Solid dispersion as well as preparation method and application thereof
A solid dispersion, mass percentage technology, applied in the field of medicine, can solve the problems of thermal decomposition of drugs and carriers, organic solvent residues, restrictions on wide application, etc., and achieve the effect of improving drug solubility, increasing solubility, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
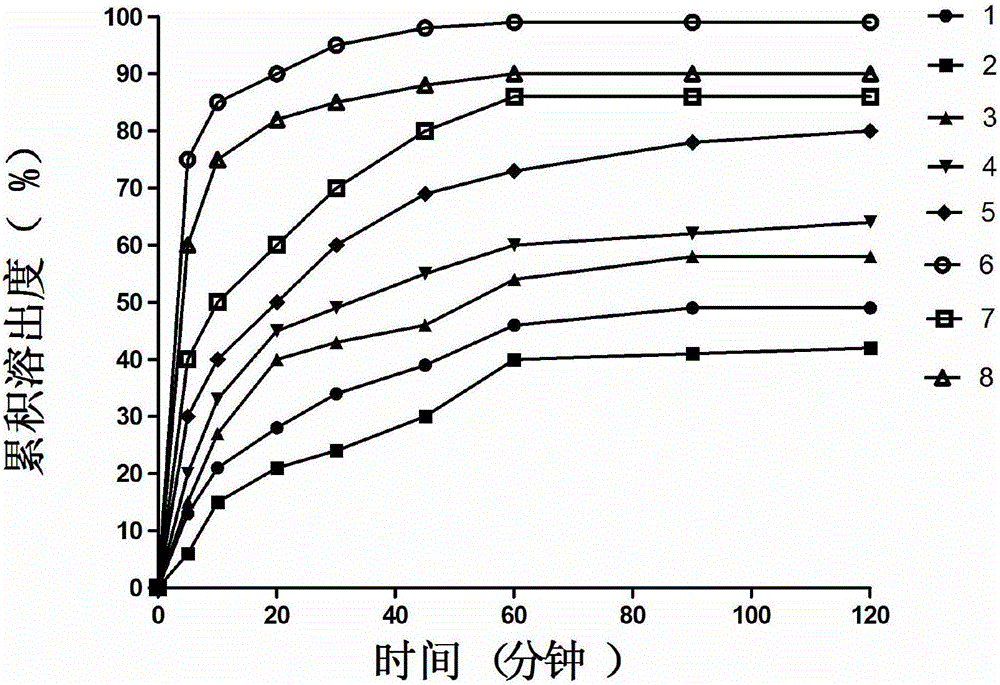
Image
Examples
Embodiment 1
[0060] Embodiment 1: Spray-drying method prepares diflunisal solid dispersion
[0061] A kind of diflunisal solid dispersion of the present embodiment is prepared by the spray drying method from the raw materials shown in the following table:
[0062]
[0063] Its preparation method specifically comprises the following steps:
[0064] (1) Take diflunisal, surfactant and water-soluble polymer material, mix evenly, add 100ml of secondary water and heat to 70°C in a water bath, stir magnetically until the solution is in a clear state, and obtain a mixed solution containing medicine .
[0065] (2) The obtained mixed solution containing medicine is spray-dried, the inlet air temperature of the spray drying is 120°C, the outlet air temperature is 75°C, and the air volume is 0.7m 3 / min, the atomization pressure is 9kPa, and the liquid flow rate is 2mL / min. During the spray drying process, the temperature of the drug-containing mixed solution is kept at 70°C.
[0066] (3) The s...
Embodiment 2
[0079] Embodiment 2: Spray-drying method prepares piroxicam solid dispersion
[0080] The preparation of a piroxicam solid dispersion in this embodiment is prepared by the spray drying method from the following raw materials: piroxicam as an active ingredient, Soluplus as a surfactant and povidone K25 as a water-soluble polymer material. into, wherein the mass content of piroxicam in the solid dispersion is 10%, and the mass ratio of Soluplus to povidone K25 is 4:6.
[0081] Its preparation method specifically comprises the following steps:
[0082] (1) Take 300mg of piroxicam, 1.08g of Soluplus and 1.62g of povidone K25, mix well, add 100ml of secondary water and heat to 75°C in a water bath, and magnetically stir until the solution is in a clear state to obtain a drug-containing mixed solution .
[0083] (2) The obtained mixed solution containing medicine is spray-dried, the inlet air temperature of the spray drying is 110°C, the outlet air temperature is 70°C, and the air...
Embodiment 3
[0094] Embodiment 3: spray drying method prepares carbamazepine solid dispersion
[0095] A kind of carbamazepine solid dispersion of the present embodiment is prepared by spray-drying method by following raw material: as the carbamazepine of active ingredient, as the Soluplus of surfactant and as the povidone K25 preparation of water-soluble macromolecular material; into, wherein the mass content of carbamazepine in the solid dispersion is 20%, and the mass ratio of Soluplus to povidone K25 is 2:8.
[0096] Its preparation method specifically comprises the following steps:
[0097] (1) Take 600mg of carbamazepine, 480mg of Soluplus and 1.92g of povidone K25, mix well, add 100ml of secondary water and heat to 80°C in a water bath, and magnetically stir until the solution is in a clear state to obtain a mixed solution containing medicine .
[0098] (2) The obtained mixed solution containing medicine is spray-dried, the inlet air temperature of spray drying is 130°C, the outle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com