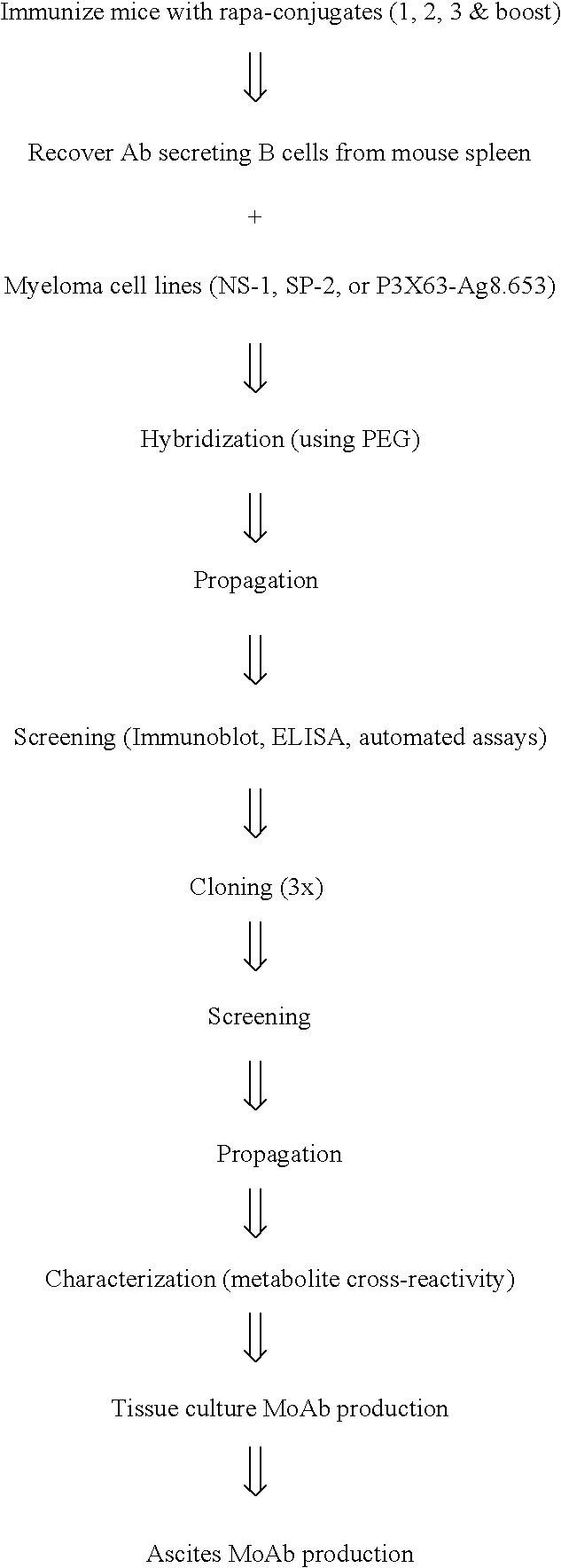
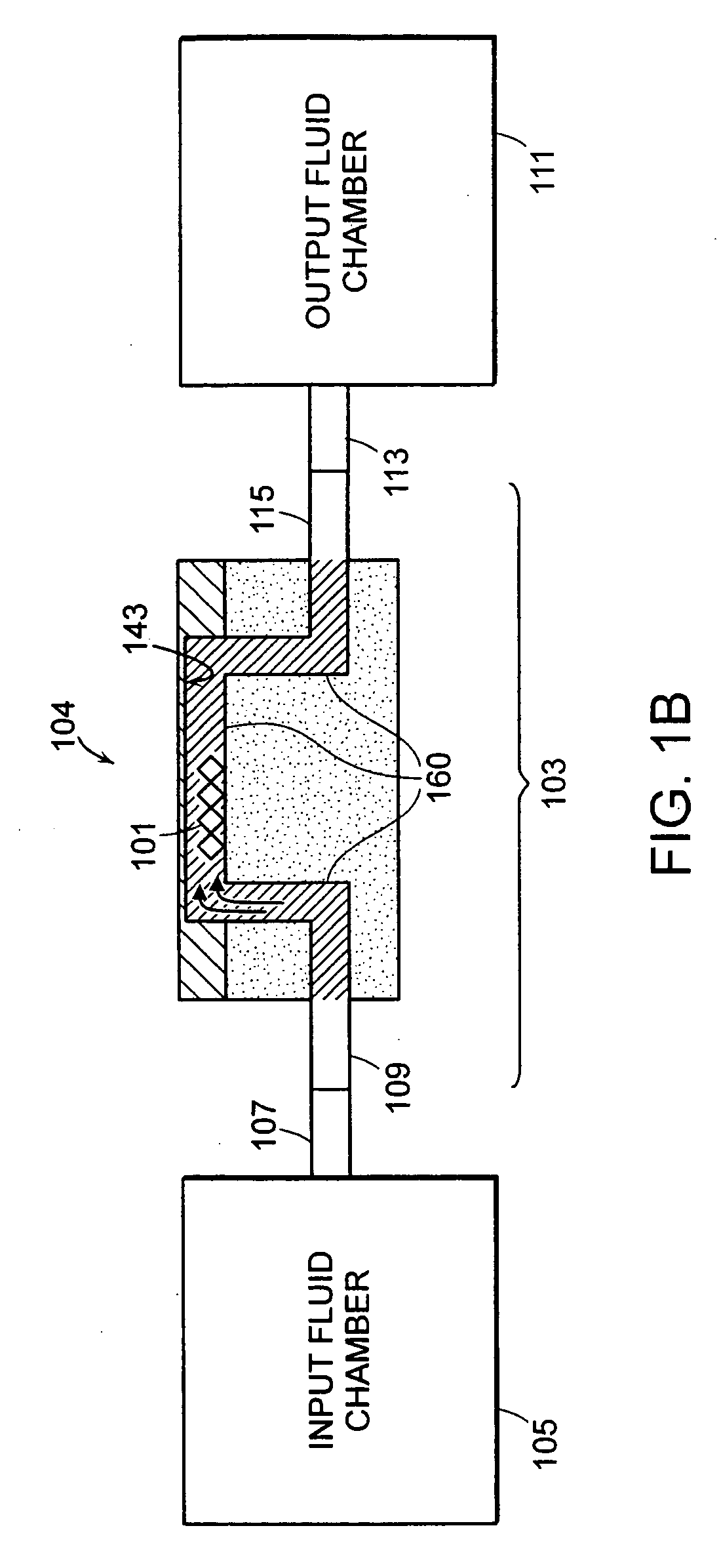
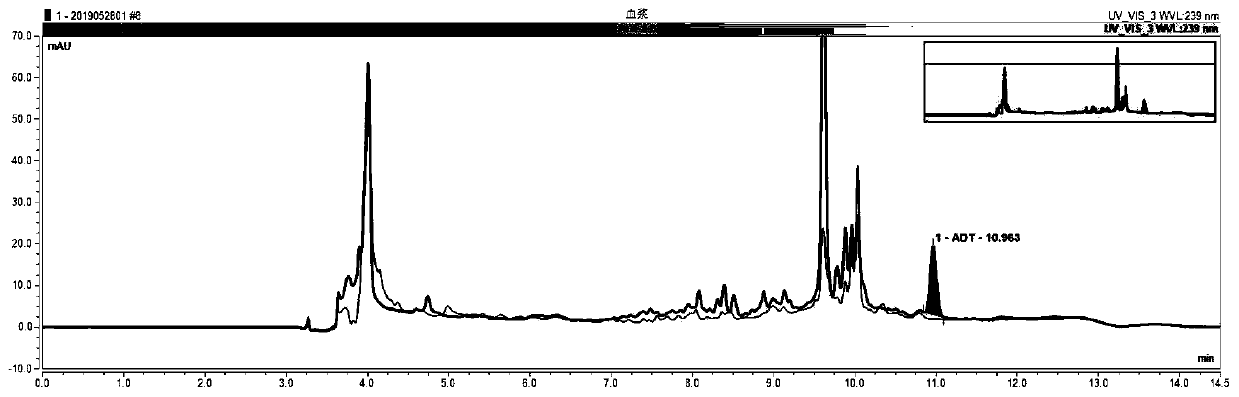
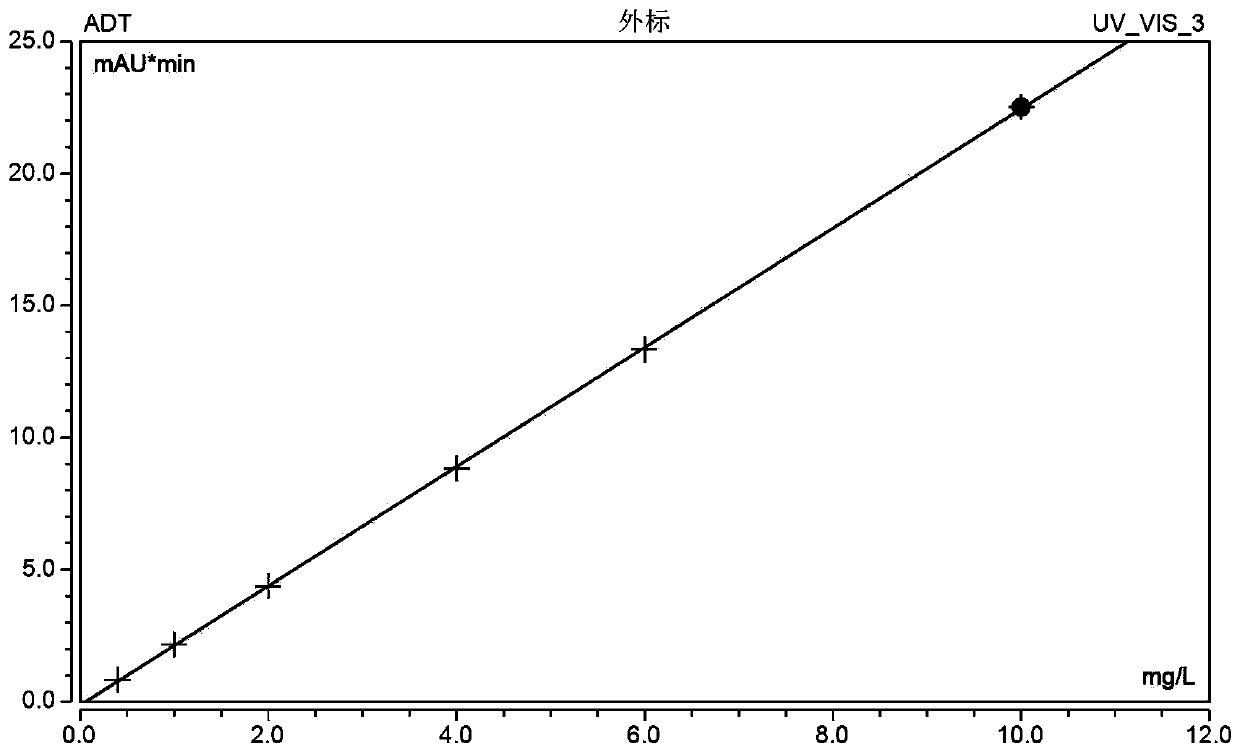
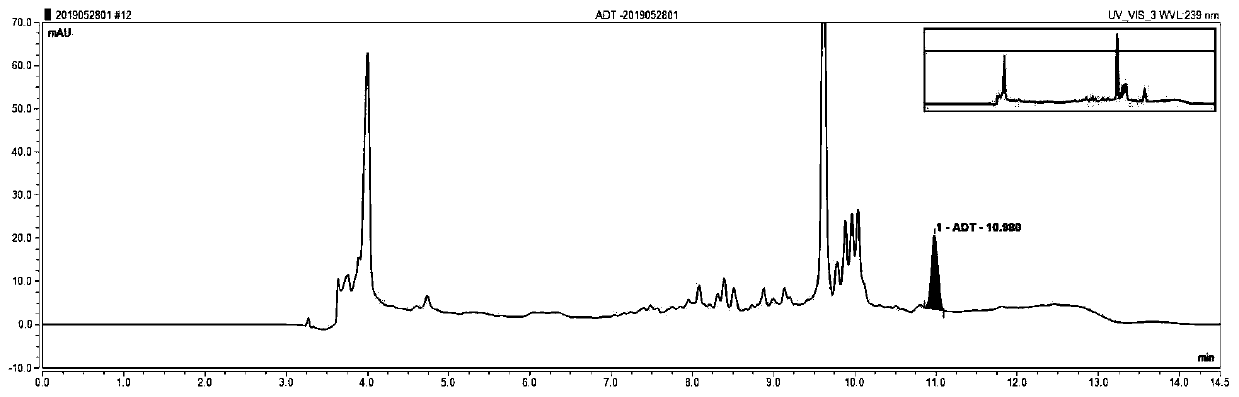
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Therapeutic drug monitoring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic drug monitoring (TDM) is a branch of clinical chemistry and clinical pharmacology that specializes in the measurement of medication concentrations in blood. Its main focus is on drugs with a narrow therapeutic window. TDM aims at improving patient care by adjusting the dose of drugs for which clinical experience or clinical trials have shown it improved outcome in the general or special populations. It can be based on a priori pharmacogenetic, demographic and clinical information, and/or on the a posteriori measurement of blood concentrations of drugs (pk/pd monitoring).
