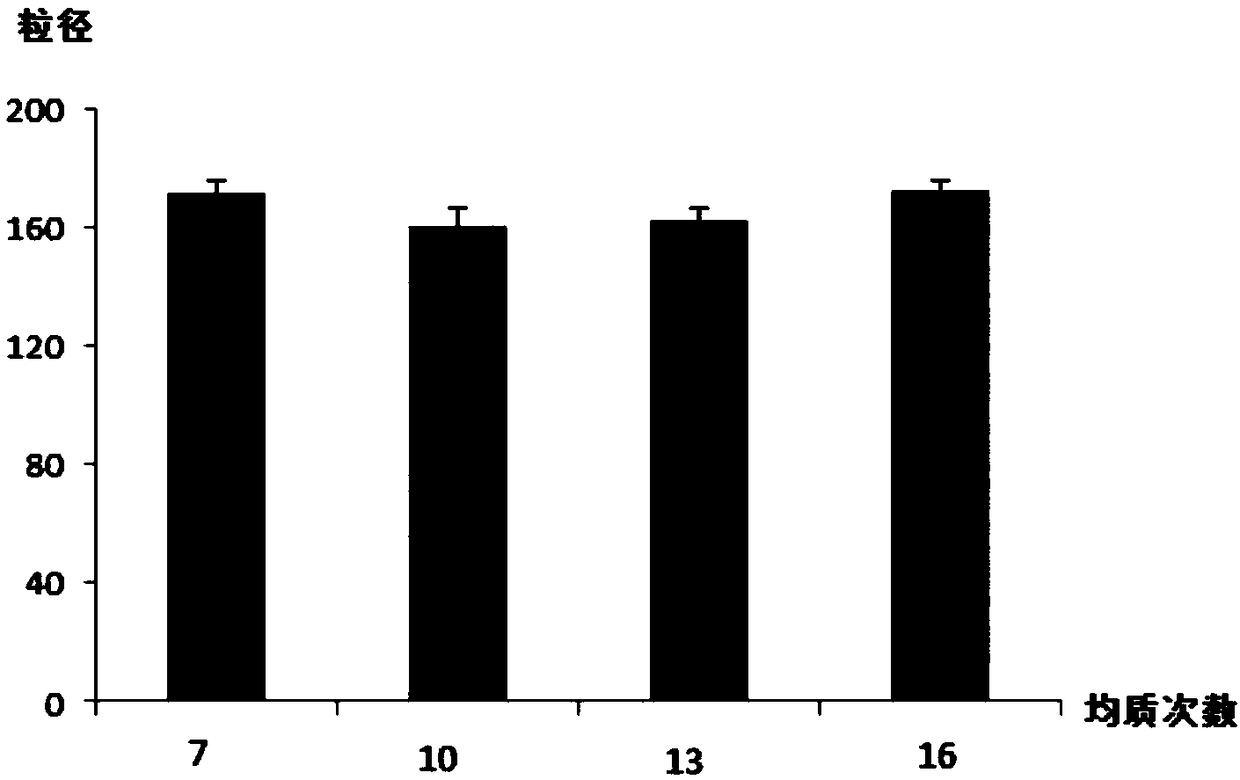
Preparation method and application of paclitaxel albumin nanoparticles
InactiveCN108524452AImprove drug solubilityGood biocompatibilityOrganic active ingredientsPowder deliveryAlbumin nanoparticlesPharmaceutical formulation
The invention relates to a preparation method and application of paclitaxel albumin nanoparticles and belongs to the field of medicinal preparations. The preparation method includes: weighing paclitaxel active pharmaceutical ingredient, and dissolving in a proper amount of organic solvent to obtain an oil phase; weighing albumin, dissolving in water, stirring, heating to enable the albumin to be fully dissolved to obtain an albumin water solution, adjusting pH of the albumin water solution to 6.0, and adding the organic solvent to obtain an aqueous phase; under action of high-speed shearing, dropwise adding the oil phase into the aqueous phase to obtain an oil-in-water emulsion; transferring the oil-in-water emulsion into a