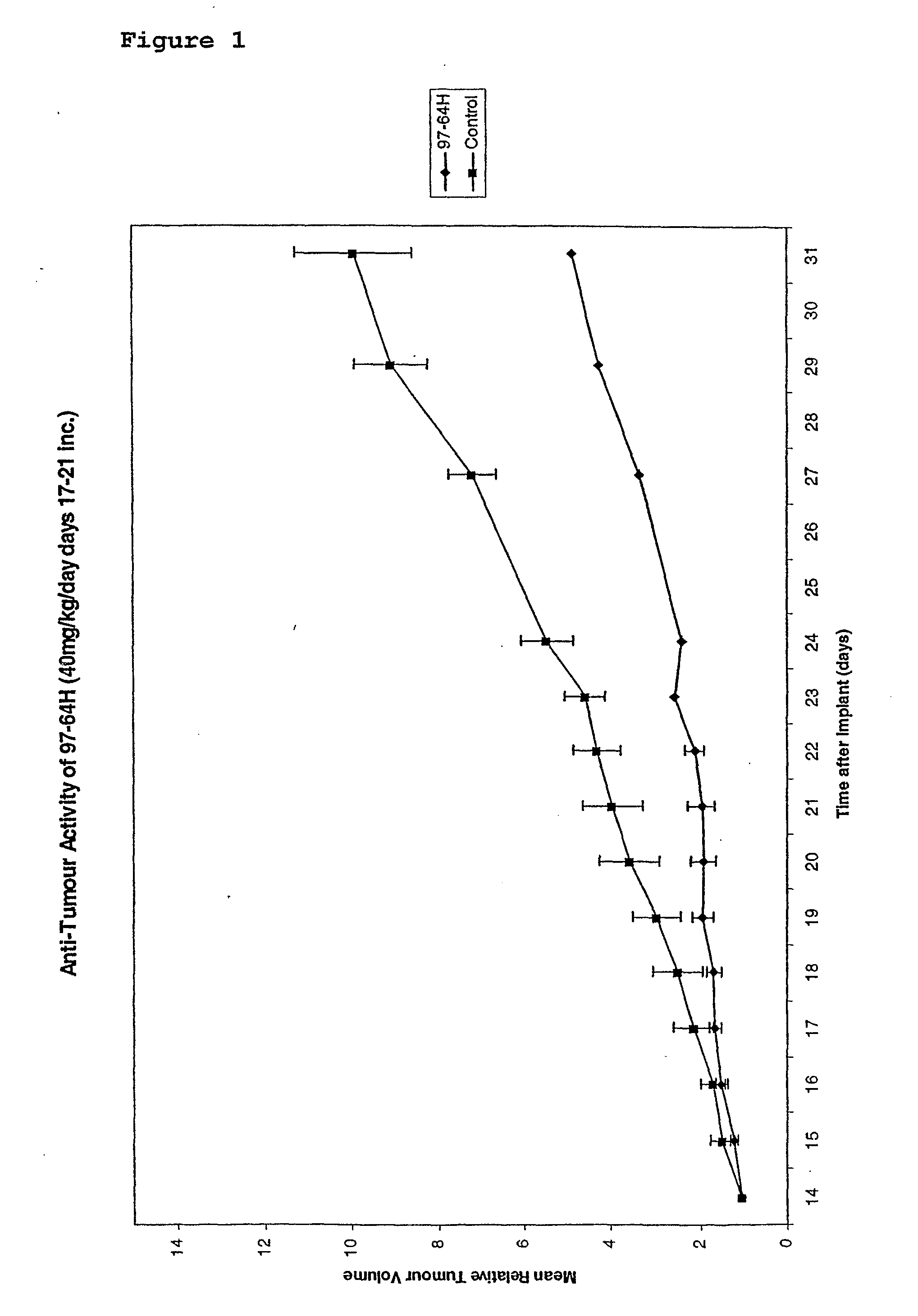
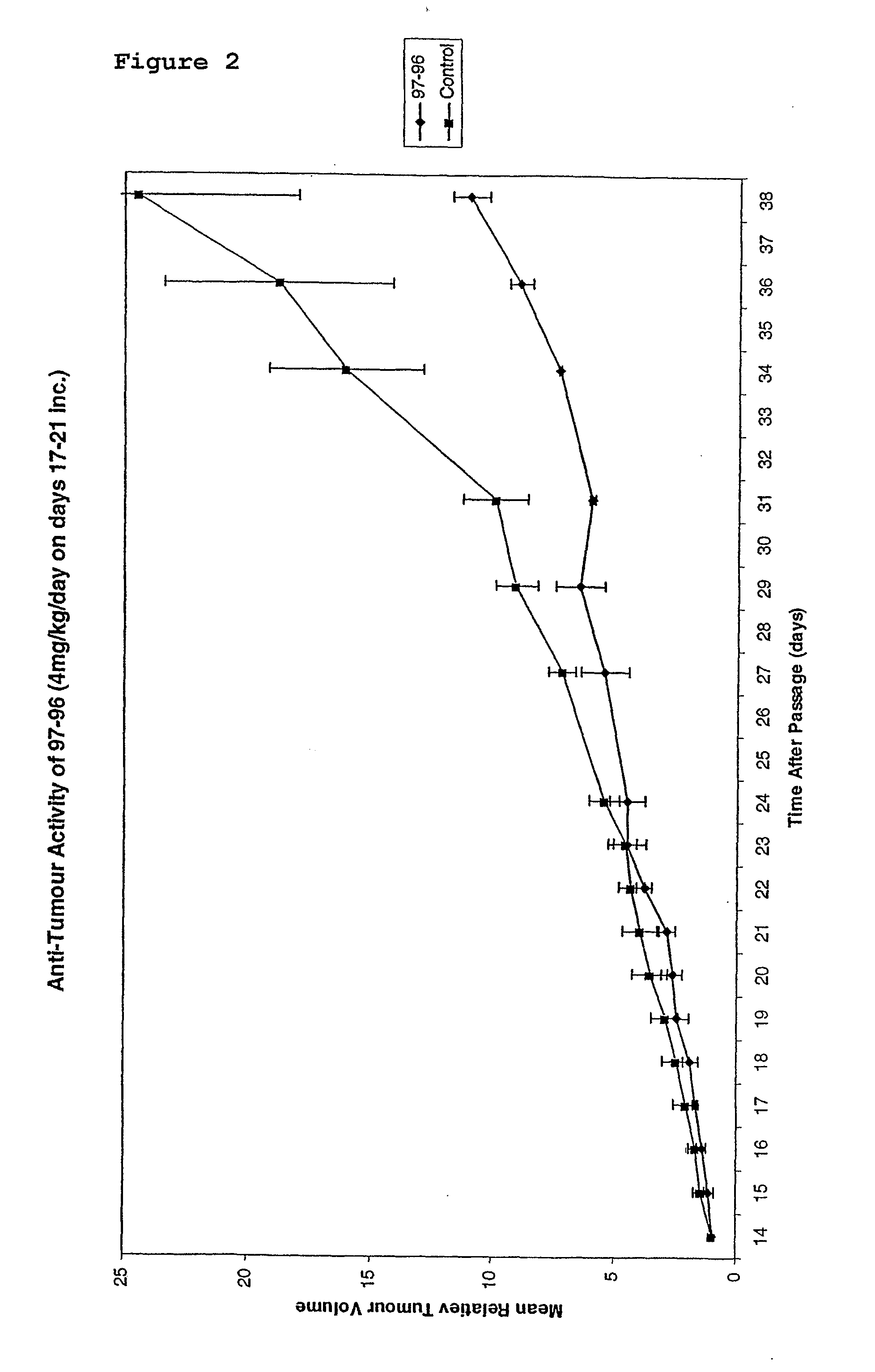
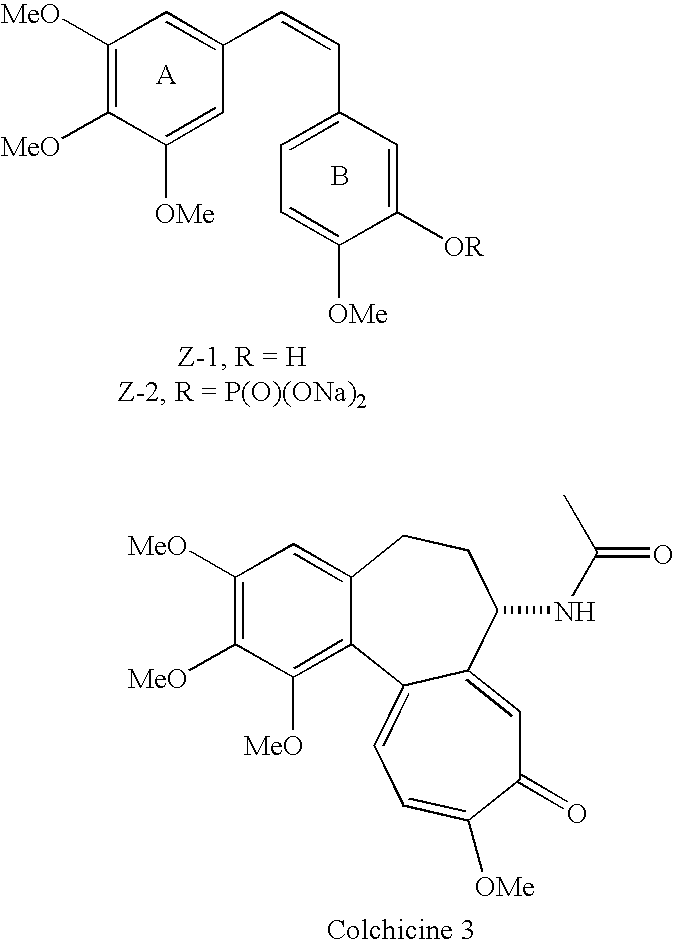
Substituted stilbenes and their reactions
a technology of stilbenes and quinones, applied in the field of substituting stilbenes and quinone compounds, can solve the problems of poor water solubility of z-1, destruction of microvessels within tumours, and severe hampered clinical use of z-1 as a clinically useful anticancer agent, and achieves the effects of improving the selectivity of irradiated e-1, good yield, and increasing the activity. rapid and rapid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Combretastatins with Alkyl Groups on the Double Bond
Z- and E-1-(3′-t-Butyldimethylsilyloxy-4′-methoxyphenyl)-2-(3″,4″,5″-trimetboxyphenyl)propene, 17a, 17b
[0109]
[0110] To a slurry of 3-t-butyldimethylsilyloxy-4-methoxybenzylphosphonium bromide, 18, (1 g, 1.69 mmol) in THF (10 ml) was added n-butyllithium (1.16 ml of 1.6 M solution, 1.86 mmol) at −15° C. The red anion was stirred for 20 minutes and 3,4,5-trimethoxyacetophenone (355 mg, 1.69 mmol) added. The resultant solution was stirred at room temperature for 1 hour and water (10 ml) carefully added. The aqueous layer was separated and extracted with ether (3×10 ml). The combined organic layers were washed with water (2×10 ml) and brine (10 ml), dried (MgSO4) and concentrated in vacuo.