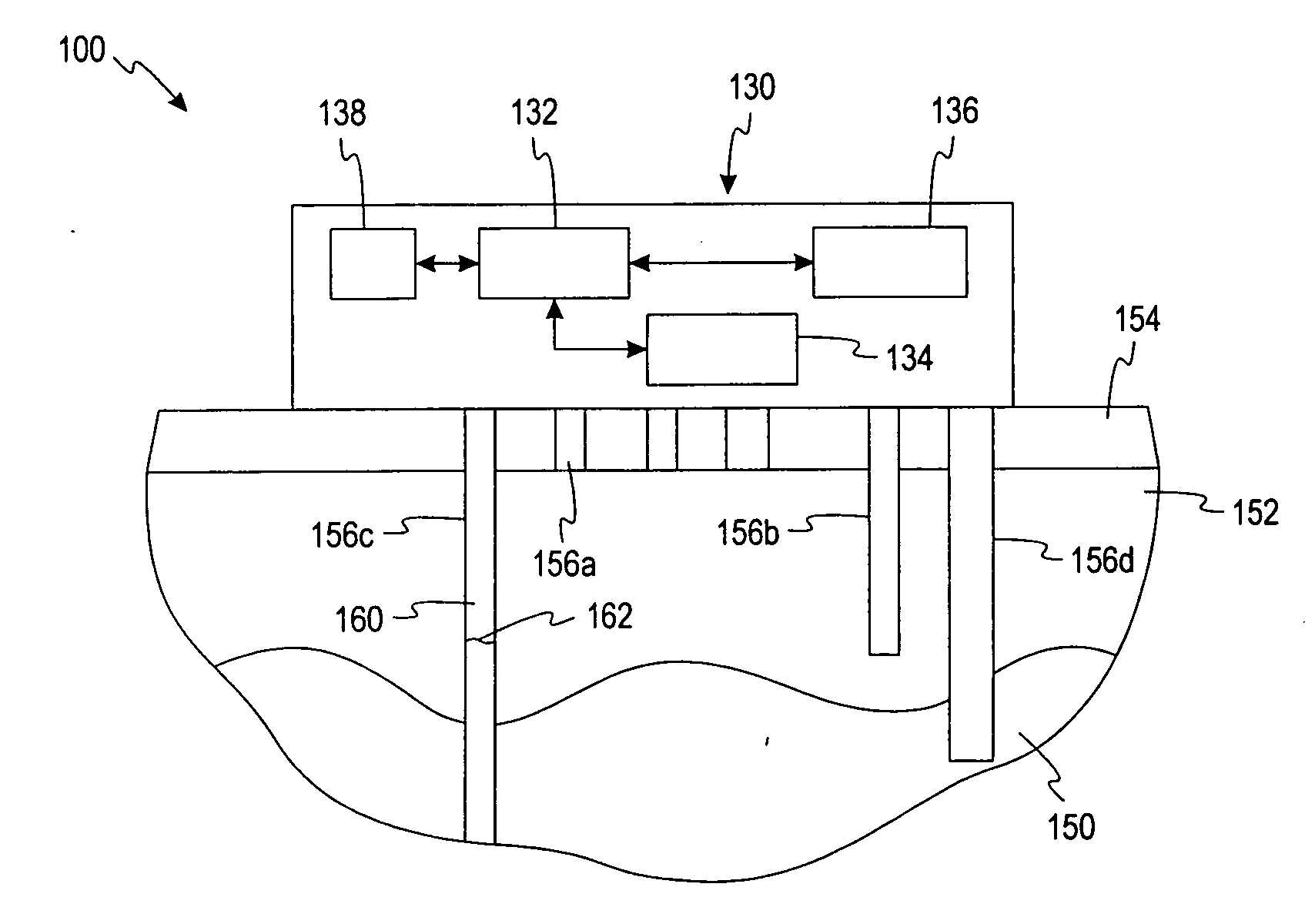
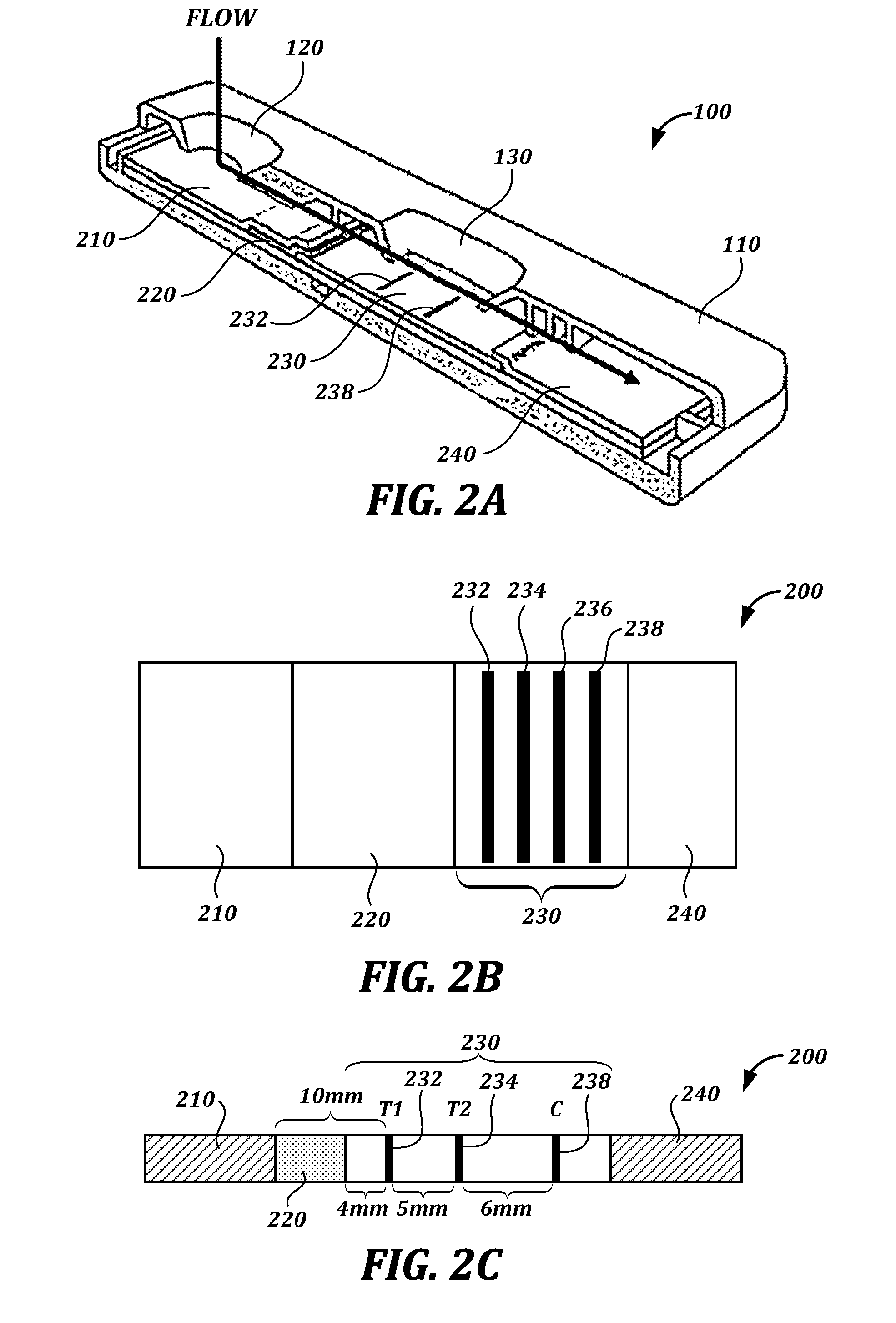
System and method for therapeutic drug monitoring
InactiveUS20050054942A1Accurate assessmentCost-effective and frequentNervous disorderElectrotherapyNoseEnvironmental health
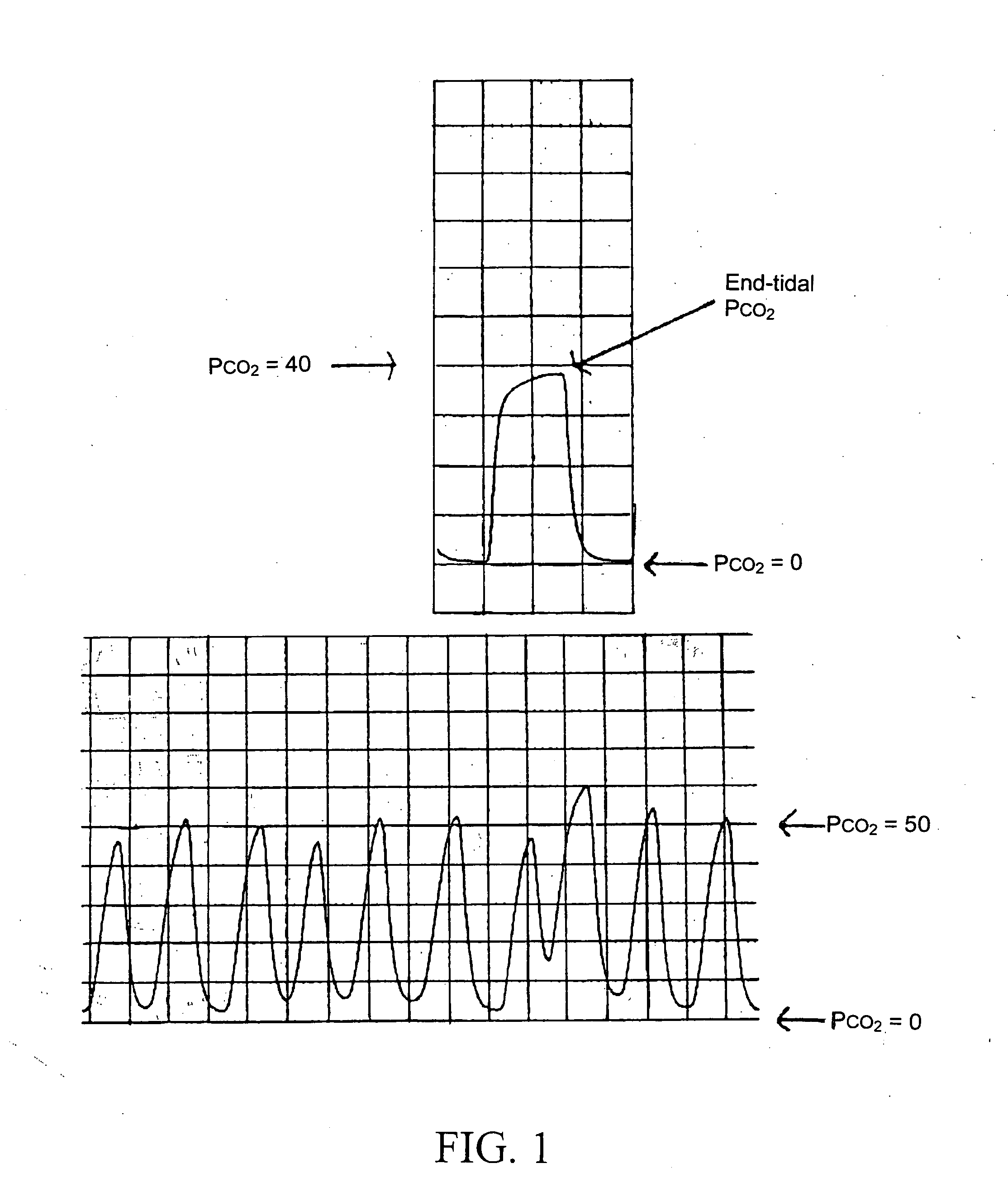
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA
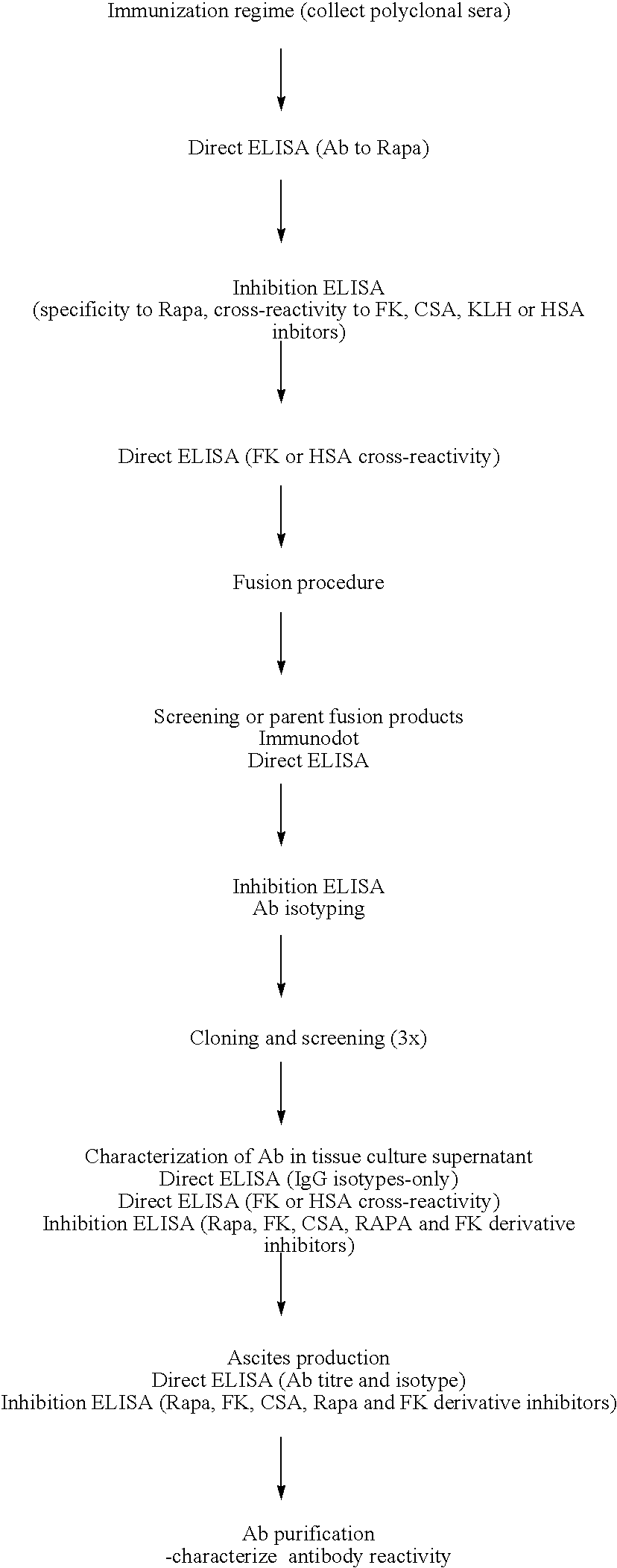
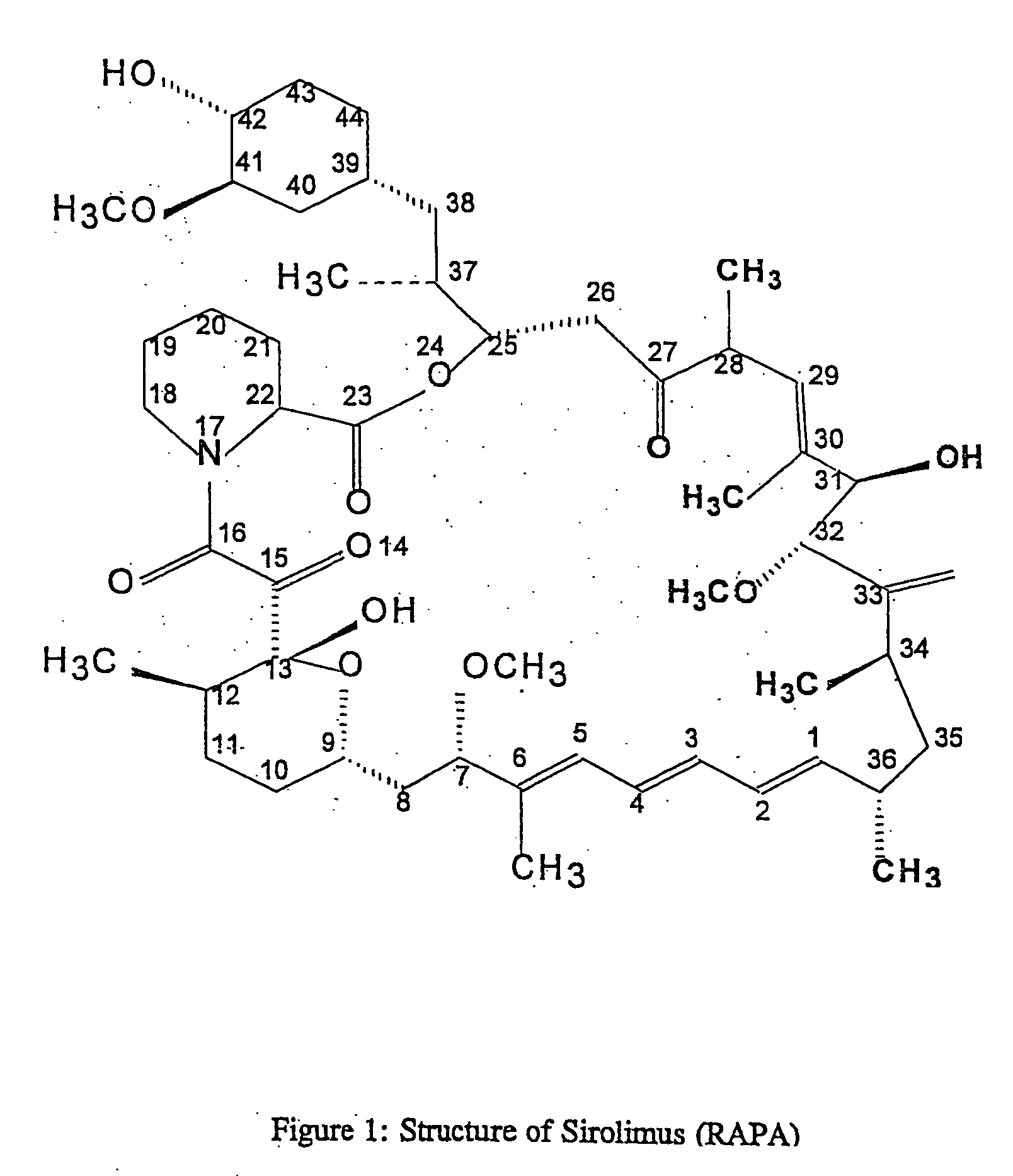
Method for production of antibodies to specific sites of rapamycin
InactiveUS6709873B1Immunoglobulins against fungi/algae/lichensBiological testingPolyclonal antibodiesDivinyl sulfone
Owner:ISODIAGNOSTIKA
Method and apparatus for therapeutic drug monitoring using an acoustic device
InactiveUS7611908B2Easy to measureMaintain curative effectMaterial nanotechnologyBioreactor/fermenter combinationsMedicineResonant sensor