high-pressure microjet nano dispersing instrument for high-pressure homogenizing, and removing the organic solvent from a system after homogenizing is completed through rotary evaporation to obtain a water solution of the paclitaxel albumin nanoparticles. Particle size of the paclitaxel albumin nanoparticles can be controlled by changing proportion of the oil phase and is 100-200nm. The preparation method is simple, controllable and suitable for industrial production.
Owner:LIAONING UNIVERSITY
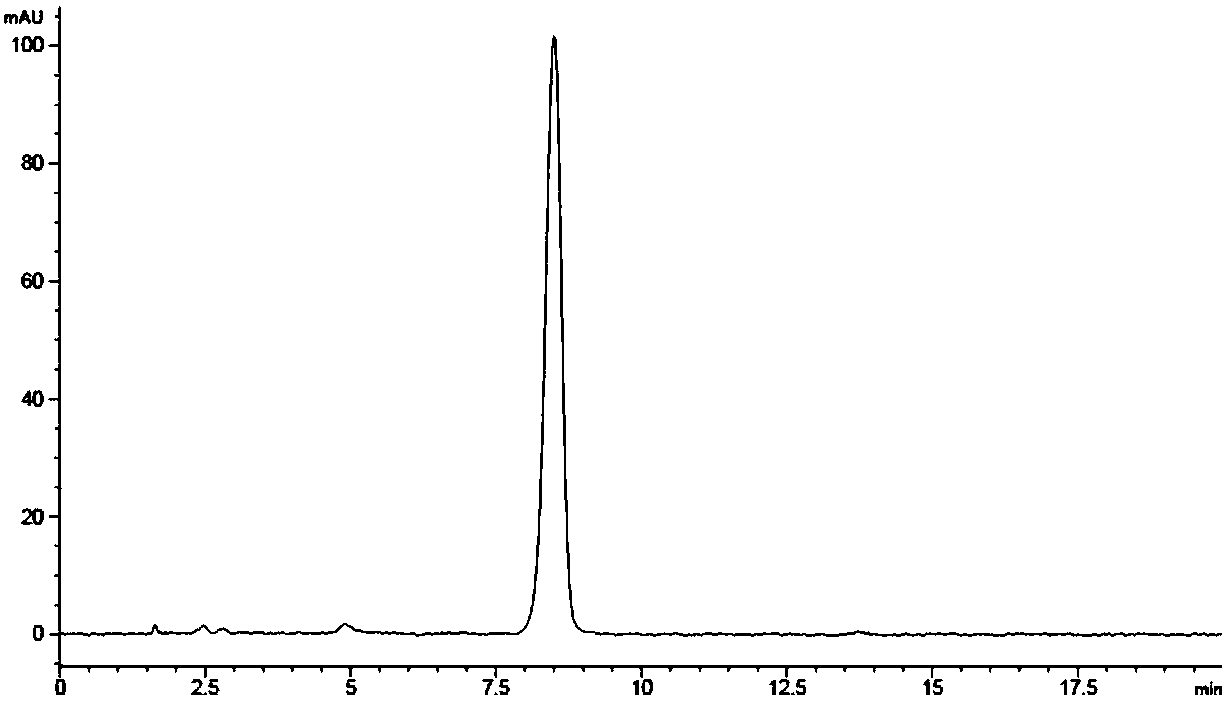
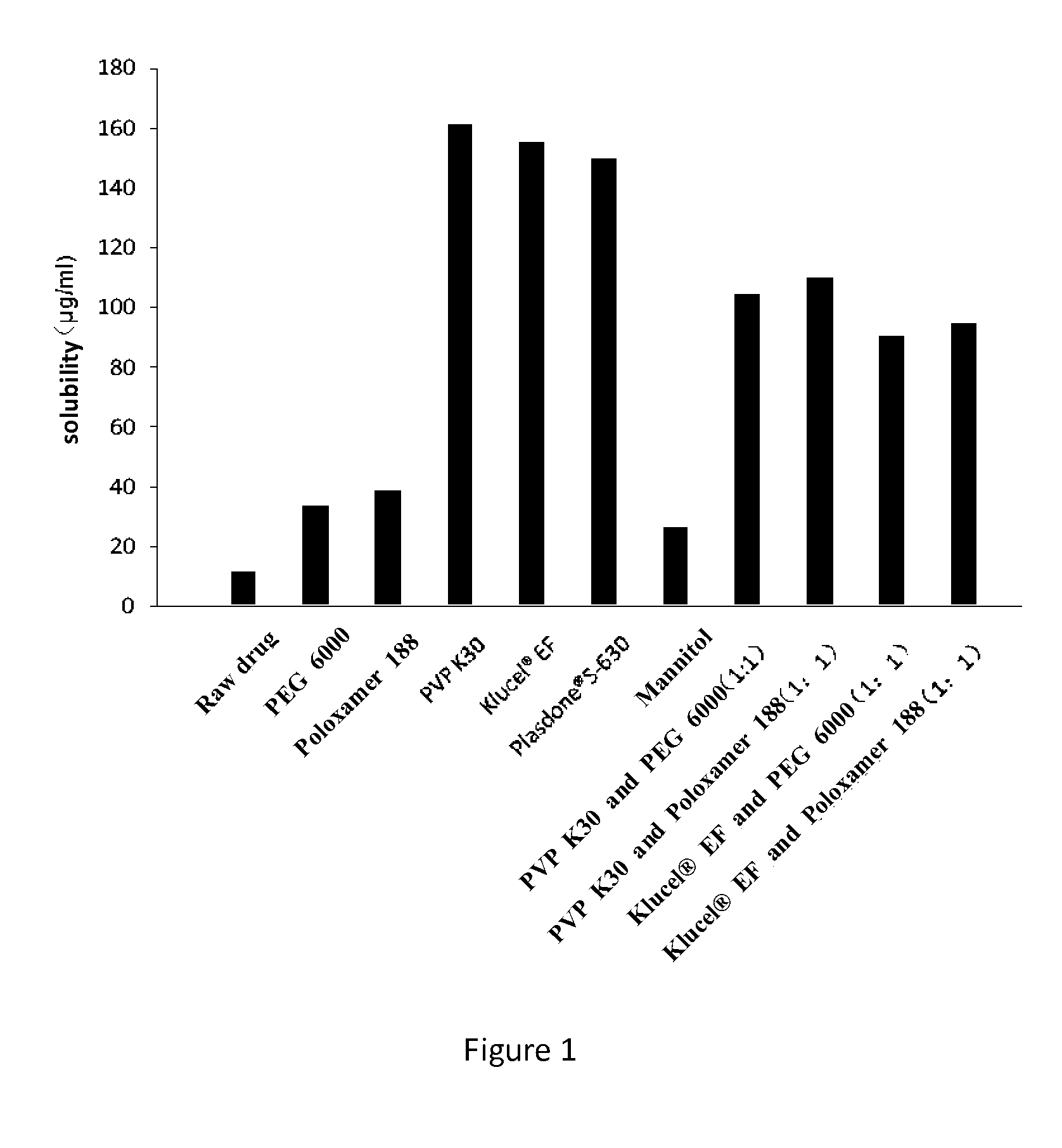
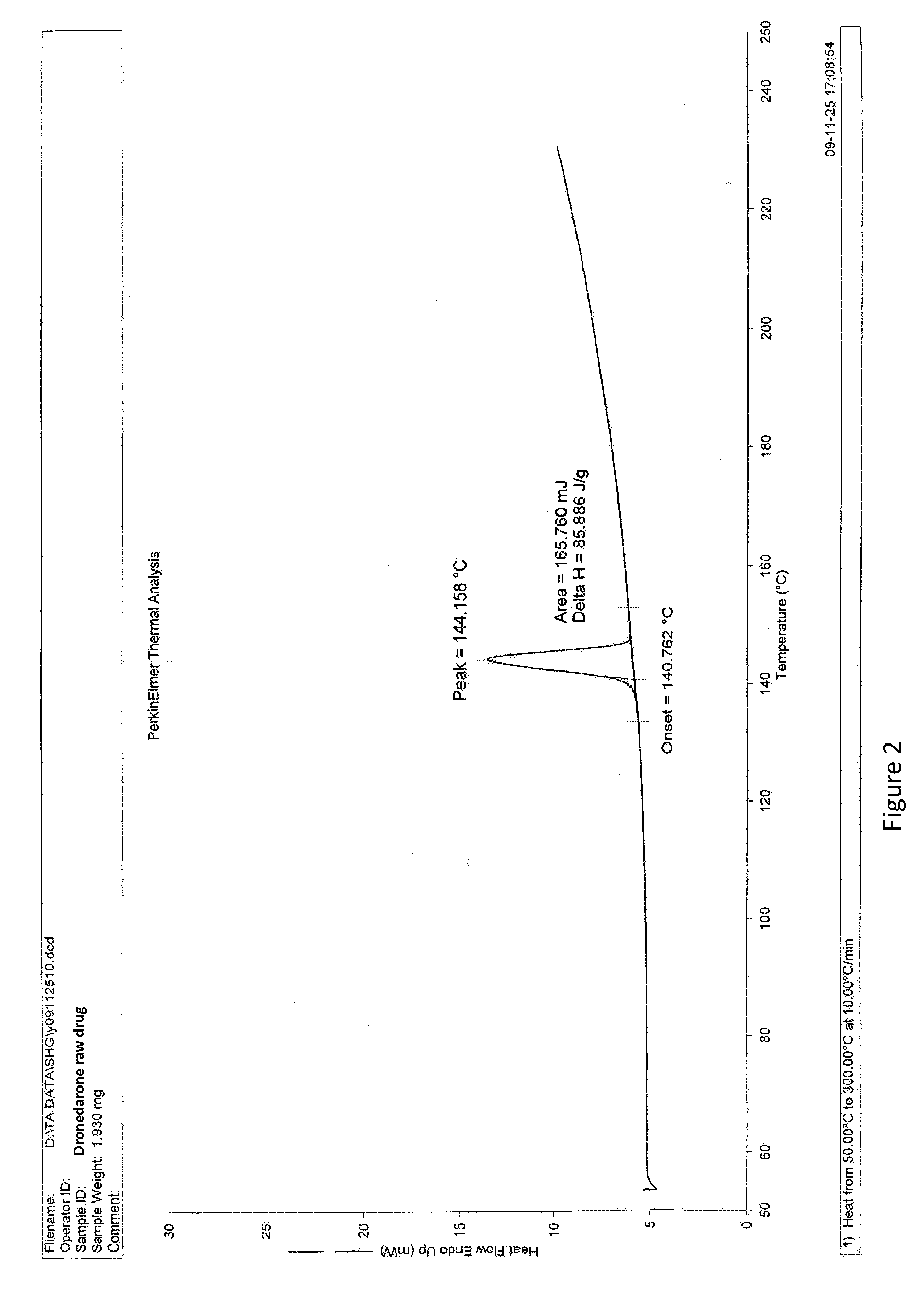
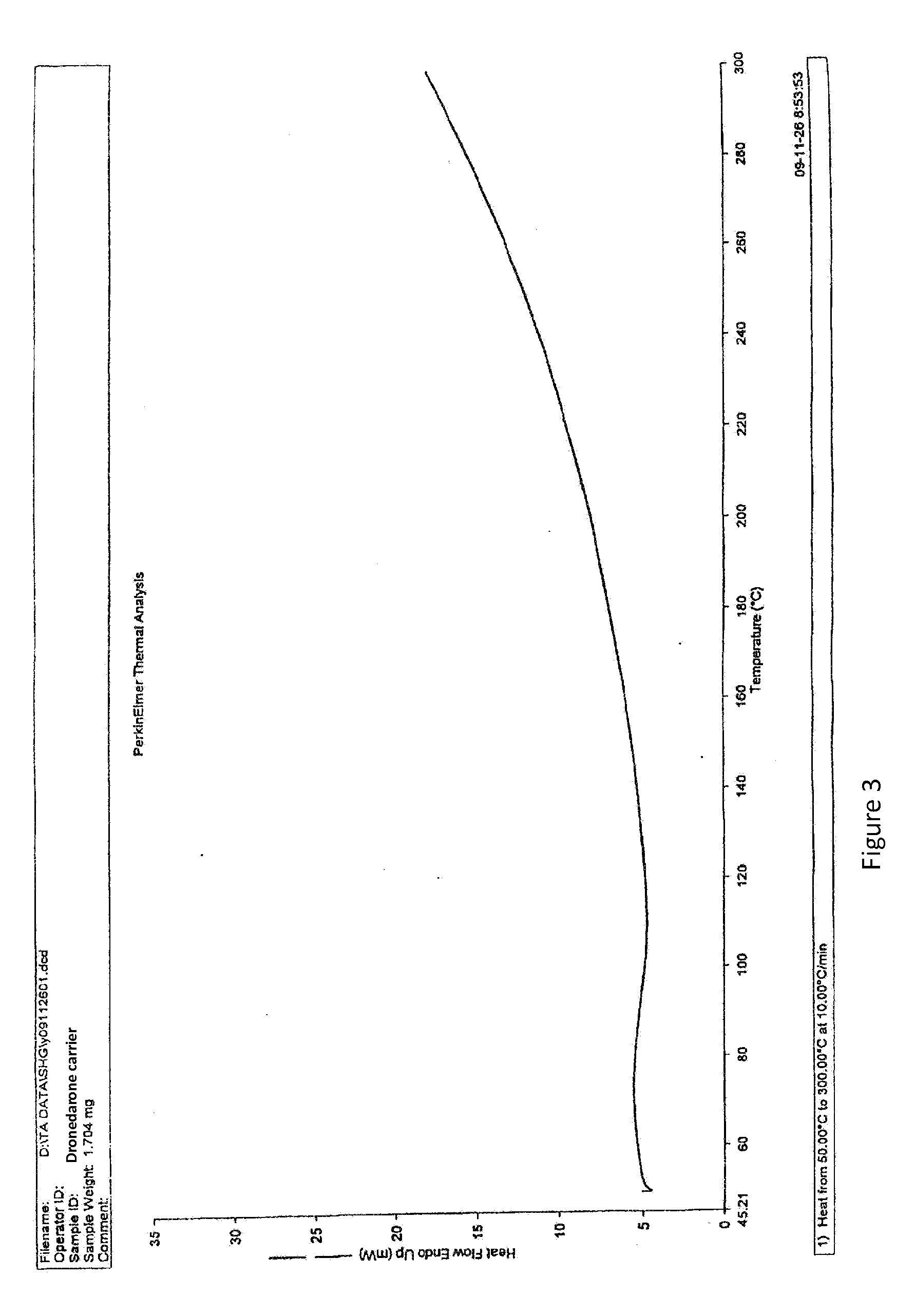
Dronedarone solid dispersion and preparation method thereof
InactiveUS20130123353A1High dissolution rateImprove solubilityBiocidePowder deliveryMedicineBULK ACTIVE INGREDIENT
A dronedarone solid dispersion and preparation method thereof are disclosed. The solid dispersion is composed of active ingredient dronedarone or its pharmaceutically