Multi-arm PEGylated dasatinib derivative and preparation thereof
A technology for dasatinib and derivatives, which is applied to multi-arm PEGylated dasatinib derivatives and their preparation and preparation, as well as the application field in the preparation of anti-tumor drugs, can solve the problem of inability to release and accumulate active ingredients in large quantities , increase the risk of pulmonary arterial hypertension, large proportion of ineffective molecules, etc., to avoid toxic side effects, increase the existence time, and enhance the water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
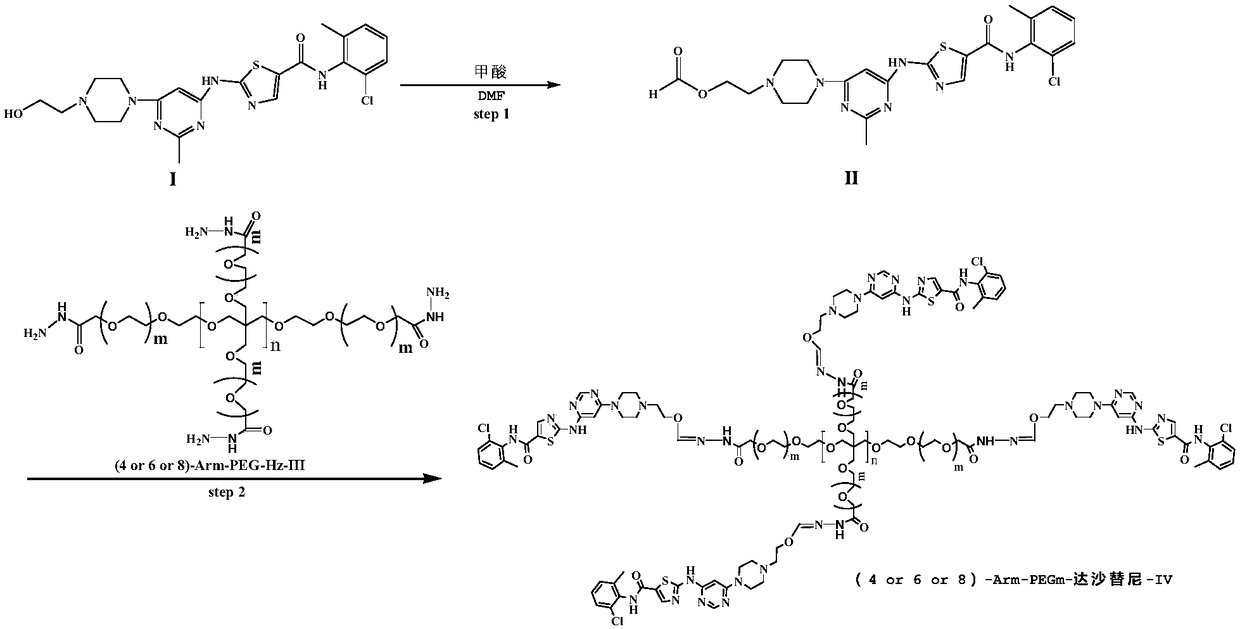
[0029] (1) Preparation of Intermediate II
[0030] Dissolve 10 mmol of dasatinib in 100 ml of DMF, add 11 mmol of formic acid and 10 mmol of DMAP at room temperature, and then stir at 100° C. for 10 h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by chromatography to obtain 9 mmol of intermediate II. Yield: 90.4%. The NMR data are as follows: 1 HNMR(400MHz,DMSO-d6)δppm 2.22(s,3H),2.38(s,3H),2.42-2.48(m,4H),2.58(t,J=5.7Hz,2H),2.7(m,4h) ,3.07(m,2H),3.21to3.38(m,4H),3.44-3.57(m,4H),4.15(t,J=5.7Hz, 2H),6.04(s,1H),7.19-7.31( m,2H),7.38(d,J=5.9Hz,1H),8.22(s,1H), 9.87(s,1H),11.48(s,1H),12.25(bs,1H).
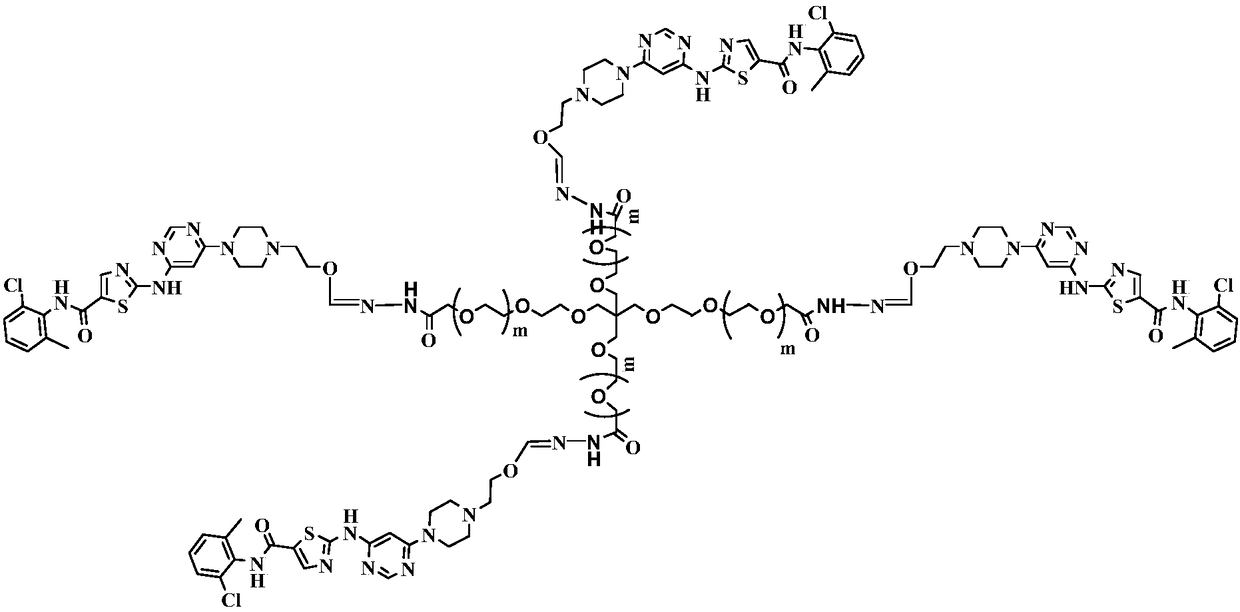
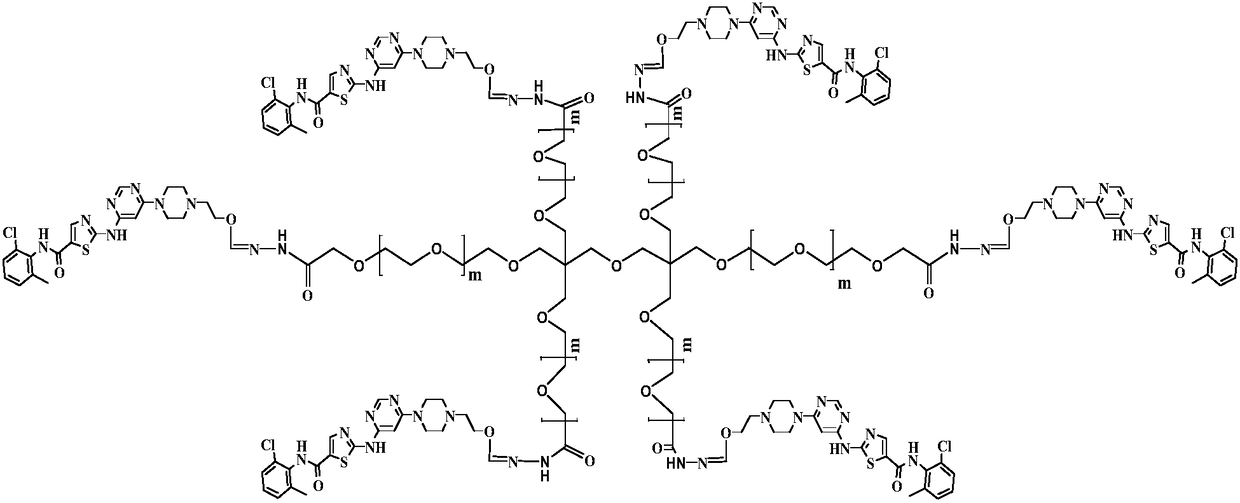
[0031] (2) Preparation of 4arm-PEG24-dasatinib (IV)
[0032] Dissolve 10mmol of intermediate II in 50ml of N,N-dimethylformamide, add 10mmol of EDCI and 10mmol of NHS, and stir at 25°C for 2h. 2 mmol of 4arm-PEG24-NH2 was added to the reaction, and the reaction was carried out at ...
Embodiment 2
[0034] (1) Preparation of Intermediate II