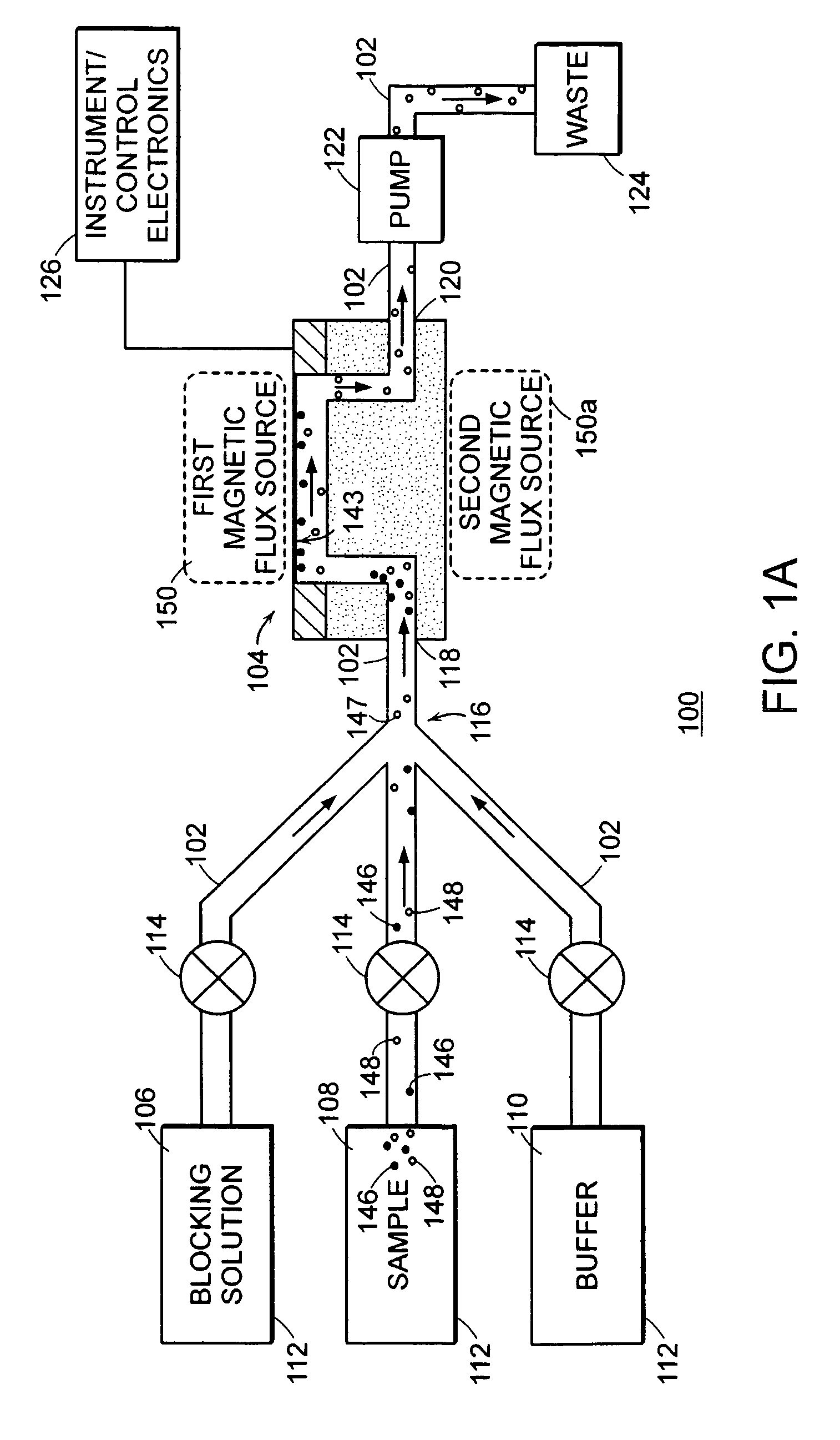
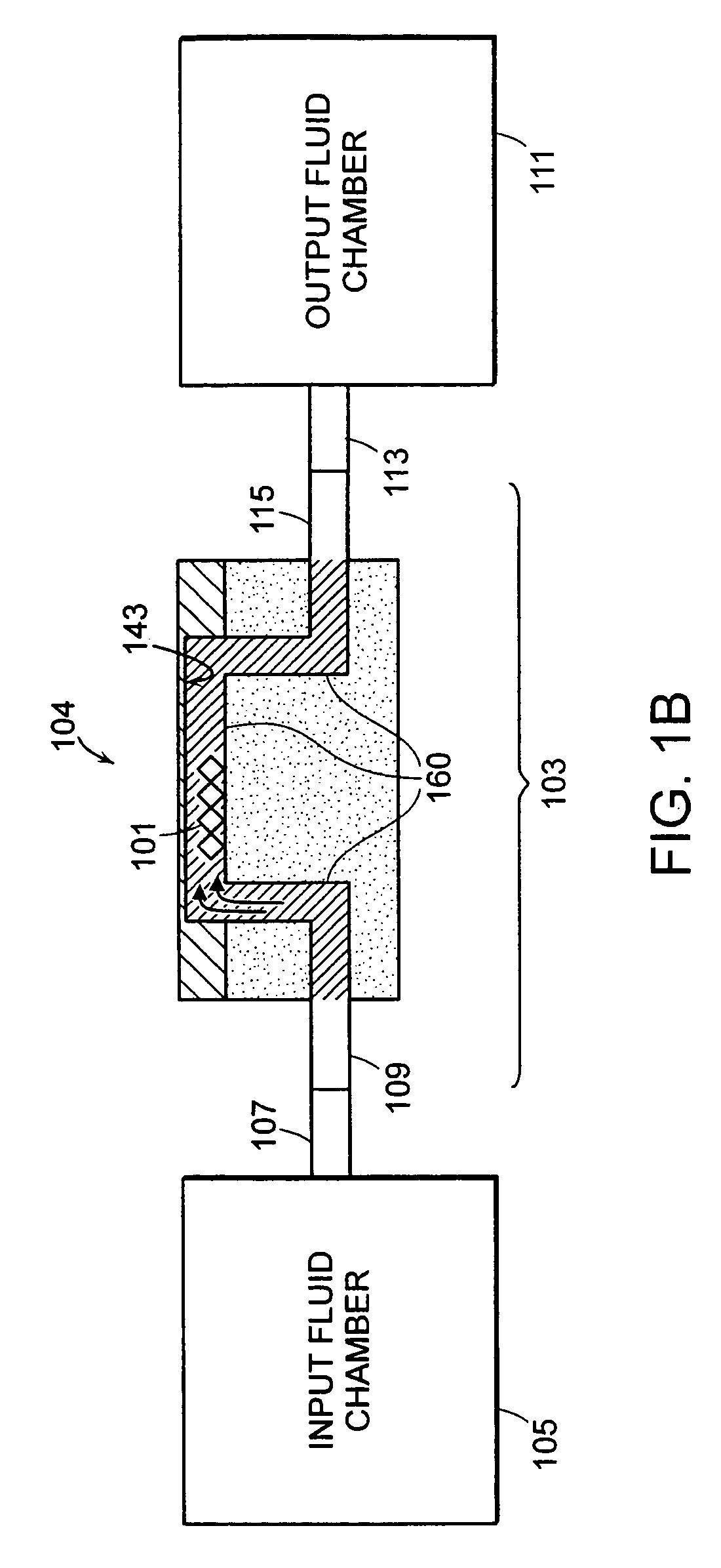
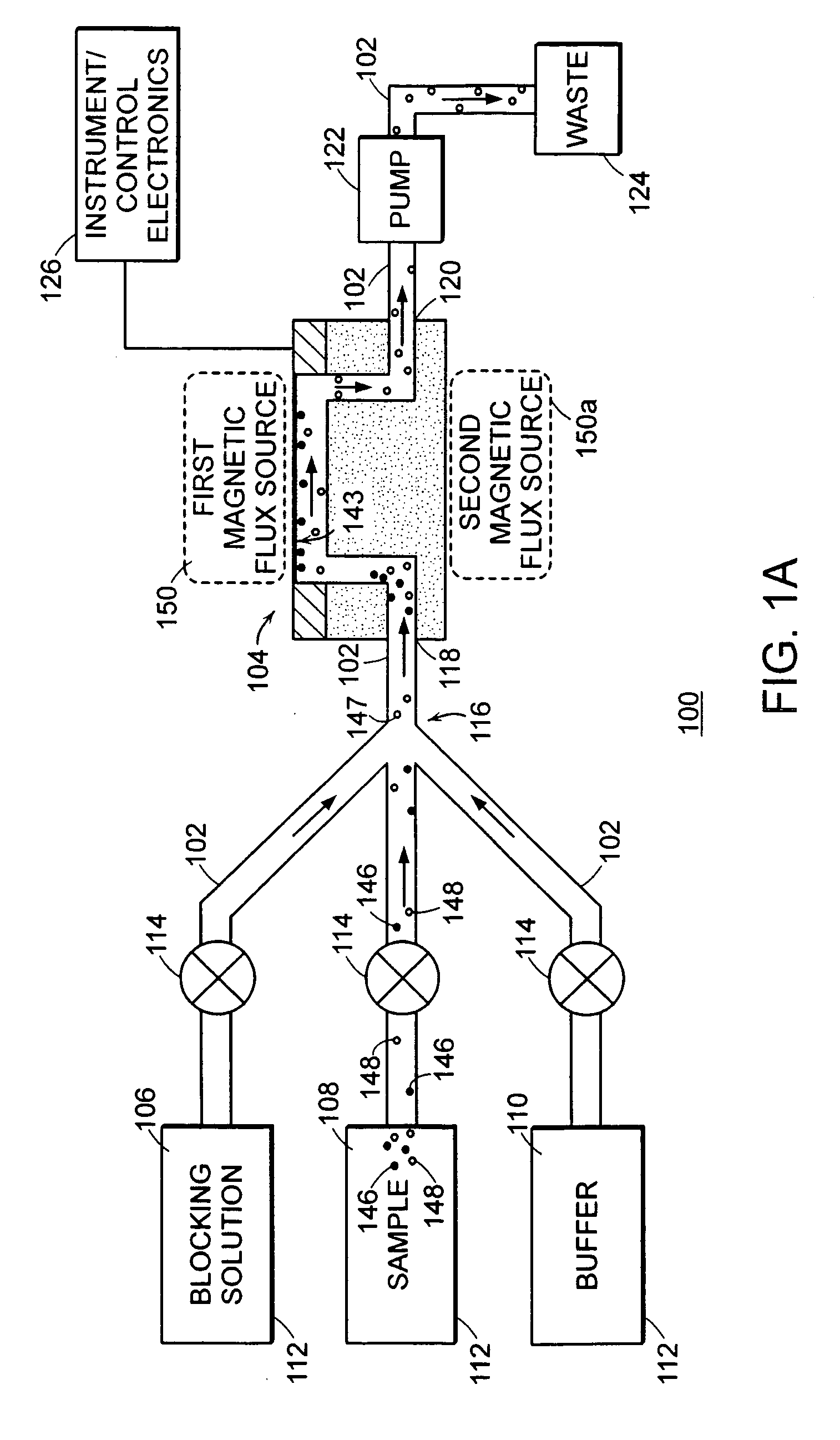
Methods for therapeutic drug monitoring are provided. A plurality of particles, each of which is coated with a capture agent capable of binding a therapeutic drug of choice is combined with the sample to form a plurality of therapeutic drug-particle complexes. The system also includes a transport arrangement for transporting the sample and / or particles to the sensor surface, and optionally a magnetic field inducing structure constructed and arranged to establish a magnetic field at and adjacent to the sensor surface. The resonant sensor produces a signal corresponding to an amount of therapeutic drug-particle complexes that are bound to the sensor surface.