acceptable salt and a carrier material, wherein the carrier material is povidone, copovidone, hydroxypropyl cellulose, or a mixture thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
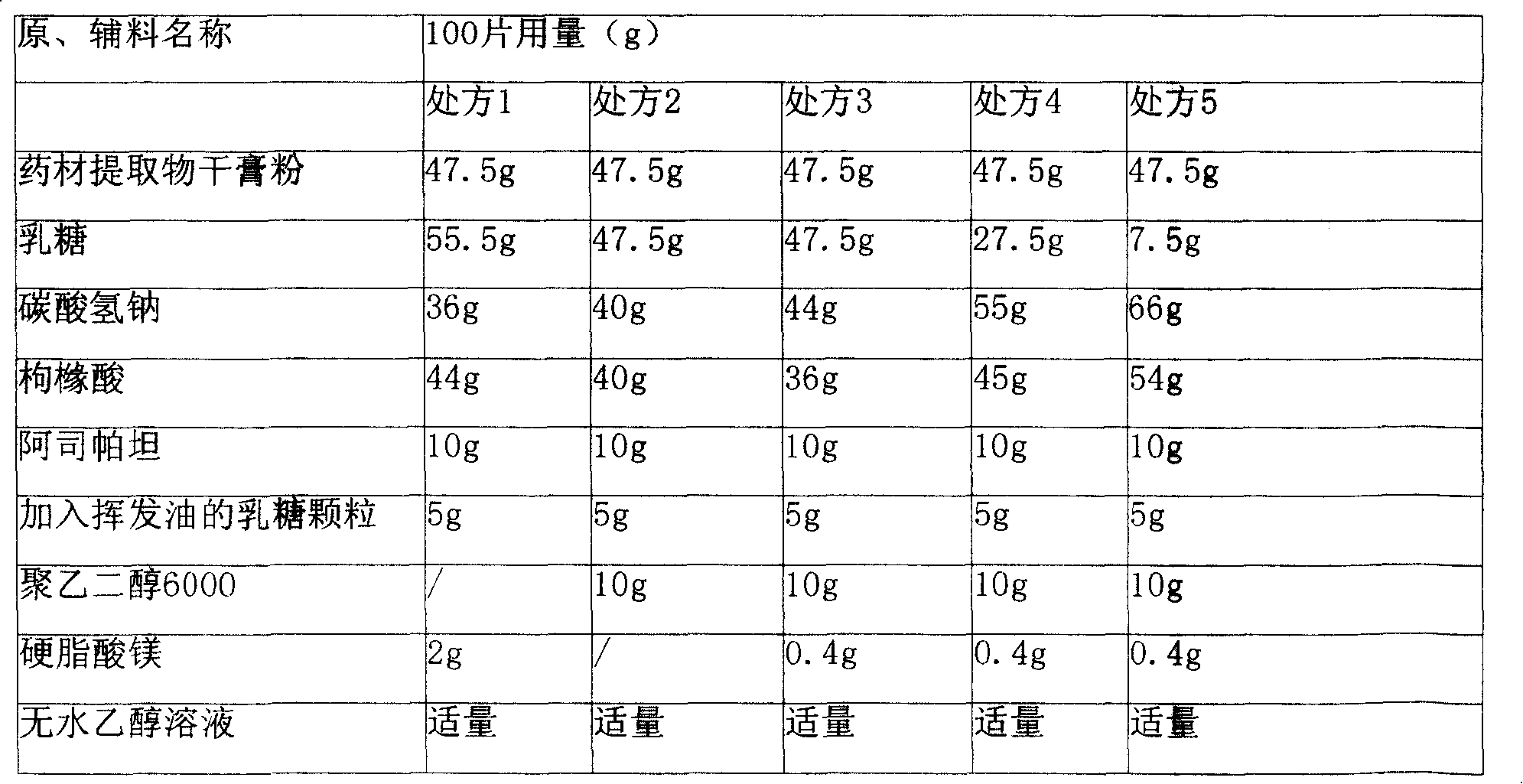
Heat-clearing effervescence tablet for infants and its preparing method
InactiveCN1813936AImprove drug solubilityQuick effectOrganic active ingredientsInorganic active ingredientsForsythiaMagnesium stearate
The present invention provides a Resuqing effervescent tablet for child and its preparation method. It is made up by using 625g of bupleurum root, 312.5g of scutellaria root, 625 g of isatis root, 312.5 g of pueraria root, 343.75 g of lonicera flower, 156.25 g of water buffalo horn, 375 g of forsythia fruit, 156.25 g of rhubarb and 125 g of lactose, 660 g of sodium hydrogen carbonate, 540 g of citric acid, 100 g of Aspatan, 100 g of polyethelene glycol 6000 and 4 g of magnesium stearate. Said effervescent tablet has obvious therapeutic effect for reducing fever.
