Sitafloxacin fumarate injection and preparation method thereof
A technology of sitafloxacin and fumaric acid, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of content decline, dark yellow color of liquid medicine, and pH value. Stability and other issues, to achieve the effect of maintaining stability, the best effect, and improving drug solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
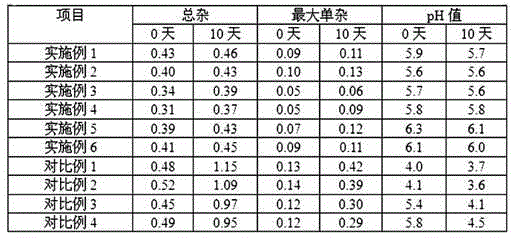
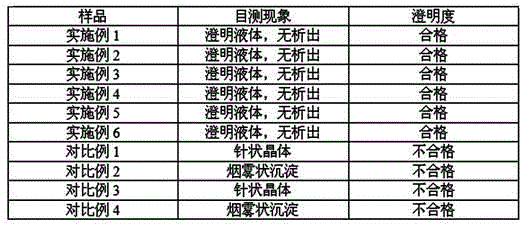
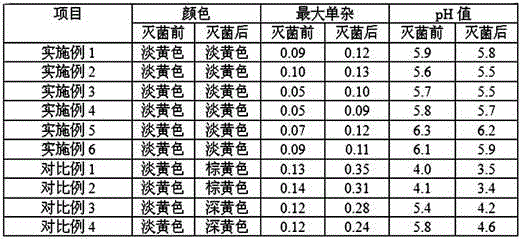
Examples
Embodiment 1
[0050] Example 1 Each 50mg / 20mL, 1000
[0051] Sitafloxacin fumarate 0.25% (calculated as sitafloxacin)
[0052] Aspartic Acid 1%
[0053] Appropriate amount of histidine, adjust pH5.5~6.5
[0054] Methionine 0.5%
[0055] Sorbitol 0.1%
[0056] The balance of water for injection.
[0057] Preparation:
[0058] (1) Weigh aspartic acid and methionine according to the ratio and dissolve them in water for injection to prepare a concentrated solution with a total weight of 20-30% (w / w), keep warm at 45°C-55°C, stir and dissolve, then add For sitafloxacin fumarate, stir to completely dissolve, add 70% of the prescription amount with water for injection to dilute, then add sorbitol, stir to dissolve, and cool to room temperature;
[0059] (2) Dissolve histidine in an appropriate amount of water for injection, slowly add it dropwise to the above solution, adjust the pH to 5.5-6.5, and replenish the water for injection to the full amount;
[0060] (3) Add 0.05% (g / ml) activated...
Embodiment 2
[0062] Example 2 Each 100mg / 20mL, 1000
[0063] Sitafloxacin fumarate 0.5% (calculated as sitafloxacin)
[0064] Aspartic Acid 1.5%
[0065] Appropriate amount of histidine, adjust pH5.5~6.5
[0066] Methionine 0.8%
[0067] Sorbitol 0.1%
[0068] The balance of water for injection.
[0069] Preparation:
[0070] (1) Weigh aspartic acid and methionine according to the ratio and dissolve them in water for injection to prepare a concentrated solution with a total weight of 20-30% (w / w), keep warm at 45°C-55°C, stir and dissolve, then add For sitafloxacin fumarate, stir to completely dissolve, add 70% of the prescription amount with water for injection to dilute, then add sorbitol, stir to dissolve, and cool to room temperature;
[0071] (2) Dissolve histidine in an appropriate amount of water for injection, slowly add it dropwise to the above solution, adjust the pH to 5.5-6.5, and replenish the water for injection to the full amount;
[0072] (3) Add 0.05% (g / ml) activat...
Embodiment 3
[0074] Example 3 Each 50mg / 20mL, 1000
[0075] Sitafloxacin fumarate 0.25% (calculated as sitafloxacin)
[0076] Citric acid 0.6%
[0077] Appropriate amount of sodium citrate, adjust pH5.5~6.5
[0078] Methionine 0.3%
[0079] Sorbitol 0.1%
[0080] The balance of water for injection.
[0081] Preparation:
[0082] (1) Weigh citric acid and methionine according to the ratio and dissolve them in water for injection to prepare a concentrated solution with a total weight of 20-30% (w / w), keep warm at 45°C-55°C, stir and dissolve, then add fuma Acid sitafloxacin, stir to make it completely dissolved, add 70% of the prescription amount with water for injection to dilute, then add sorbitol, stir to dissolve, and cool to room temperature;
[0083] (2) Dissolve sodium citrate in an appropriate amount of water for injection, slowly add it dropwise to the above solution, adjust the pH to 5.5-6.5, and add water for injection to the full amount;
[0084] (3) Add 0.05% (g / ml) activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com