Owner:BIOSCALE
Method of therapeutic drug monitoring
InactiveUS20080152592A1Facilitated DiffusionMonitor effectivenessVaccination/ovulation diagnosticsSensorsMetaboliteContinuous monitoring
A method of using a diffusion-based, continuous-monitoring system to monitor the effectiveness of delivering a drug includes creating and maintaining a diffusion channel in an area of skin. The levels of the drug, metabolite, or affected substance of the drug are continuously monitored in the area of the skin for a desired duration via a diffusion-based, continuous-monitoring device. The levels of the drug, the metabolite, or affected substance is analyzed to determine the effectiveness of delivering the therapeutic drug.
Owner:ASCENSIA DIABETES CARE HLDG AG
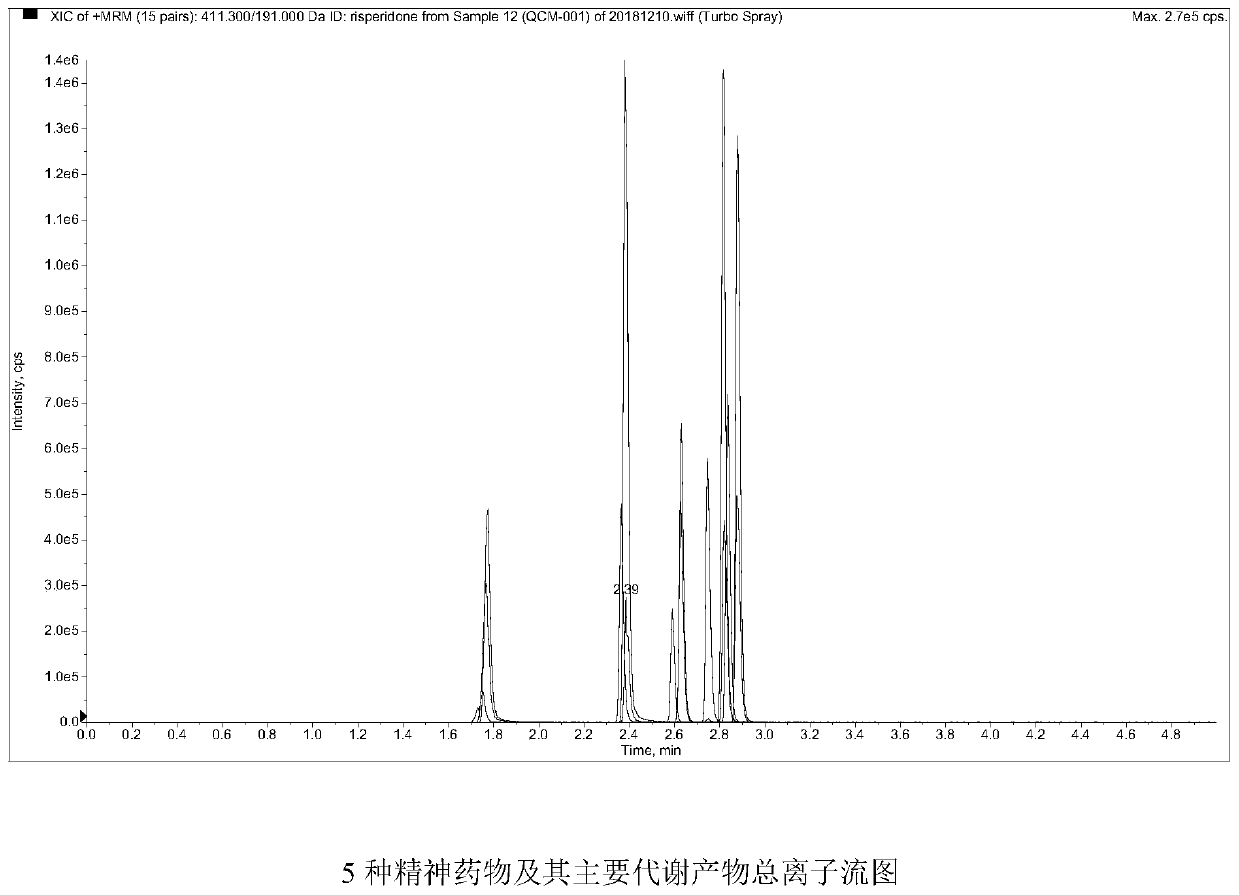
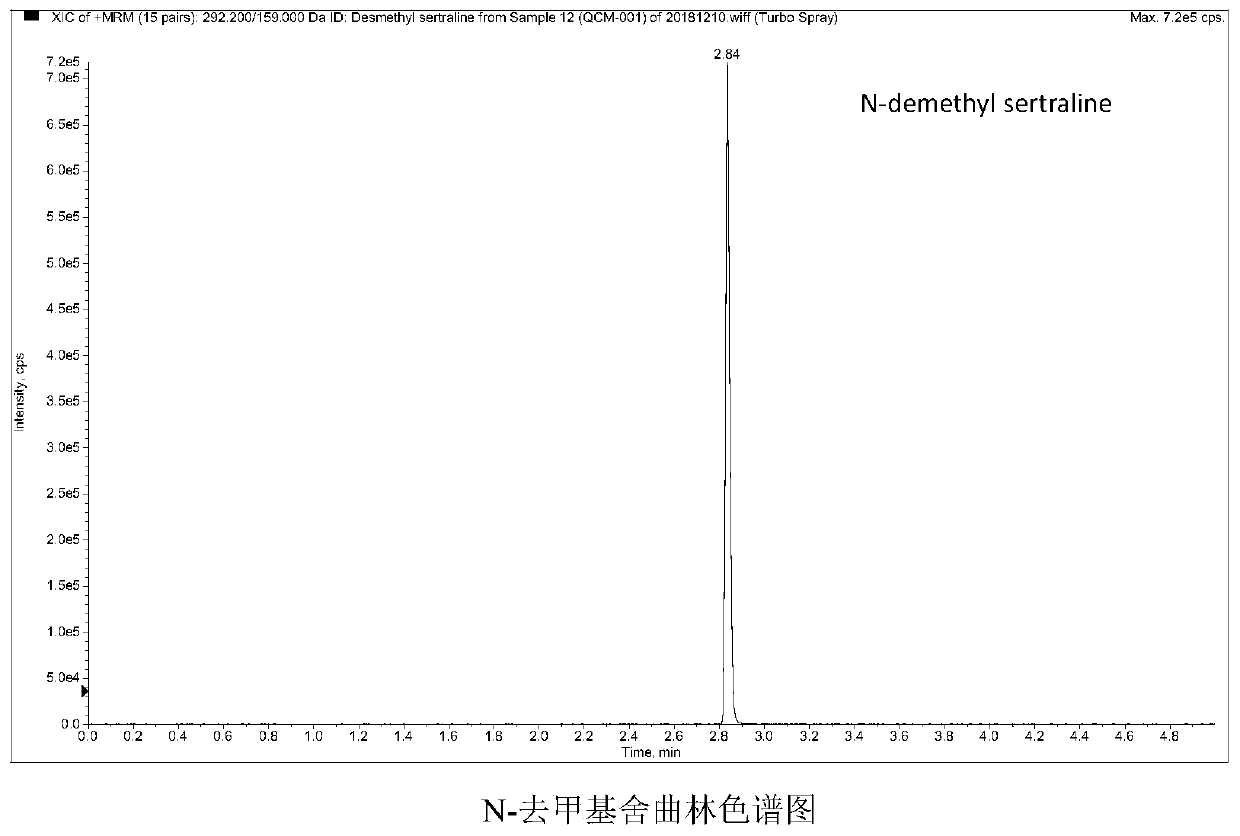
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
Anti-retroviral analysis by mass spectrometry
InactiveUS20050032042A1Fast and simple and accurateFast and simple and accurate analysisMicrobiological testing/measurementIsotope separationAnalyteMedicine
Methods for the simultaneous or sequential analysis and quantification of a plurality of antiretroviral analytes in a complex biological matrix by mass spectrometry are disclosed. The methods require minimal sample size, minimal preparation time and allow for rapid through-put. The system is particularly useful in therapeutic drug monitoring.
Owner:CHILDRENS NAT MEDICAL CENT
Blood aripiprazole drug concentration monitoring kit and detection method thereof
InactiveCN110361486AReduce operating errorsImprove accuracyComponent separationMedicineMedication monitoring
The invention relates to the field of medical detection and in particular to a blood aripiprazole therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquid chromatography analytical technology, more specifically to a method for accurately and quantitatively detecting aripiprazole drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. Thetherapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has variousmethodological indicators that satisfy the requirement for monitoring the blood aripiprazole therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
Method for producing rapamycin-specific antibodies
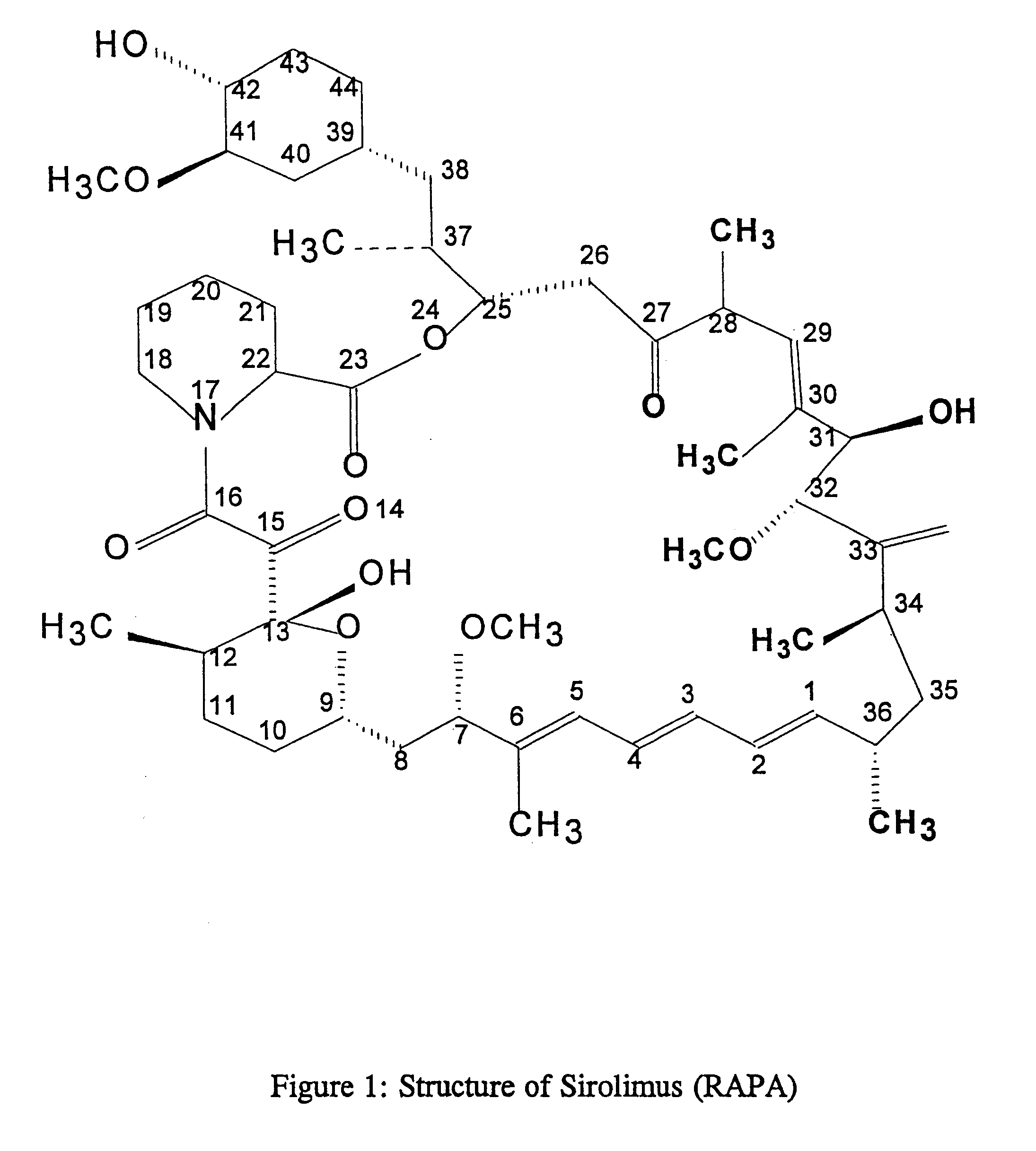