Owner:葵花药业集团(贵州)宏奇有限公司
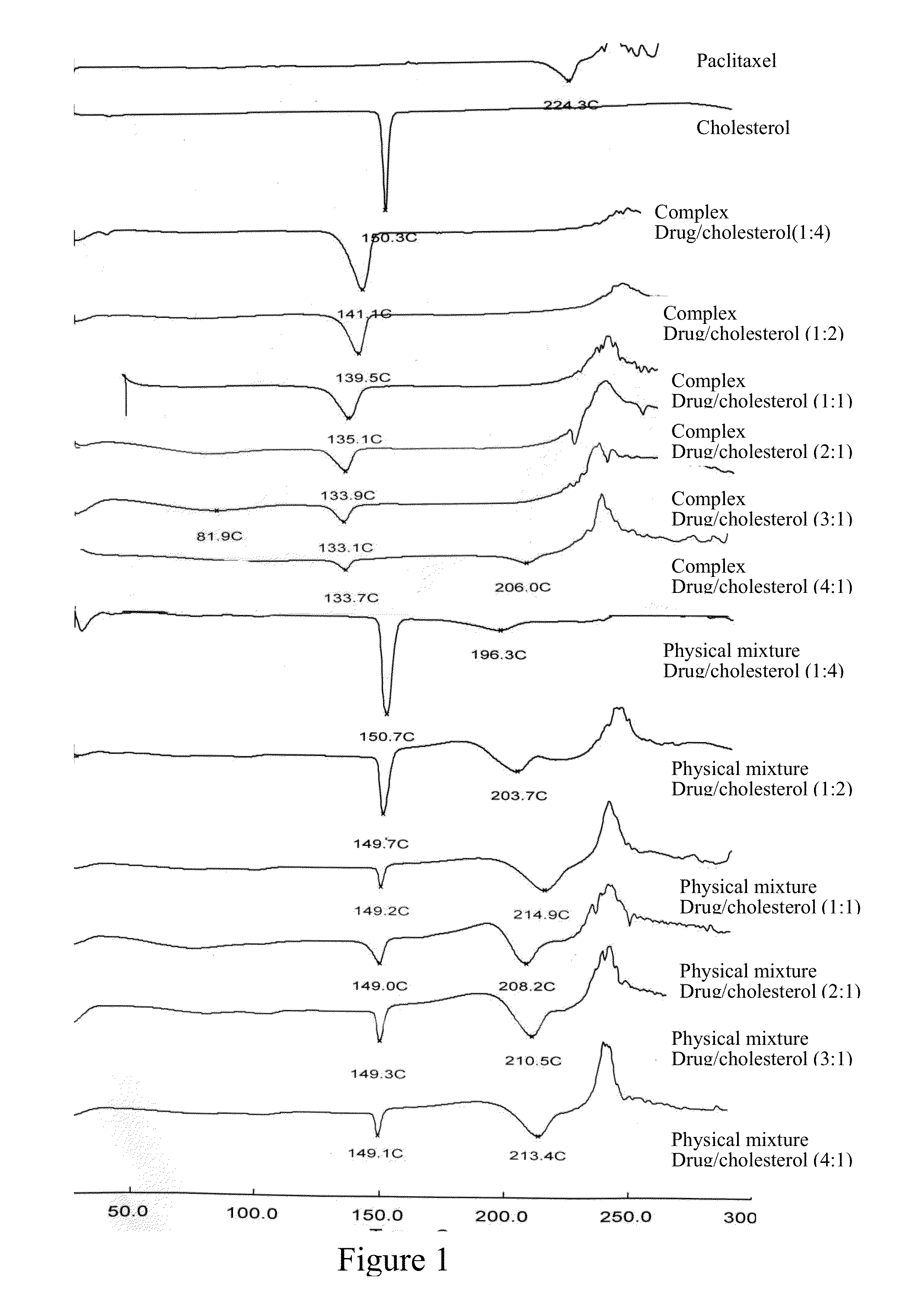
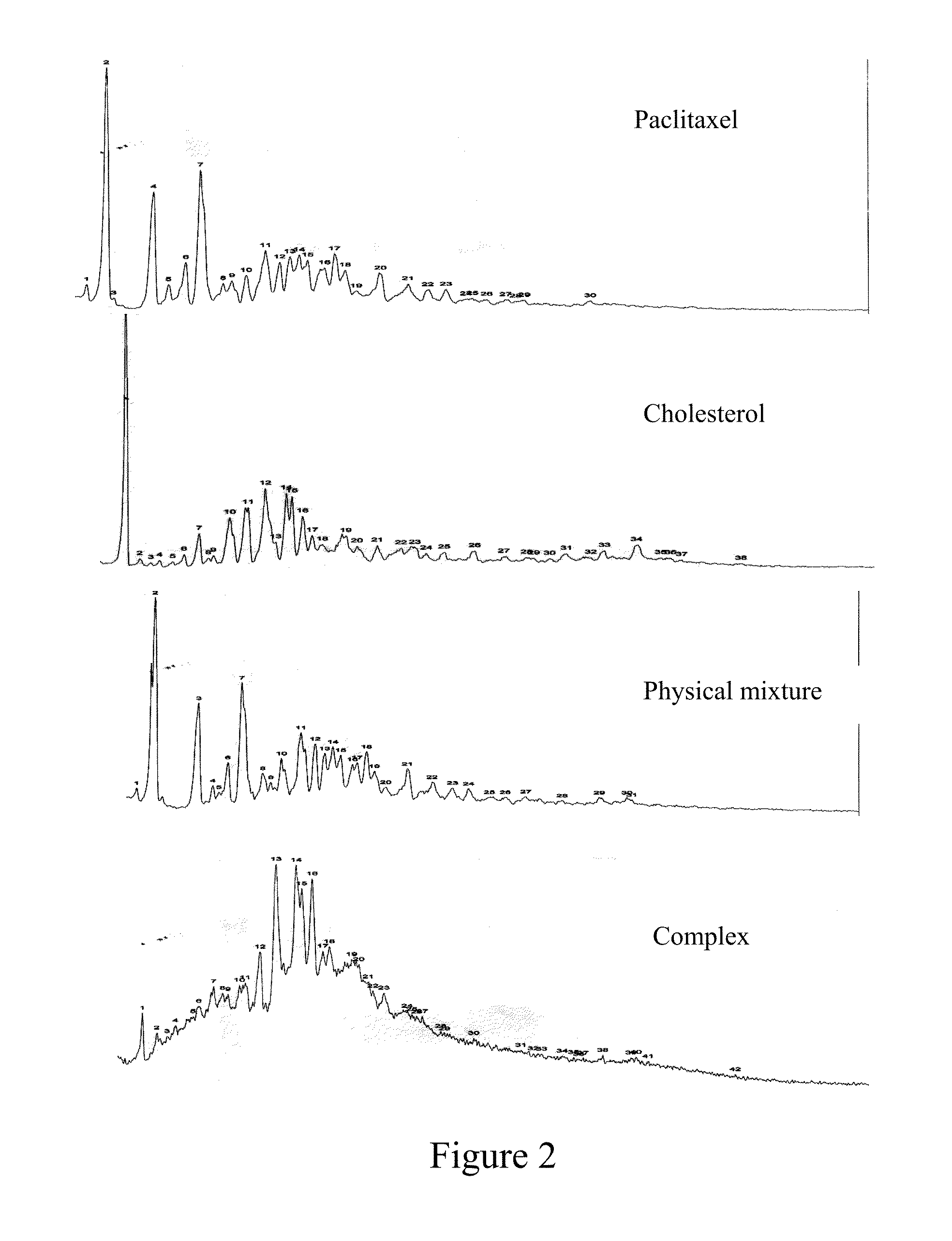
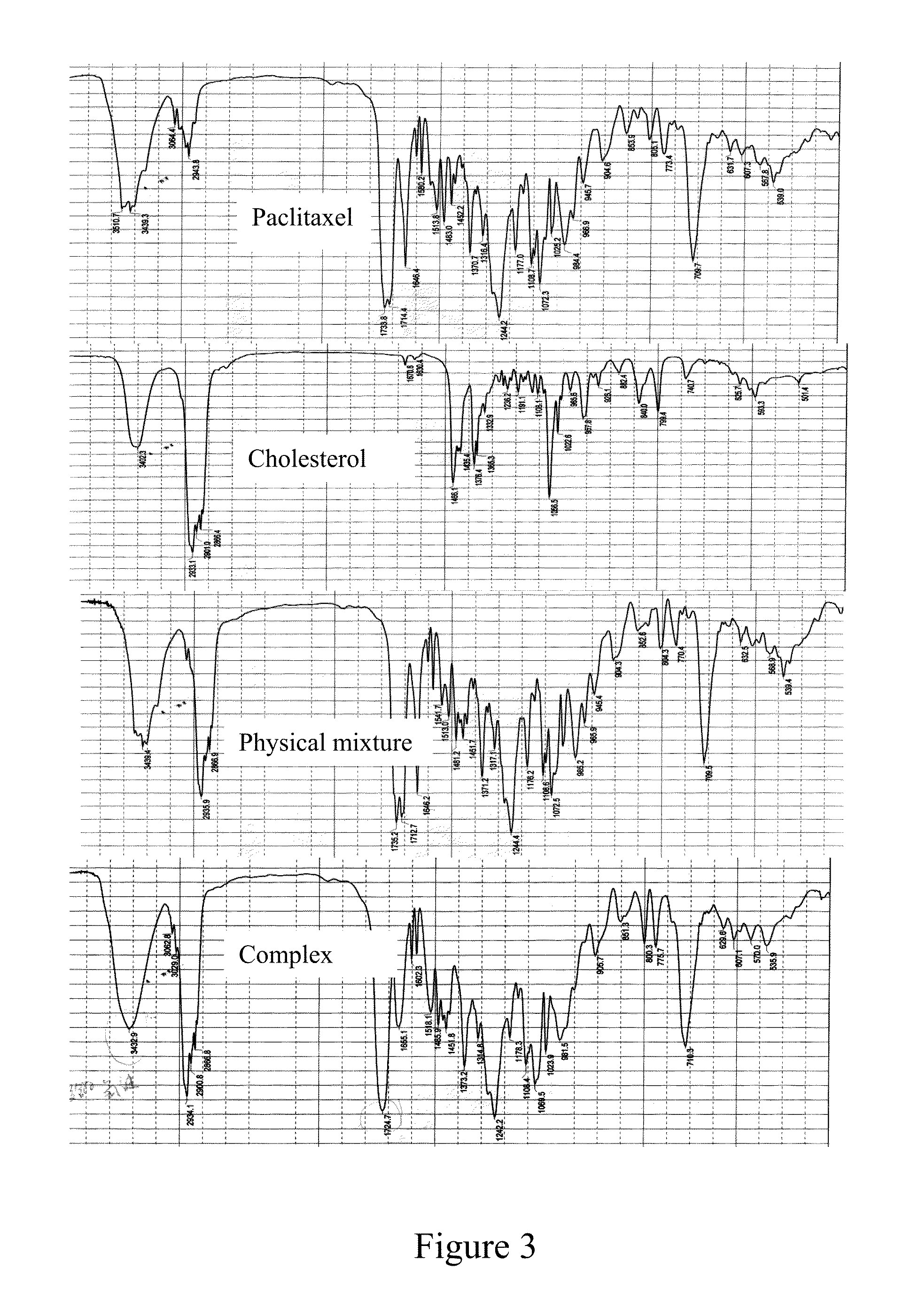
Paclitaxel/steroidal complex
InactiveUS20130150335A1Reduce amountEasy loadingBiocideOrganic active ingredientsEmulsionPhotochemistry
A paclitaxel / steroid complex comprising paclitaxel and steroid is disclosed. The molar ratio of paclitaxel to steroid is 1:0.2˜4, preferably 1:0.25˜2. A process for the preparation thereof and the use thereof in the manufacture of submicron emulsion, dry emulsion, self-microemulsifying system are also disclosed.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Sitafloxacin fumarate injection and preparation method thereof
ActiveCN105769756AImprove drug solubilityGood effectAntibacterial agentsOrganic active ingredientsSolventMethionine biosynthesis
The invention discloses a sitafloxacin fumarate injection. Additive composition of the injection is simple. But stability is obviously raised under high temperature sterilization and high-light exposure, and the preparation can be effectively avoided from being precipitated out under a low-temperature condition during the storage process. The injection is composed of the following ingredients: sitafloxacin fumarate and methionine are used as main drugs; a cosolvent is one ingredient selected from aspartic acid, citric acid and lactic acid; a pH regulator is one ingredient