[0111] Following flash column chromatography (SiO2 petrol:EtOAc 19:1) the Z stilbene, 17a, was isolated as a colourless oil (109 mg, 15%). Rf=0.72 (SiO2 petrol:EtOAc 1:1); δH (300 M) 0.20 [6H, s, (CH3)2], 1.03 [9H, s, (CH3)3], 2.28 (3H...
example 2
Synthesis of Combretastatins with Alkyl Groups Replacing the Methoxy Groups on the A Ring
E-2-(3′,4′,5′-trimethylphenyl)-3-(3″-(2′″,3′″,5′″,6′″-tetrafluoropyridoxy)-4″-methoxyphenyl)prop-2-enoic acid, 24
[0122]
[0123] A mixture of 3-(2′,3′,5′,6′-tetrafluoropyridoxy)-4-benzaldehyde 25 (2 g, 6.64 mmol), 3,4,5-trimethylphenylacetic acid 26 (2.37 g, 13.3 mmol) acetic anhydride (6 ml) and triethylamine (3 ml) were heated under reflux for 3 h. After acidification with concentrated hydrochloric acid (9 ml), the solid was filtered off and recrystallised from ethanol to give E-2-(3′,4′,5-trimethyl)-3-(3″-(2′″,3′″,5′″,6′″-tetrafluoropyridoxy)-4″-methoxyphenyl)prop-2-enoic acid 24 as a yellow crystalline solid (700 mg, 1.52 mmol, 23%). m.p. 184-6° C. δH (300 MHz, DMSO) 2.11, (3H, s, CH3), 2.14 (6H, s, (CH3)2), 3.83 (3H, s, OCH3), 6.61 (1H, d, J=1.5, H-2″), 6.71 (2H, s, H-2′,6′), 7.13 (1H, d, J=8.7, H-5″), 7.19 (1H, dd, J=8.7, 1.5, H-6″), 7.61, (1H, s, olefinic H), 12.52, (1H, s OH).