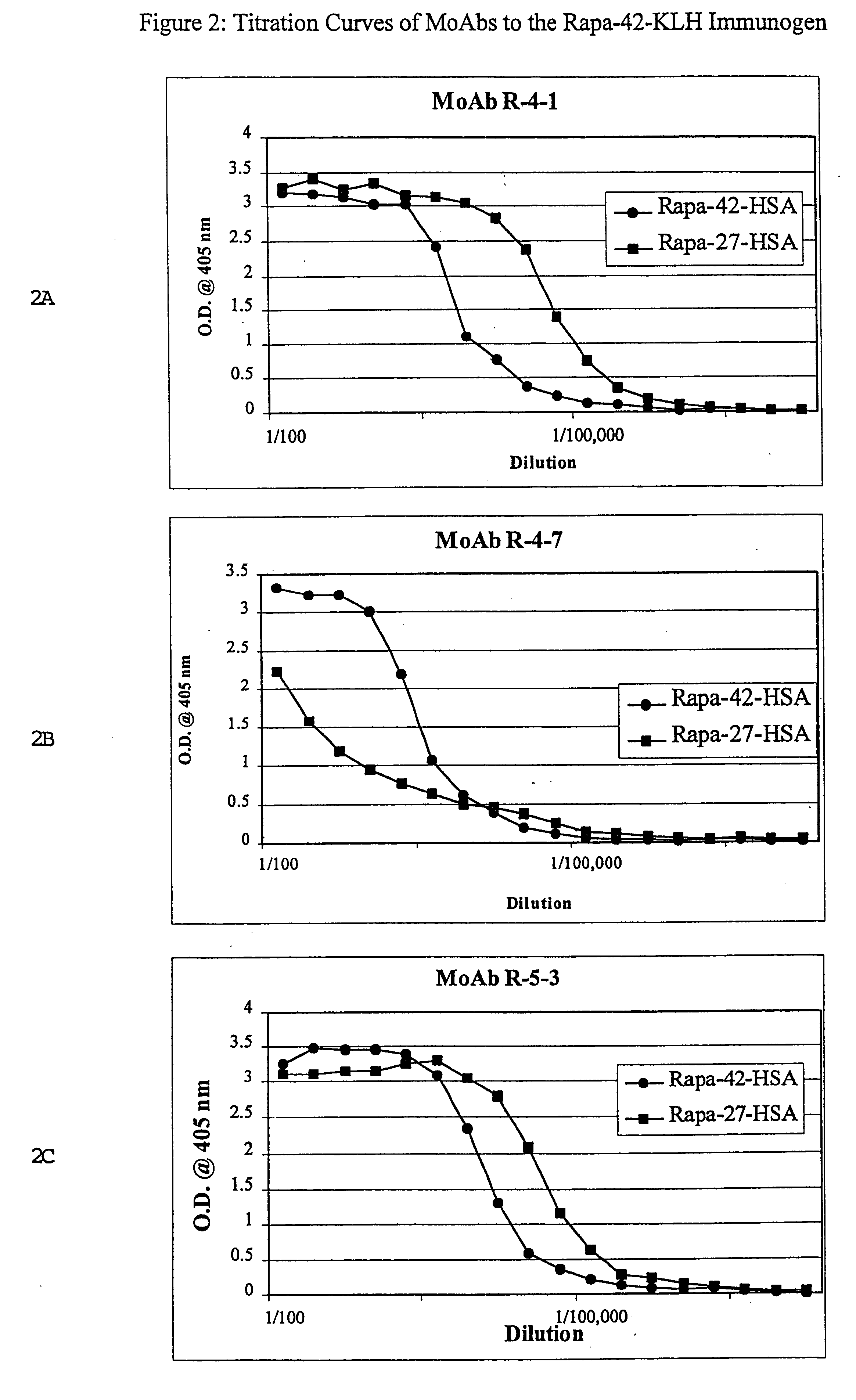
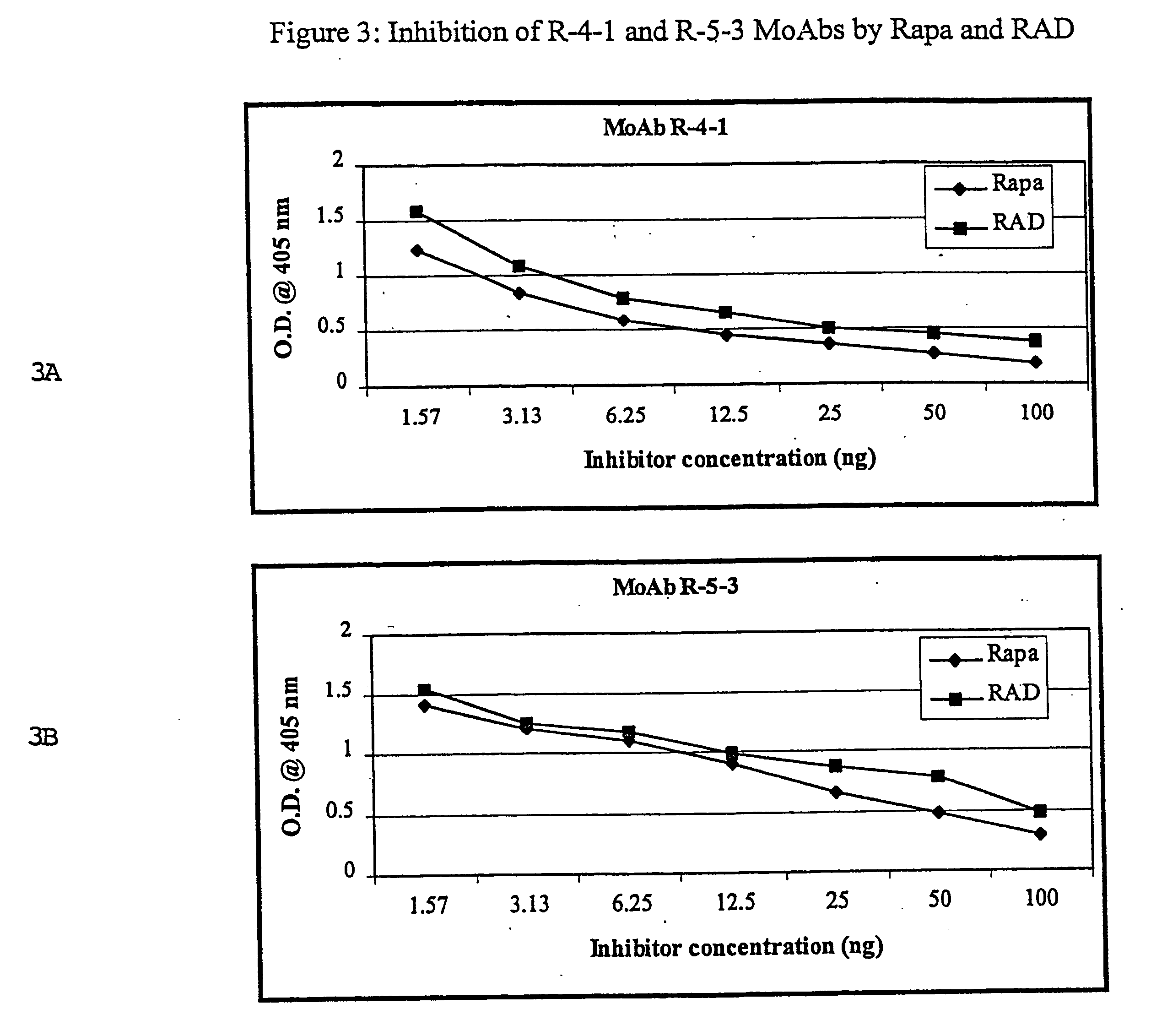
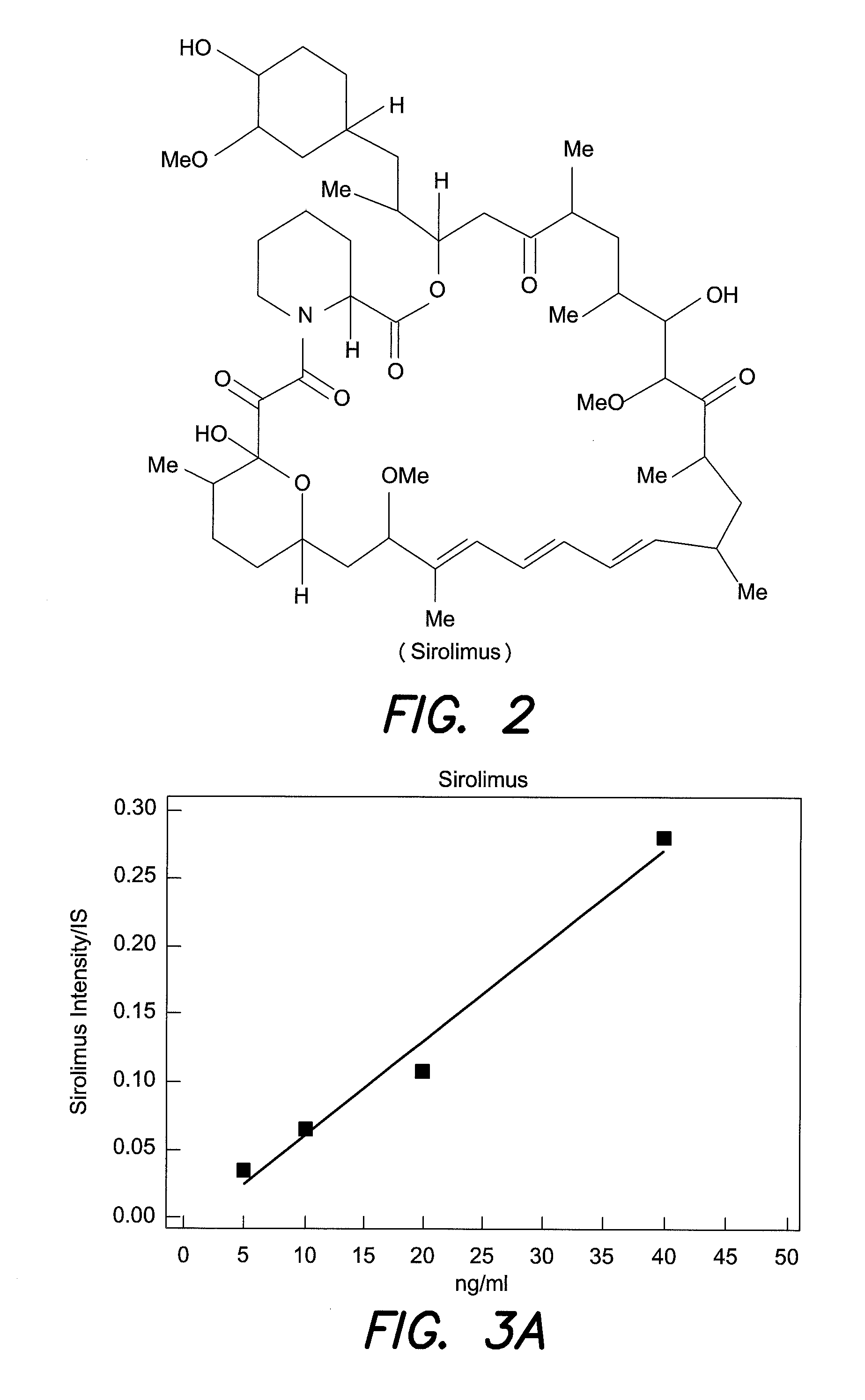
This invention relates to the production of polyclonal and monoclonal antibodies to specific sites of rapamycin (Sirolimus). The reactivity of these poly and monoclonal antibodies make them particularly useful for immunoassays for therapeutic drug monitoring (TDM). These immunoassays or TDM kits may include polyclonal or monoclonal antibodies to specific sites of rapamycin. These kits may also include various combinations of polyclonal antibodies, polyclonal and monoclonal antibodies or a panel of monoclonal antibodies. Rapamycin conjugate immunogens are prepared for the immunization of a host animal to produce antibodies directed against specific regions of the rapamycin molecule. By determining the specific binding region of particular antibody, immunoassays which are capable of distinguishing between the parent molecule, active metabolites, inactive metabolites and other structurally similar immunosuppressant compounds are developed. The use of divinyl sulfone (DVS) as the linker arm molecule for forming rapamycin-protein conjugate immunogens is described. DVS-linked rapamycin-protein conjugates were found to elicit antibodies with greater specificity to the rapamycin molecule than succinate linked conjugates.
Owner:YATSCOFF RANDALL W +2
Methods, devices, and reagents for monitoring paclitaxel concentration in plasma for pharmacokinetic-guided dosing of paclitaxel
InactiveUS20150285827A1High sensitivityImprove dynamic rangeDisease diagnosisBiological testingPharmaceutical drugBlood plasma
Methods, devices, and compositions for assaying therapeutic agents. In one aspect, methods, devices, and compositions for assaying paclitaxel to provide therapeutic drug monitoring guided therapy of paclitaxel.
Owner:AUTOTELIC
Methods and compositions for therapeutic drug monitoring and dosing by point of care pharmacokinetic profiling
InactiveUS20140349862A1Good curative effectReduce dosageOrganic active ingredientsLibrary screeningPoint of careSelf sampling
Disclosed are methods and kits for pharmacokinetic profiling employing point-of-care or point of service self-sampling and allowing for dosage adjustments based on the pharmacokinetic profiles.