selected from histidine, sodium citrate or disodium hydrogen phosphate; D-sorbitol is used as an antifreezing agent capable of greatly preventing low-temperature crystallization; and water for injection is also included. The injection can be applied in the form of a small-volume injection, a large-volume infusion or freeze-dried powder injection. During the preparation process of the injection, a specific addition order is applied such that dosage of the cosolvent for dissolving the main drug sitafloxacin fumarate is minimized as much as possible; and with addition of methionine and sorbitol, stability of the injection during high temperature sterilization, high-light exposure and low-temperature storage is raised. The preparation technology of the injection can completely meet industrial production and quality requirements.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Self-assembled nanoparticle releasing soluble microneedle structure and preparation method therefor
PendingUS20190117561A1Reduce deliveryImprove drug solubilityOrganic active ingredientsGenetic material ingredientsSolubilityNanoparticle
The present invention relates to a self-assembled nanoparticle releasing microneedle structure which is formed of biocompatible amphiphilic block copolymers containing a drug, and a preparation method therefor. The microneedle structure according to the present invention can deliver a water-soluble or hydrophobic drug while being carried in a microneedle. In particular, since a fat-soluble drug is delivered while being carried by micelle-type self-assembled nanoparticles which are formed as the structure is dissolved, it is possible to greatly increase the solubility in an aqueous solution. As such, existing drugs with poor absorption can be delivered through the skin of a body.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
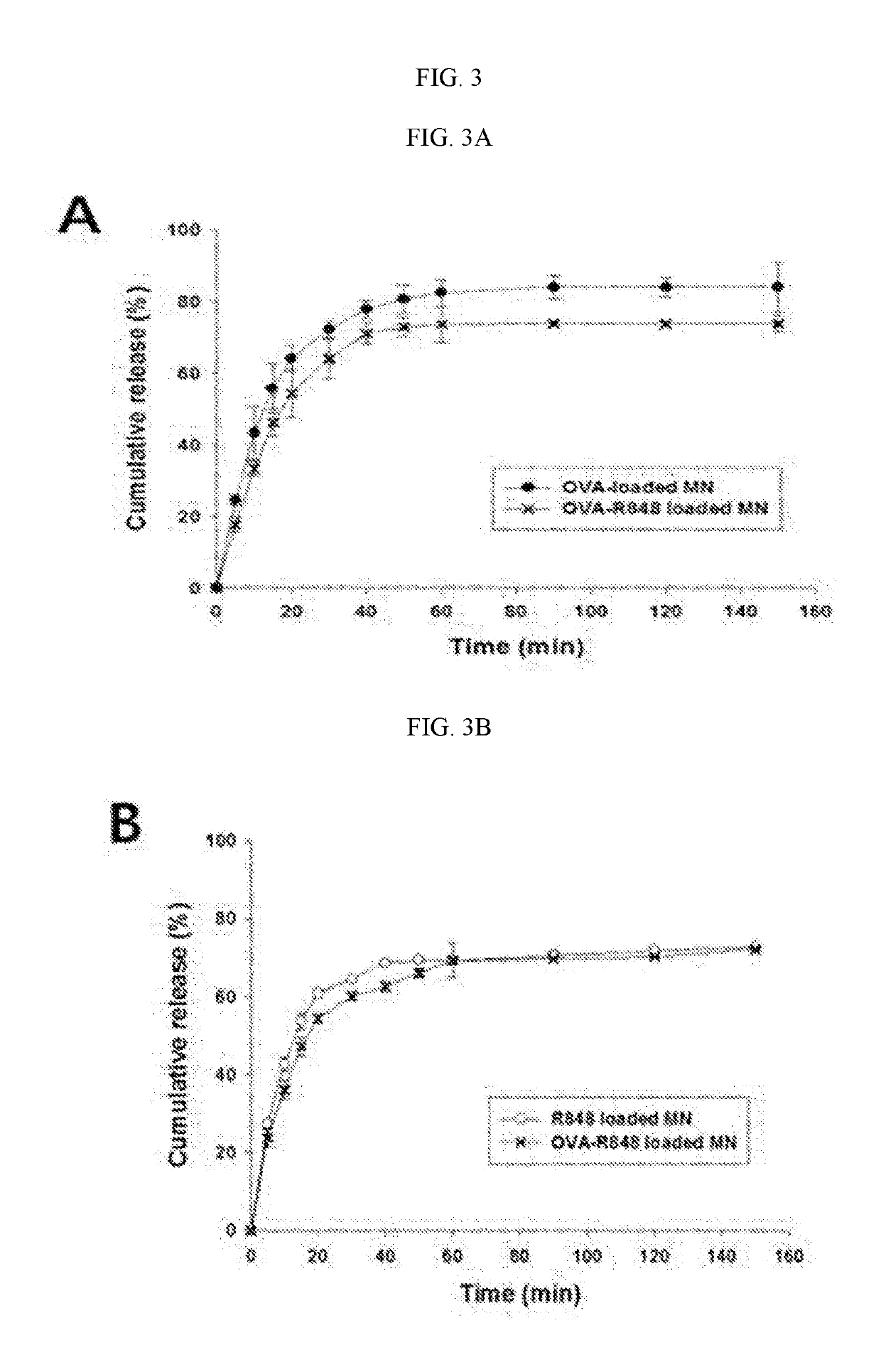
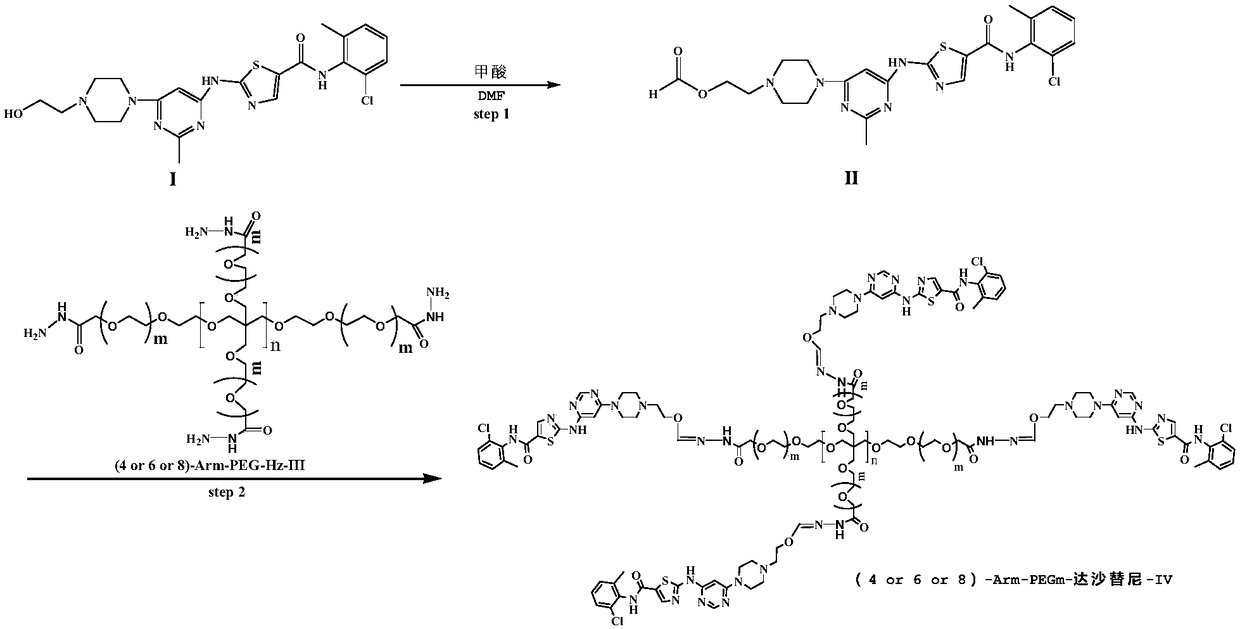
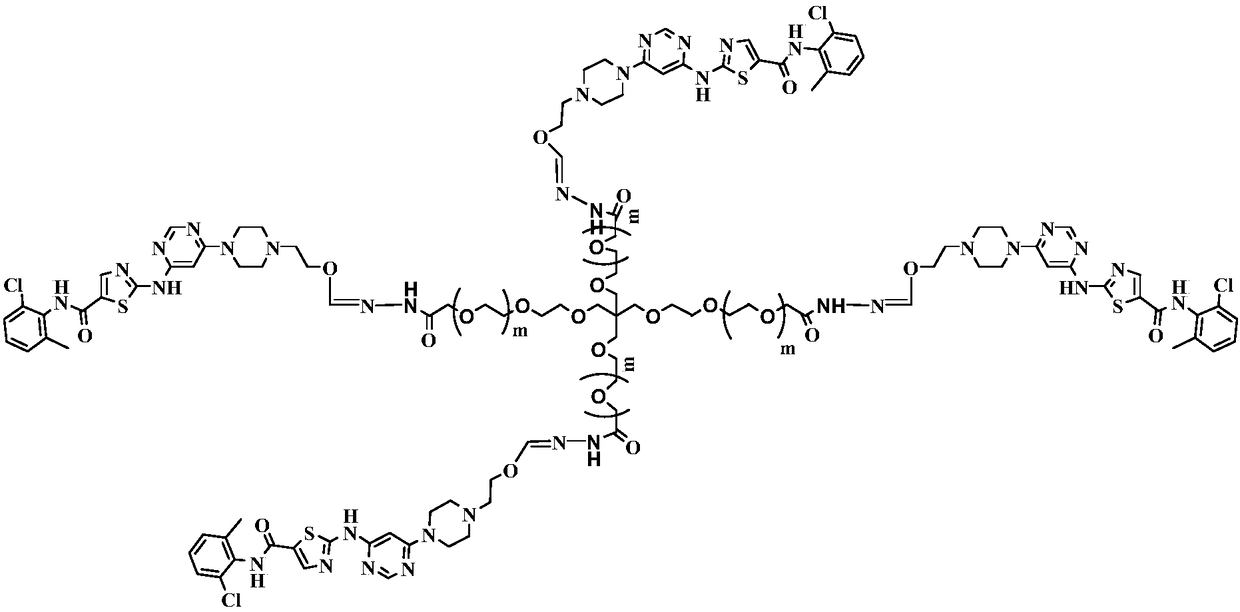
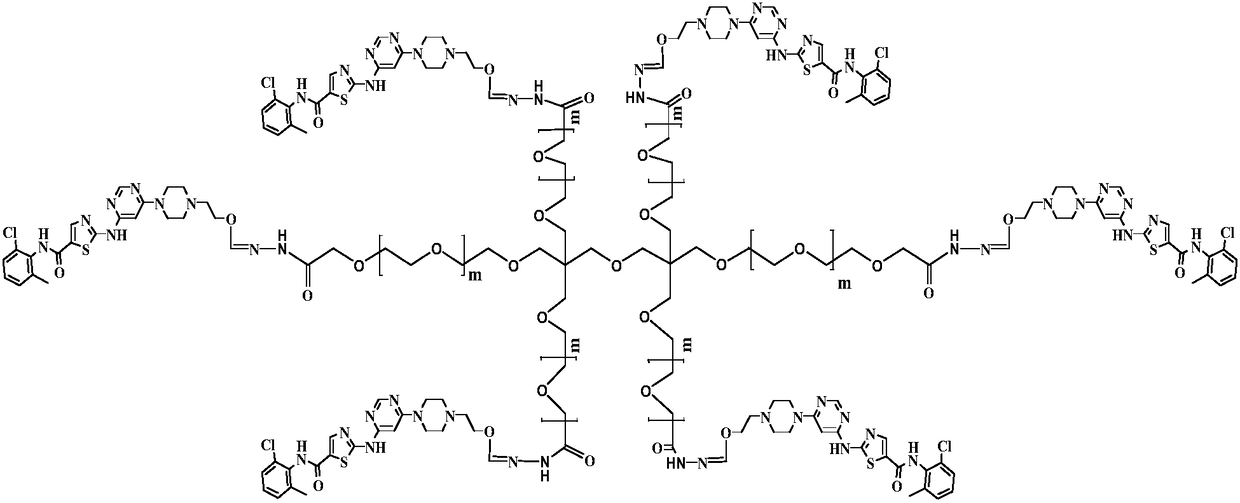
Multi-arm PEGylated dasatinib derivative and preparation thereof
InactiveCN108939088AImprove drug solubilityLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsTreatment effectDasatinib
Based on the characters that multi-arm PEG is toxic-free and is easy to be combined, tetra-arm PEG, hexa-arm PEG and octa-arm PEG are respectively connected with dasatinib. The multi-arm PEG-supporteddasatinib predrug has excellent water solubility; and what is more, one multi-arm PEG chain can be connected with a plurality of dasatinib residues, so that supporting rate of the medicine is greatlyincreased. In addition, the half-life period of the medicine is greatly prolonged, so that the medicine is significantly increased in existing time in blood plasma, thereby improving treatment effect.