(Z)-1-(3′,4...
example 3
Synthesis of Combretastatins with a 3,4,5 Trialkoxy Group
Z- and E-1-(3′,4′,5′-triethoxyphenyl)-2-(3″-t-butyldimethylsilyloxy-4″-methoxyphenyl)ethene, 30a, 30b
[0128] To a slurry of 3,4,5-triethoxybenzylphosphonium bromide 29 (2 g, 3.24 mmol) in THF (30 ml) was added n-butyllithium (2.5 ml of 1.6M solution in hexanes, 4 mmol) at −15° C. under argon. The red anion was stirred for 20 min and 3-O-t butyldimethylsilyl-4-methoxybenzaldehyde 6 (0.86 g, 3.24 mmol) added. The resultant solution was stirred for 1 h at room temperature and water (10 ml) was carefully added. The aqueous layer was separated and extracted with ethyl acetate (3×100 ml). The combined organic layers were washed with water (2×100 ml), brine (100 ml), dried (MgSO4) and concentrated in vacuo. Flash column chromatography afforded the cis stilbene 30a as a colourless oil (0.23 g, 15%). Rf=0.65 (petrol:ethyl acetate 9:1); δH (300 MHz) 0.08 (6H, s, Si(CH3)2), 0.95 (9H, s, 3×CH3), 1.35 (9H m, 3×OCH2CH3), 3.79 (3H, s, OCH3)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com