Owner:AUTOTELIC
Blood donepezil drug concentration monitoring kit and detection method thereof
The invention relates to the field of medical detection and in particular to a blood donepezil therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquidchromatography analytical technology, more specifically to a method for accurately and quantitatively detecting donepezil drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood donepezil therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
Blood vancomycin drug concentration monitoring kit and detection method thereof
InactiveCN110361481AReduce operating errorsImprove accuracyComponent separationMedicineQuality control
The invention relates to the field of medical detection and in particular to a blood vancomycin therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquid chromatography analytical technology, more specifically to a method for accurately and quantitatively detecting vancomycin drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood vancomycin therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
Immunosuppressant monitoring by maldi mass spectrometry
InactiveUS20120074308A1Wide linear responseImprove accuracyBiological material analysisIsotope separationEverolimusMacrolide resistance
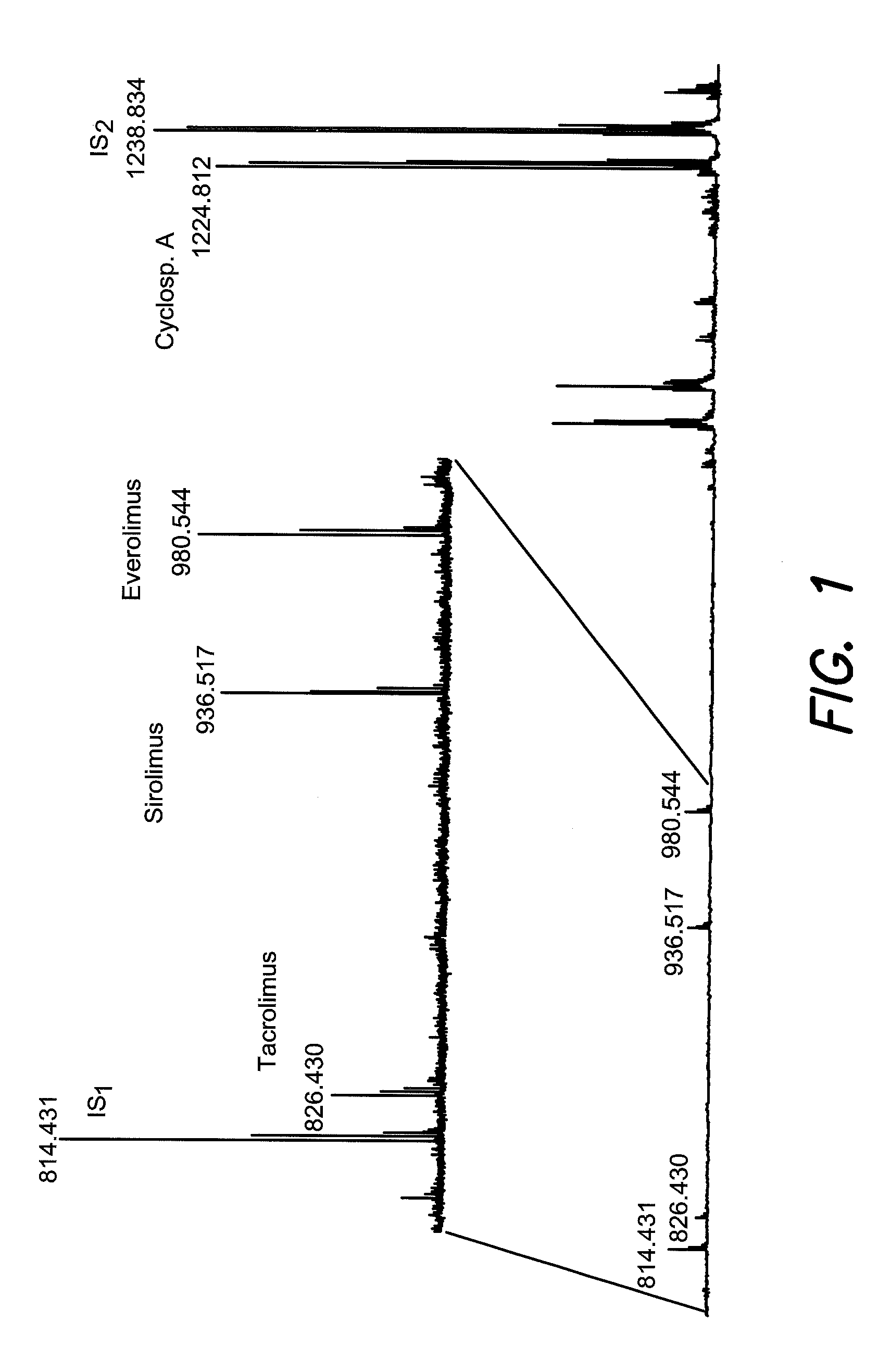
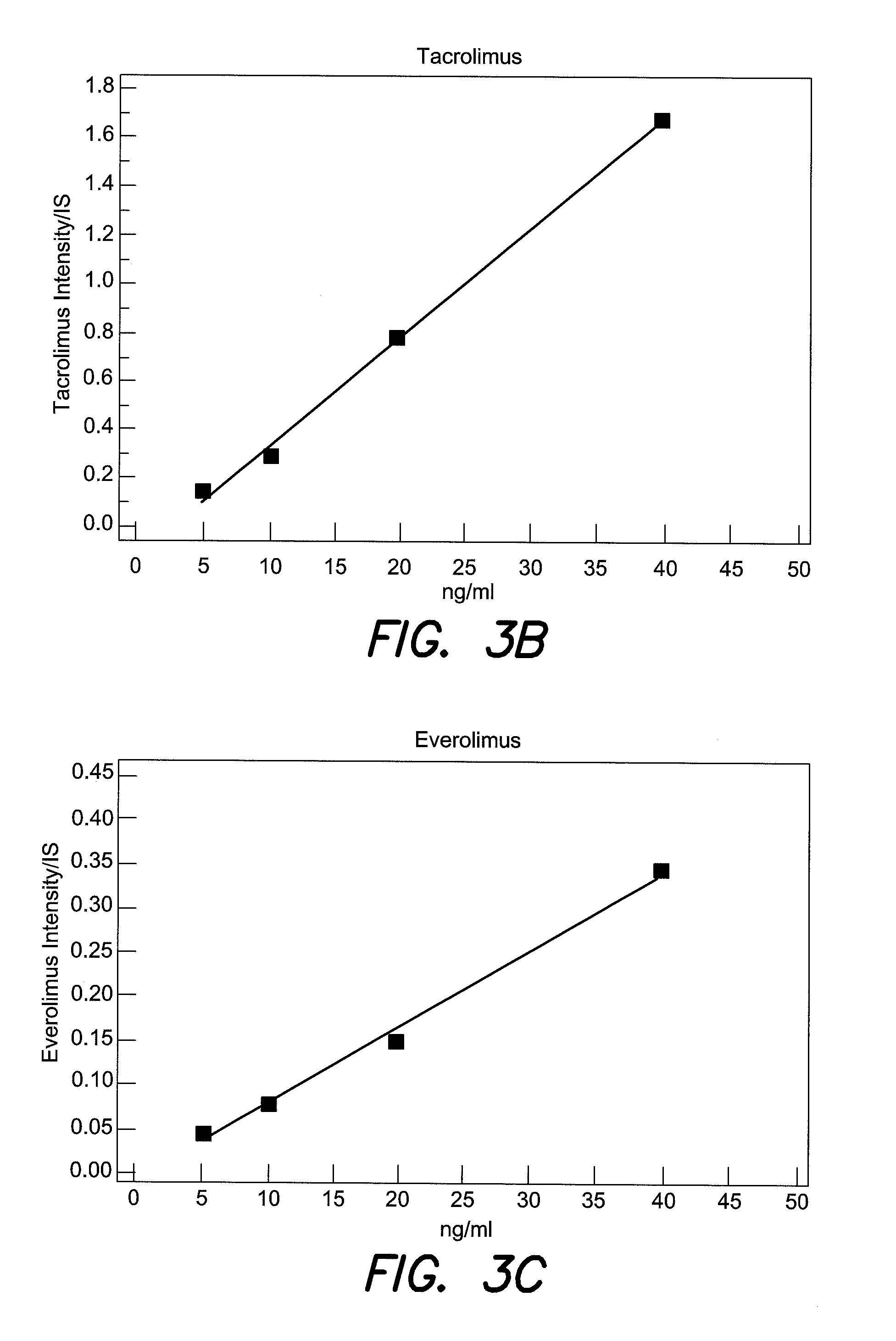
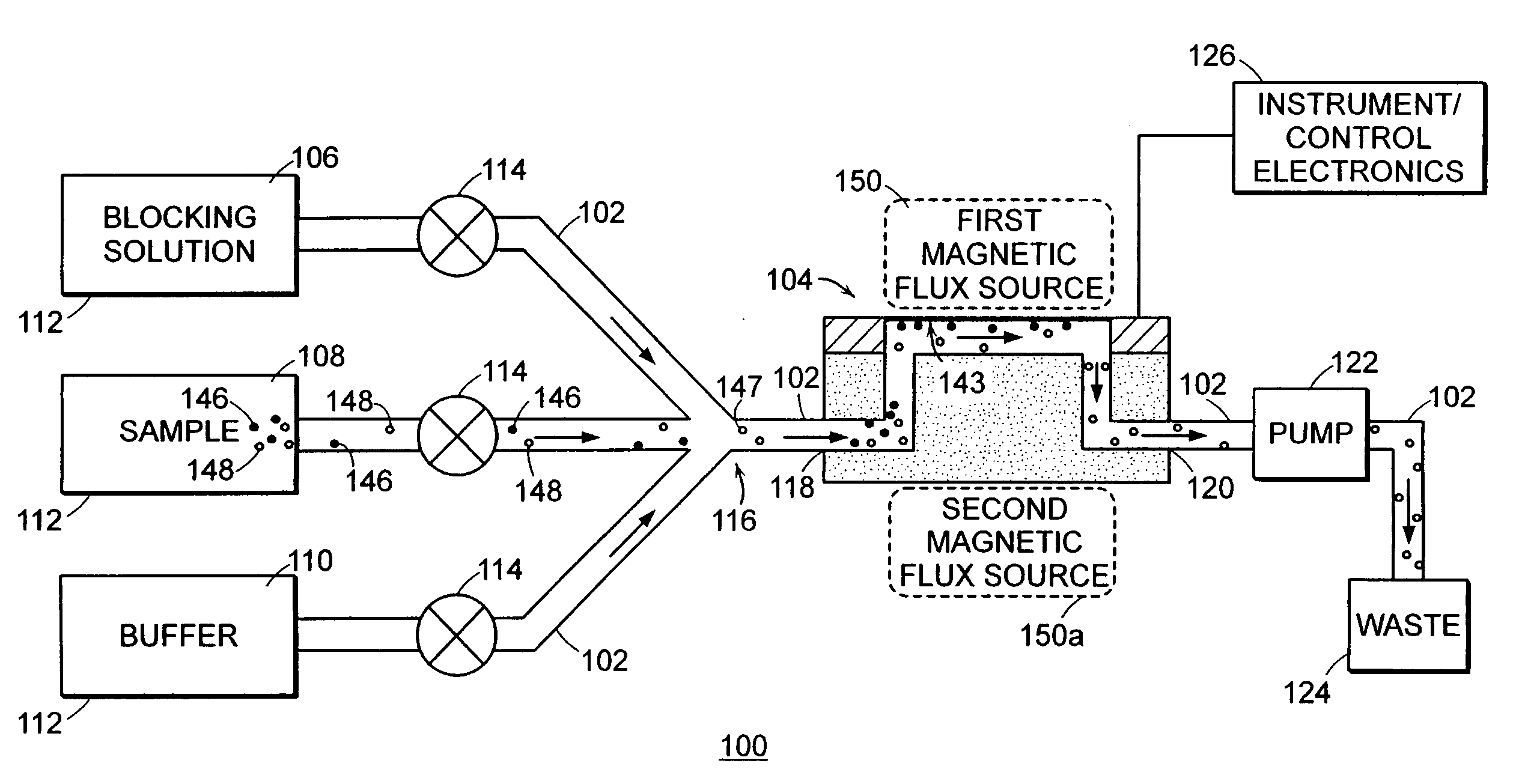
The invention relates to therapeutic drug monitoring (TDM) by mass spectrometry, particularly to the monitoring of immunosuppressant levels in blood of patients with transplanted organs. A liquid phase extraction procedure reproducibly extracts the therapeutic drug molecules from whole blood and mass spectrometric analysis on MALDI instruments, with a matrix substance for highest sensitivity and special sample deposition procedure for a reproducible ionization of the therapeutic drug molecules. Suitable internal standard substances added to the blood in exact amounts ensure a correct absolute quantification. The method is particularly suitable for immunosuppressants belonging to the class of macrocyclic lactones (sirolimus, tacrolimus, everolimus) and cyclic polypeptides (cyclosporin A), and even works as a multiplex method for all four immunosuppressants simultaneously.
Owner:BRUKER DALTONIK GMBH
Methods and apparatus for therapeutic drug monitoring using an acoustic device
InactiveUS20090148856A1Low level of analyteImprove detection limitMaterial nanotechnologyBioreactor/fermenter combinationsMedicineResonant sensor
Methods for therapeutic drug monitoring are provided. A plurality of particles, each of which is coated with a capture agent capable of binding a therapeutic drug of choice is combined with the sample to form a plurality of therapeutic drug-particle complexes. The system also includes a transport arrangement for transporting the sample and / or particles to the sensor surface, and optionally a magnetic field inducing structure constructed and arranged to establish a magnetic field at and adjacent to the sensor surface. The resonant sensor produces a signal corresponding to an amount of therapeutic drug-particle complexes that are bound to the sensor surface.