Owner:湖南华腾制药有限公司
Transdermally absorbable preparation
PendingUS20190000774A1Improve drug solubilitySuperior in releasabilityPharmaceutical non-active ingredientsSheet deliveryElastomerIrritation
The present invention provides a transdermal absorption preparation in which a drug-containing adhesive layer is formed on a support, the aforementioned adhesive layer contains at least a thermoplastic elastomer and a higher fatty acid ester, and a content of a tackifier is not more than 10 wt %, which is superior in drug solubility and releasability, as well as adhesiveness to the skin and low irritation to the skin.
Owner:KM TRANSDERM LTD
External preparation
InactiveUS20070134311A1Promote transdermal absorptionImprove drug solubilityBiocideAnimal repellantsSolubilityTopical preparation
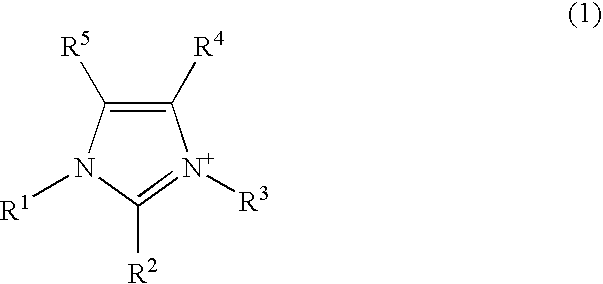
An external preparation having excellent percutaneous absorption ability for ionic drugs is provided. An external preparation which comprises the following components (a) and (b); component (a): an ionic drug, component (b): an ionic liquid containing a cation and an anion. By this construction, the ionic liquid functions as a solubilizing agent for the ionic drug and thereby the solubility of the drug in the external preparation increases, so that percutaneous absorption ability of the ionic drug is accelerated.
Owner:NITTO DENKO CORP
Heat-clearing effervescence tablet for infants and its preparing method
InactiveCN100408059CImprove drug solubilityQuick effectOrganic active ingredientsInorganic active ingredientsTherapeutic effectForsythia
The present invention provides a Resuqing effervescent tablet for child and its preparation method. It is made up by using 625g of bupleurum root, 312.5g of scutellaria root, 625 g of isatis root, 312.5 g of pueraria root, 343.75 g of lonicera flower, 156.25 g of water buffalo horn, 375 g of forsythia fruit, 156.25 g of rhubarb and 125 g of lactose, 660 g of sodium hydrogen carbonate, 540 g of citric acid, 100 g of Aspatan, 100 g of polyethelene glycol 6000 and 4 g of magnesium stearate. Said effervescent tablet has obvious therapeutic effect for reducing fever.
Owner:葵花药业集团(贵州)宏奇有限公司
Ampicillin capsule and preparation method thereof
ActiveCN103462929AFast dissolutionImprove drug solubilityAntibacterial agentsPharmaceutical non-active ingredientsDrugPrill
The invention discloses an ampicillin capsule and a preparation method thereof. The formulation is formed by uniformly mixing a drug-containing granule and a lubricating agent and then filling into a capsule shell; the drug-containing granule comprises the following components in parts by weight: 1 part of ampicillin and 0.1-0.5 part of tartaric acid, and the drug-containing granule is obtained by screening and uniformly mixing the ampicillin and the tartaric acid and then adopting a wet method for granulation. The ampicillin capsule disclosed by the invention has the advantages of fast dissolution, good stability, less auxiliary material variety and simple preparation process.