[0035] Dissolve 10 mmol of dasatinib in 100 ml of DMF, add 11 mmol of formic acid and 10 mmol of DMAP at room temperature, and then stir at 100° C. for 8 h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by chromatography to obtain 9 mmol of intermediate II. Yield: 90%. The NMR data are as follows: 1 HNMR(400MHz,DMSO-d6)δppm 2.22(s,3H),2.38(s,3H),2.42-2.48(m,4H),2.58(t,J=5.7Hz,2H),2.7(m,4h) ,3.07(m, 2H),3.21to3.38(m,4H),3.44-3.57(m,4H),4.15(t,J=5.7Hz,2H),6.04(s, 1H),7.19-7.31( m,2H),7.38(d,J=5.9Hz,1H),8.22(s,1H),9.87(s,1H), 11.48(s,1H),12.25(bs,1H).
[0036] (2) Preparation of 4arm-PEG124-dasatinib (IV)
[0037]Dissolve 10mmol of intermediate II in 50ml of N,N-dimethylformamide, add 10mmol of EDCI and 10mmol of NHS, and stir at 25°C for 2h. 2 mmol of 4arm-PEG124-NH2 was added to the reaction, and the reaction was carried out at 3...
Embodiment 3
[0039] (1) Preparation of Intermediate II
[0040] Dissolve 10 mmol of dasatinib in 100 ml of DMF, add 11 mmol of formic acid and 10 mmol of DMAP at room temperature, and then stir at 120° C. for 10 h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by chromatography to obtain 9 mmol of intermediate II. Yield: 90%. The NMR data are as follows: 1 HNMR(400MHz,DMSO-d6)δppm 2.22(s,3H),2.38(s,3H),2.42-2.48(m,4H),2.58(t,J=5.7Hz,2H),2.7(m,4h) ,3.07(m, 2H),3.21to3.38(m,4H),3.44-3.57(m,4H),4.15(t,J=5.7Hz,2H),6.04(s, 1H),7.19-7.31( m,2H),7.38(d,J=5.9Hz,1H),8.22(s,1H),9.87(s,1H), 11.48(s,1H),12.25(bs,1H).
[0041] (2) Preparation of 4arm-PEG240-dasatinib (IV)
[0042] Dissolve 10mmol of intermediate II in 50ml of N,N-dimethylformamide, add 10mmol of EDCI and 10mmol of NHS, and stir at 25°C for 2h. 2 mmol of 4arm-PEG240-NH2 was added to the reaction, and the reaction was carried out at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com