Owner:BIOSCALE
Blood amiodarone drug concentration monitoring kit and detection method thereof
InactiveCN110361484AExplain the relationship between drug concentration-effect therapy-adverse reactionsReduce operating errorsComponent separationMedicineQuality control
The invention relates to the field of medical detection and in particular to a blood amiodarone therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquid chromatography analytical technology, more specifically to a method for accurately and quantitatively detecting amiodarone drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood amiodarone therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
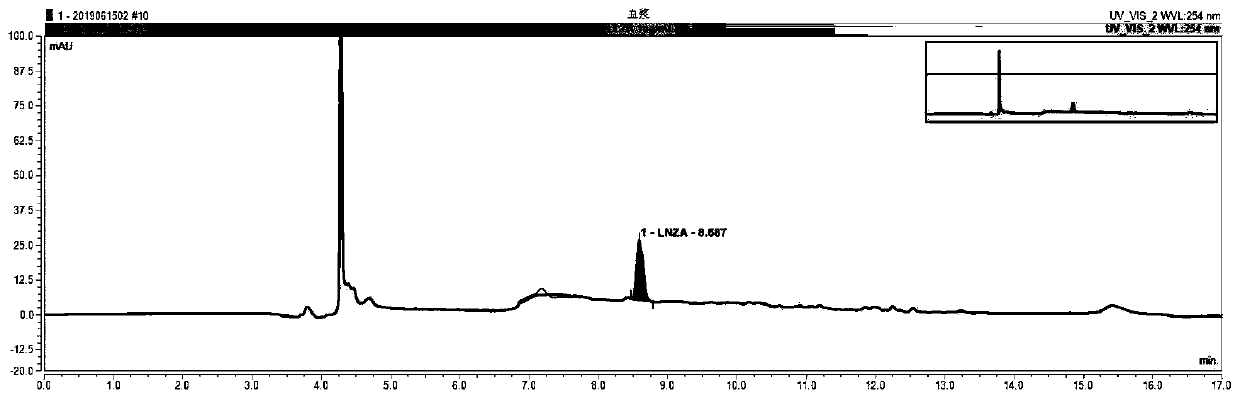
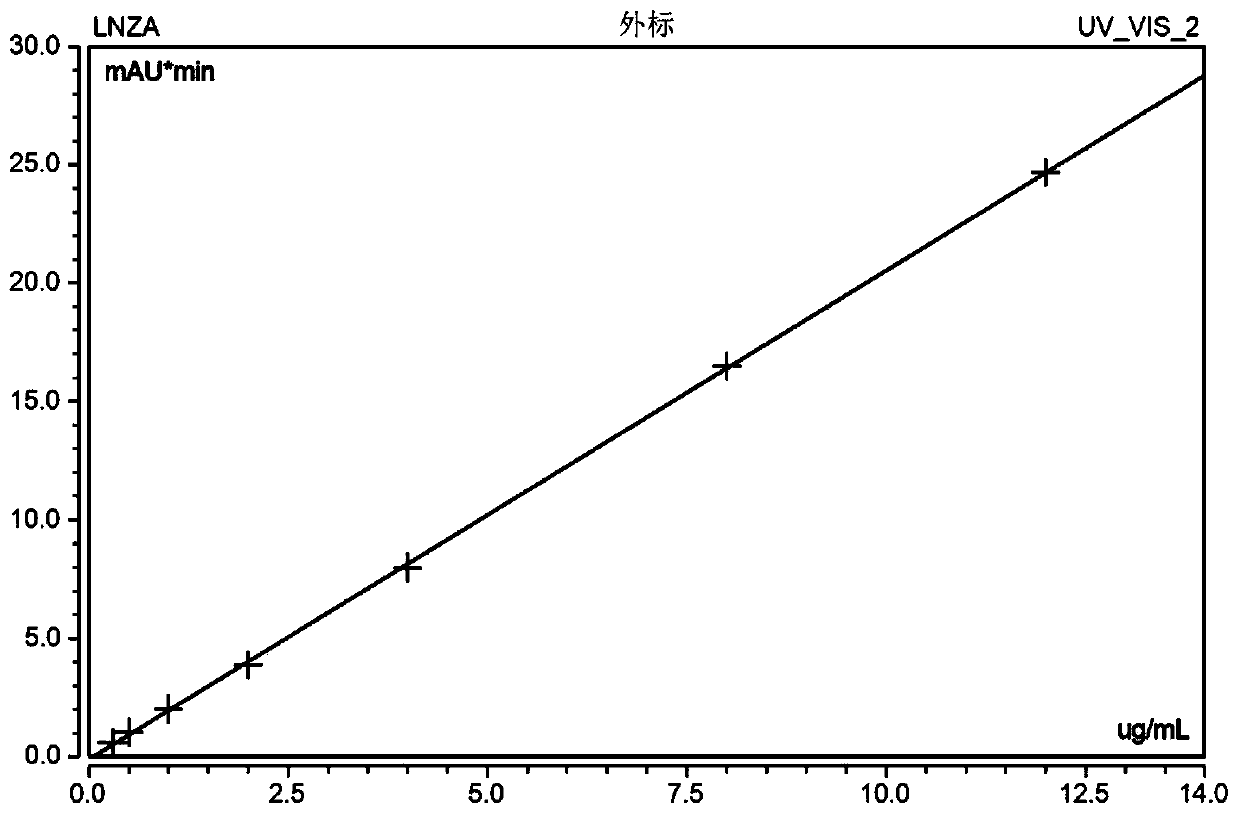
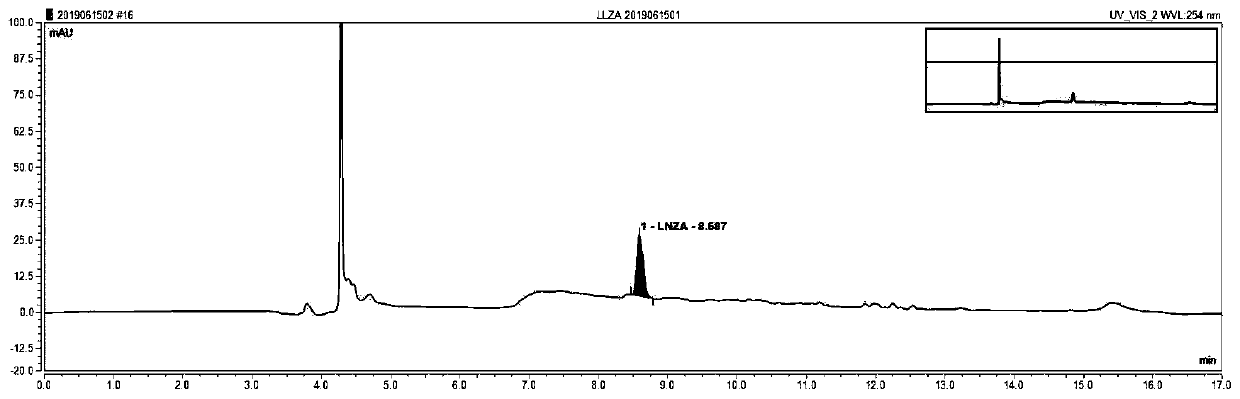
Blood linezolid drug concentration monitoring kit and detection method thereof
InactiveCN110361482AExplain the relationship between drug concentration-effect therapy-adverse reactionsReduce operating errorsComponent separationMedicineQuality control
The invention relates to the field of medical detection and in particular to a blood linezolid therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquidchromatography analytical technology, more specifically to a method for accurately and quantitatively detecting linezolid drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood linezolid therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
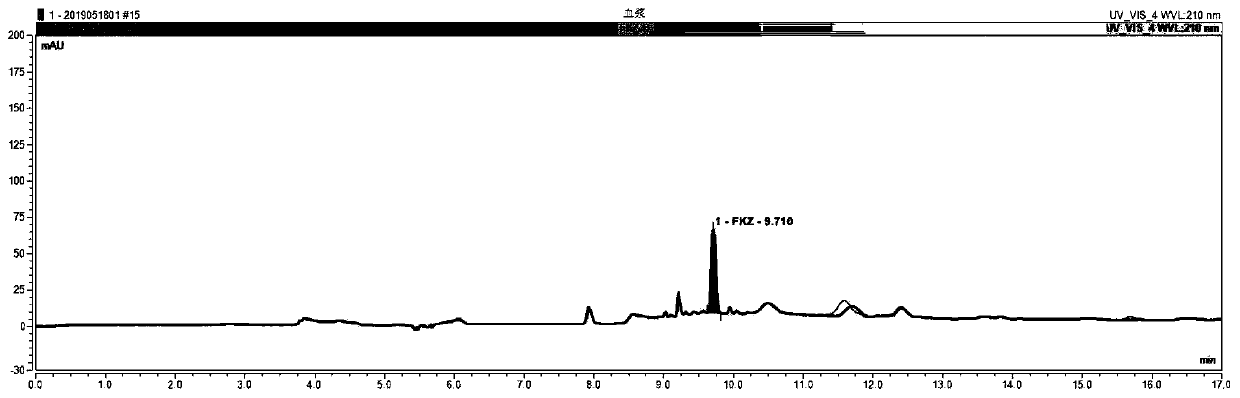
Kit for monitoring concentration of fluconazole drug in blood and detection method for kit
InactiveCN110470775AExplain the relationship between drug concentration-effect therapy-adverse reactionsReduce operating errorsComponent separationMedicineMedication monitoring
The invention relates to the field of medicine detection, in particular to a kit, for therapeutic drug monitoring of fluconazole in blood, developed on the basis of a multi-dimensional online solid phase extraction and liquid chromatography analysis technology, and more specifically discloses a method for carrying out accurate quantitative detection on the concentration of a fluconazole drug in blood by adopting an online solid phase extraction and multi-dimensional liquid chromatography analysis technology. The kit comprises a calibrator reagent, a quality control material reagent, treating fluid, extracting fluid, cleaning fluid and an eluent. According to the kit for therapeutic drug monitoring disclosed by the invention, only centrifugal treatment is needed for a blood sample, so thatthe repeated detection variability is small, the accuracy and the stability are high, the detection cost is low, and various methodology indicators all can meet the requirements of therapeutic drug monitoring of the fluconazole in the blood, and the kit is easy to promote.
Owner:苏州艾迪迈医疗科技有限公司
Method for quantitatively determining abiraterone in blood and application thereof
InactiveCN112379026AEasy to operateReduce labor costsComponent separationDrug interactionAbiraterone
The invention discloses a method for quantitatively determining abiraterone in blood and application thereof. According to the invention, a direct protein precipitation method is adopted, sample introduction determination is carried out after centrifugation, and the concentration or content of abiraterone in blood plasma is obtained through a standard curve of abiraterone. According to the methodfor quantitatively determining abiraterone in blood plasma and the application thereof, high-throughput sample treatment is facilitated, and the method is free of toxicity and safe to operate; the method has good specificity, sensitivity, precision and accuracy; a plasma matrix does not influence an analysis result; sample consumption is low; a pretreatment process is simple, economical and rapid;analysis time is short; analysis efficiency is high; an extraction recovery rate is high; and the method can be used for new preparation development, therapeutic drug monitoring, drug interaction research and the like of abiraterone and has extremely high application value.