Owner:吉林显锋科技制药有限公司
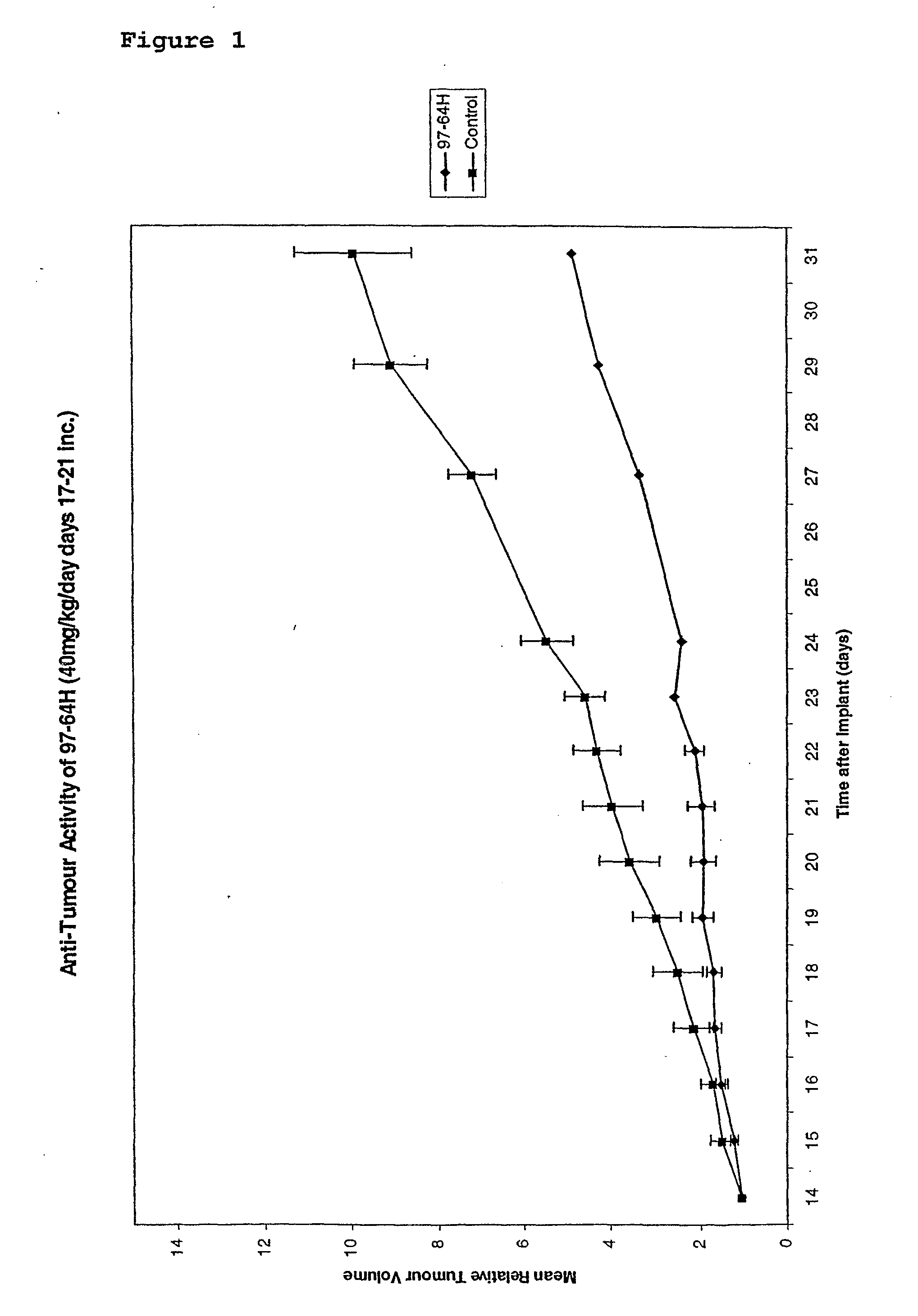
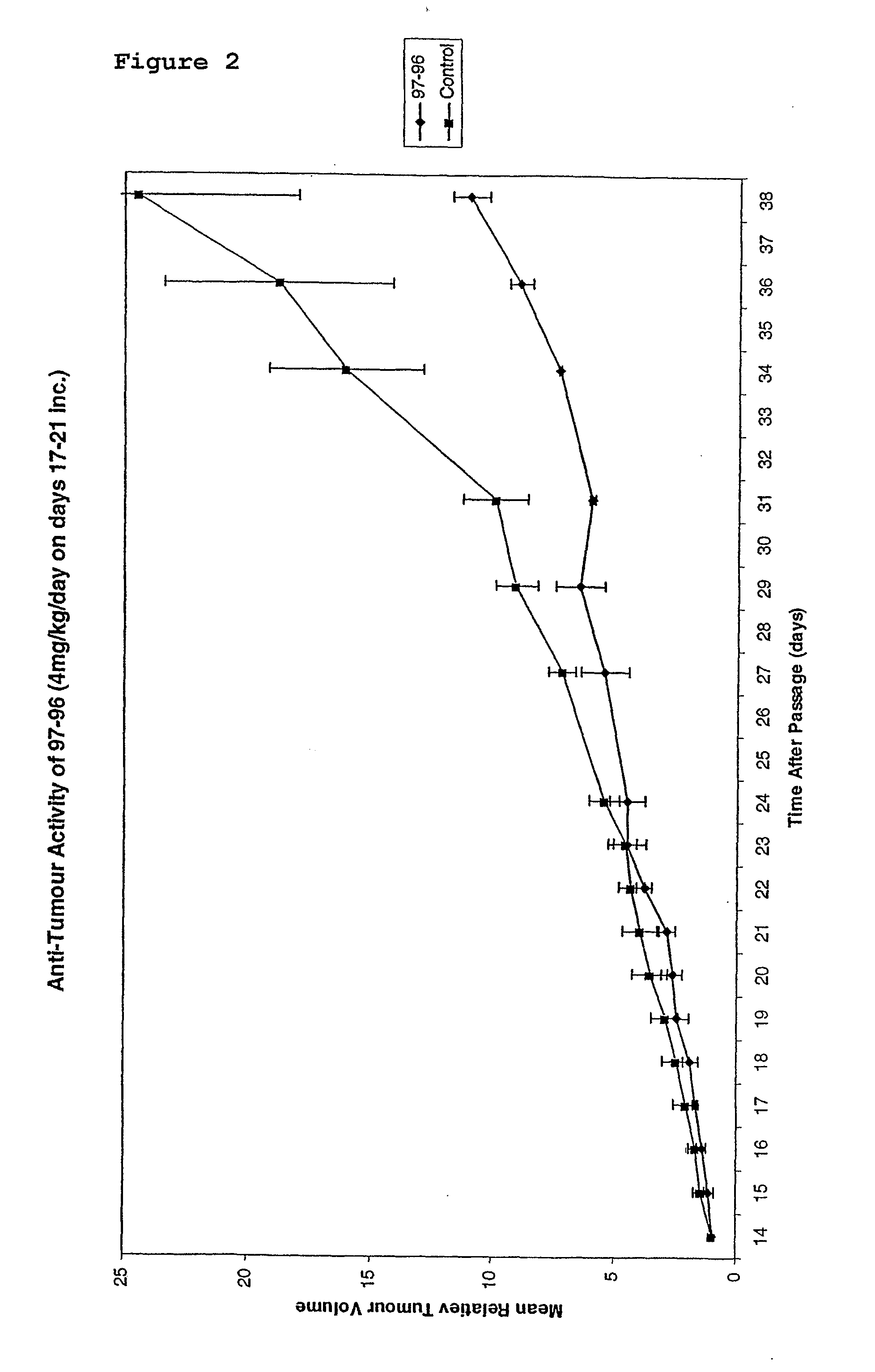
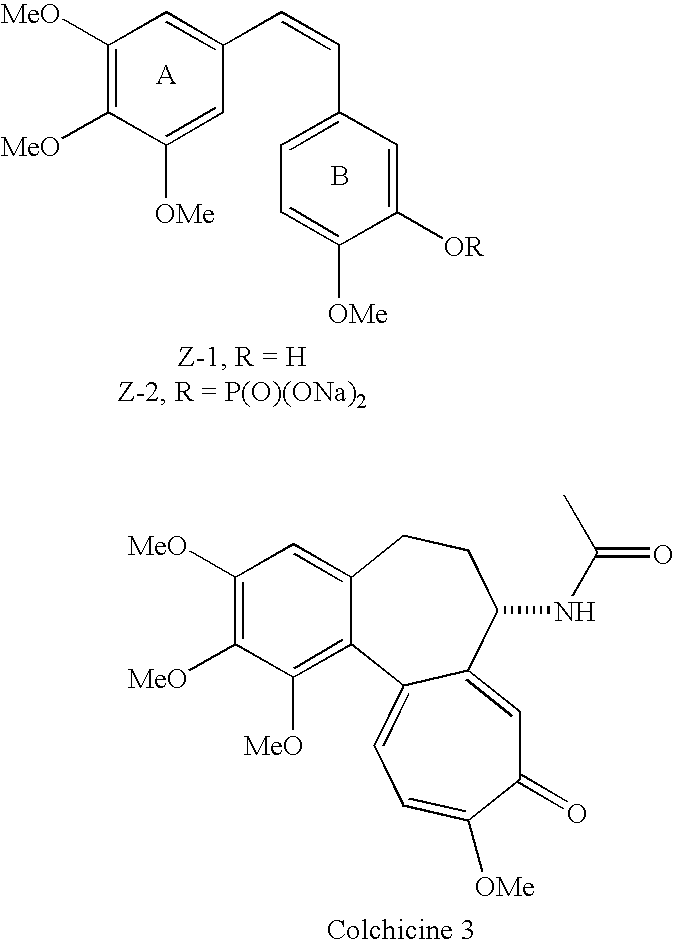
Substituted stilbenes and their reactions
Owner:UNIV UTRECHT UU HLDG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com