Owner:南京广祺医药科技有限公司
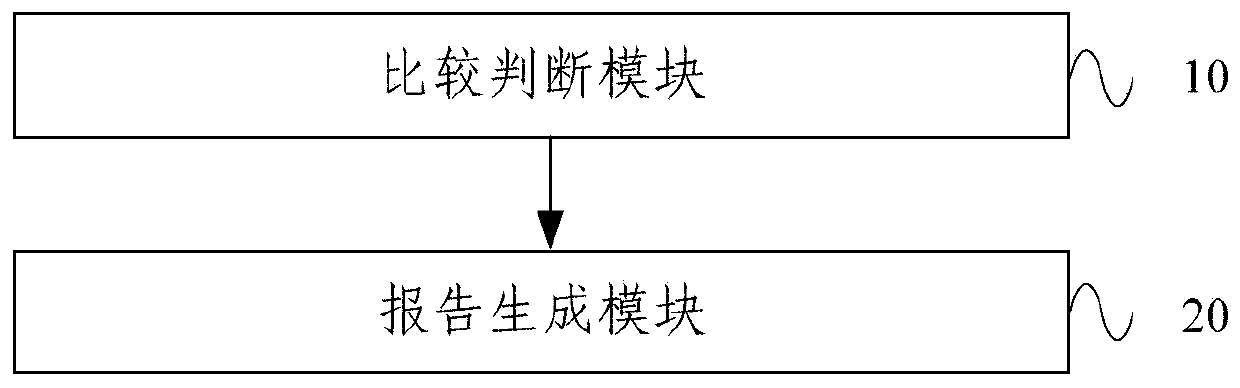
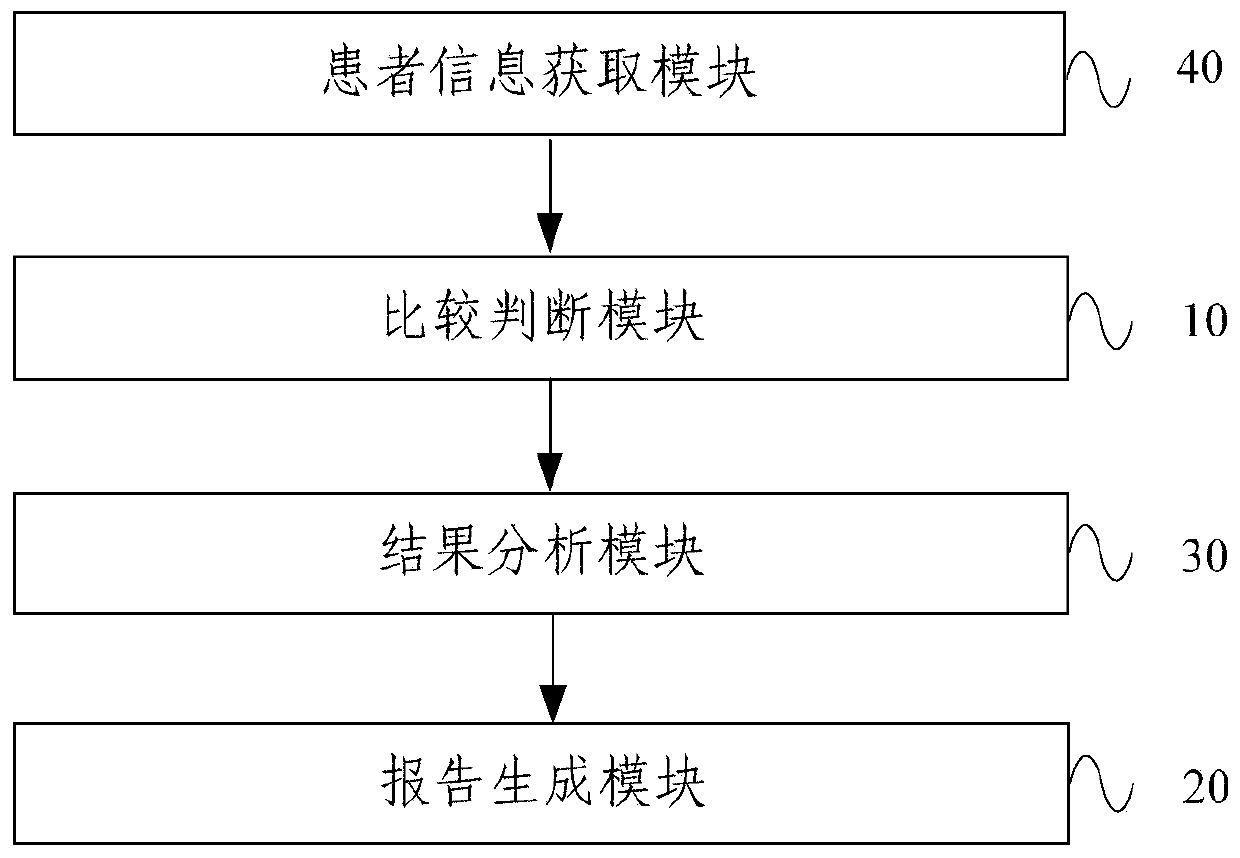
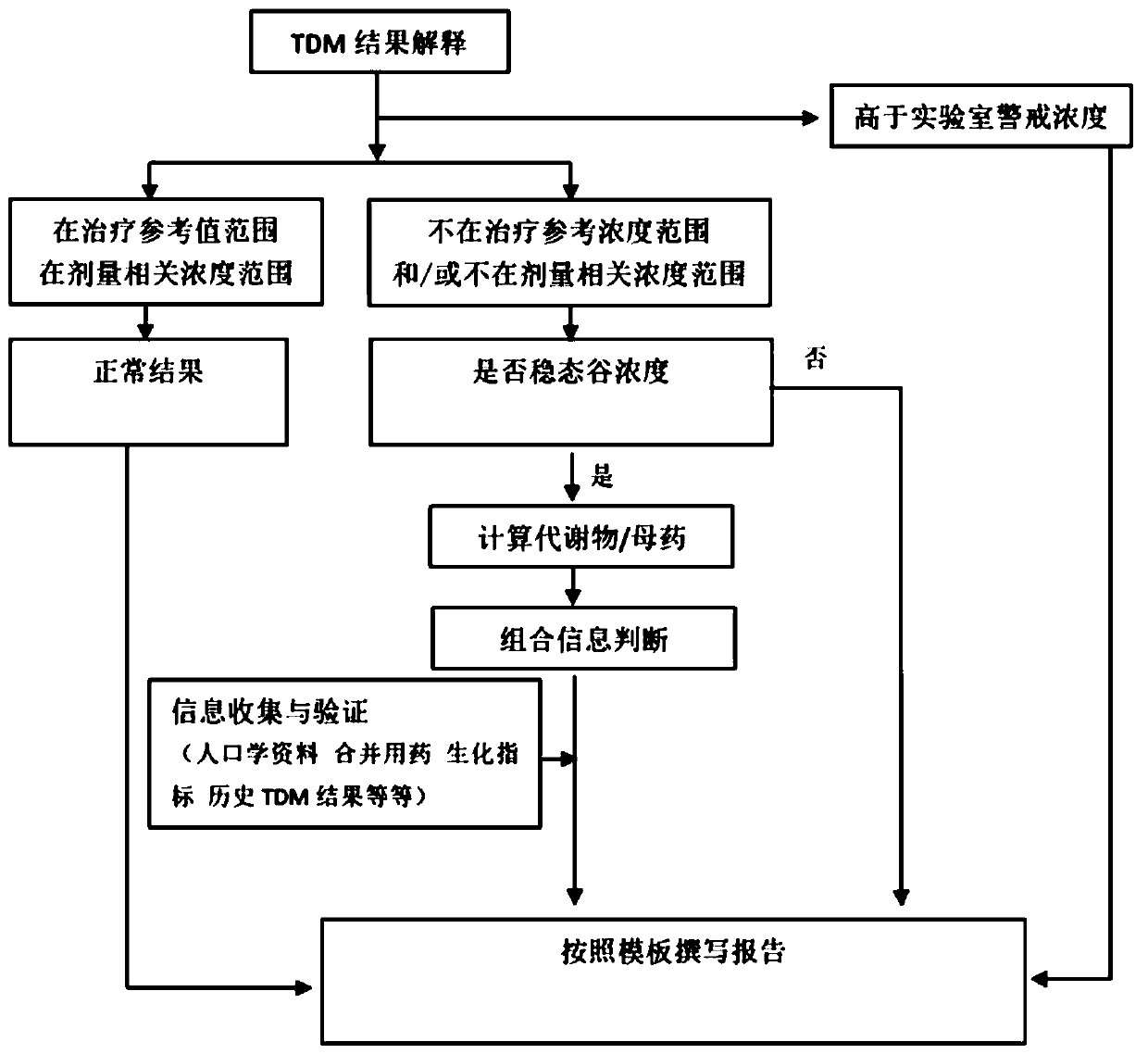
A therapeutic drug monitoring result interpretation system
PendingCN111489822AAchieve standardizationFully automatedMedical automated diagnosisMedical reportsMedication monitoringPharmaceutical drug
The embodiment of the invention provides a therapeutic drug monitoring result interpretation system, which comprises a comparison and judgment module used for comparing a therapeutic drug monitoring result with at least one preset first reference concentration range; and / or obtaining at least one preset statistical result according to the therapeutic drug monitoring result, and comparing the preset statistical result with a corresponding preset second reference concentration range to obtain a therapeutic drug monitoring comparison result; and a report generation module used for generating a therapeutic drug monitoring result interpretation report according to the therapeutic drug monitoring comparison result and a corresponding preset report template. The embodiment of the invention provides a therapeutic drug monitoring result interpretation system. A therapeutic drug monitoring comparison result is obtained through preset comparison, and a therapeutic drug monitoring result interpretation report is generated according to the therapeutic drug monitoring comparison result and the corresponding preset report template, so that standardization and automation of generation of the therapeutic drug monitoring result interpretation report are realized, and the generation efficiency is improved.
Owner:BEIJING ANDING HOSPITAL CAPITAL MEDICAL UNIV
Methods and compositions for therapeutic drug monitoring and dosing by point-of-care pharmacokinetic profiling
Disclosed are methods and kits for pharmacokinetic profiling employing point-of-care or point of service self-sampling and allowing for dosage adjustments based on the pharmacokinetic profiles.
Owner:奥德特里克有限责任公司
Blood oxcarbazepine drug concentration monitoring kit and detection method thereof
InactiveCN110361485AReduce operating errorsImprove accuracyComponent separationMetaboliteMedication monitoring
The invention relates to the field of medical detection and in particular to a blood oxcarbazepine and its metabolite therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquid chromatography analytical technology, more specifically to a method for accurately and quantitatively detecting oxcarbazepine and its metabolite drug concentration in blood byonline solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood oxcarbazepine and its metabolite therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
Blood lamotrigine drug concentration monitoring kit and detection method thereof
InactiveCN110361488AExplain the relationship between drug concentration-effect therapy-adverse reactionsReduce operating errorsComponent separationMedicineQuality control
The invention relates to the field of medical detection and in particular to a blood lamotrigine therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquid chromatography analytical technology, more specifically to a method for accurately and quantitatively detecting lamotrigine drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood lamotrigine therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
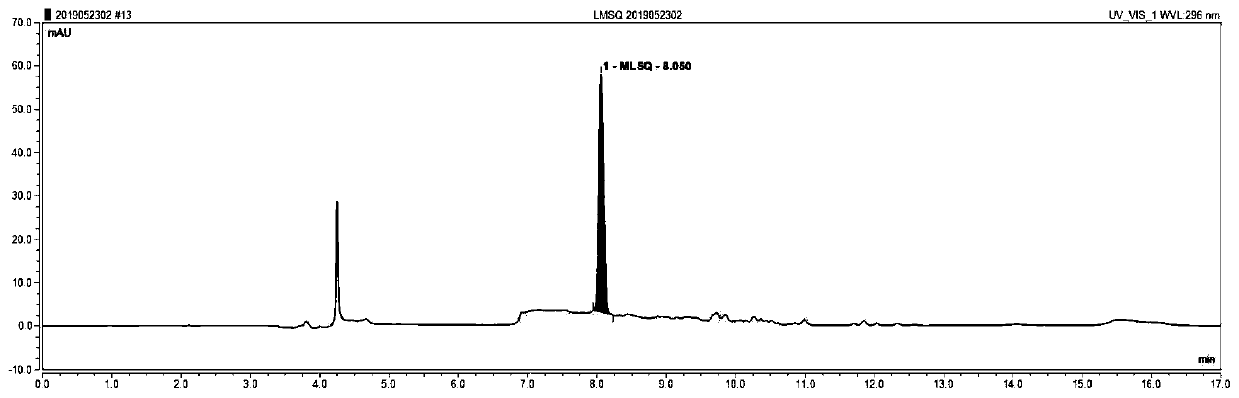
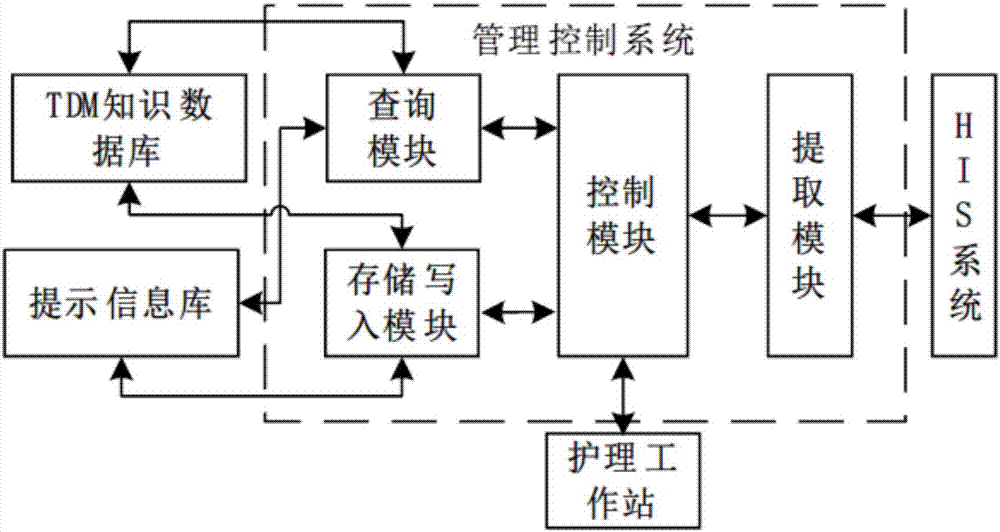
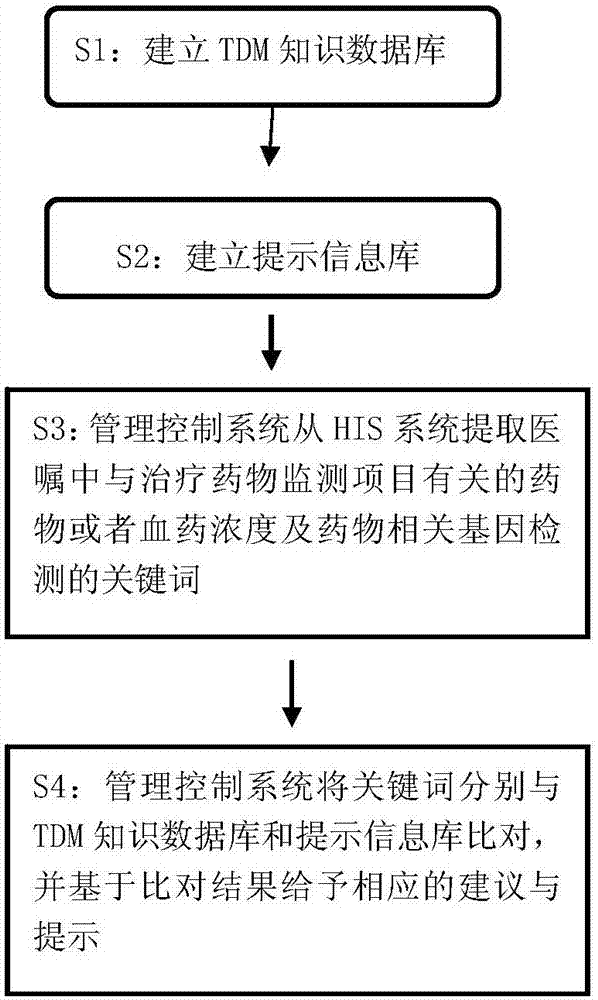
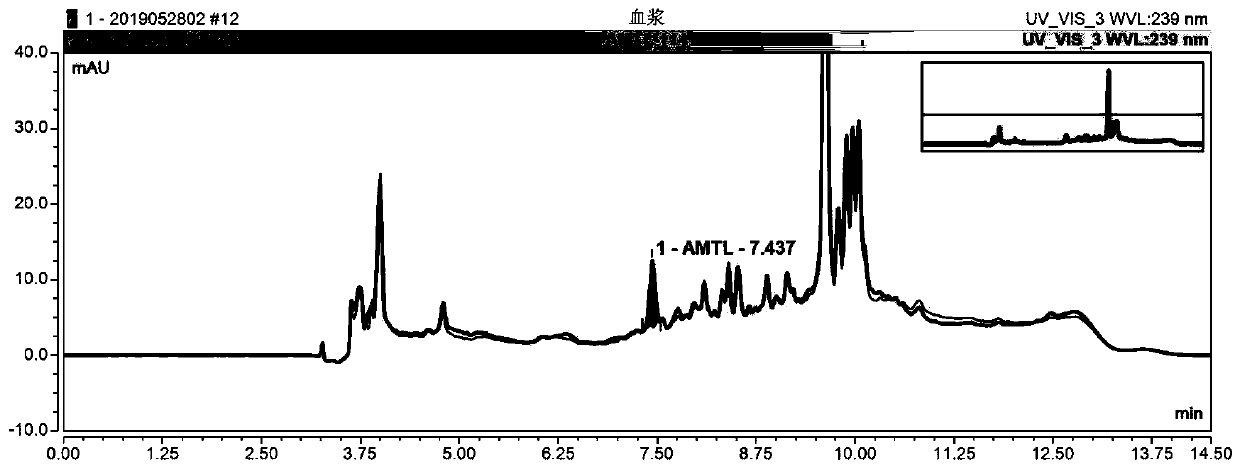
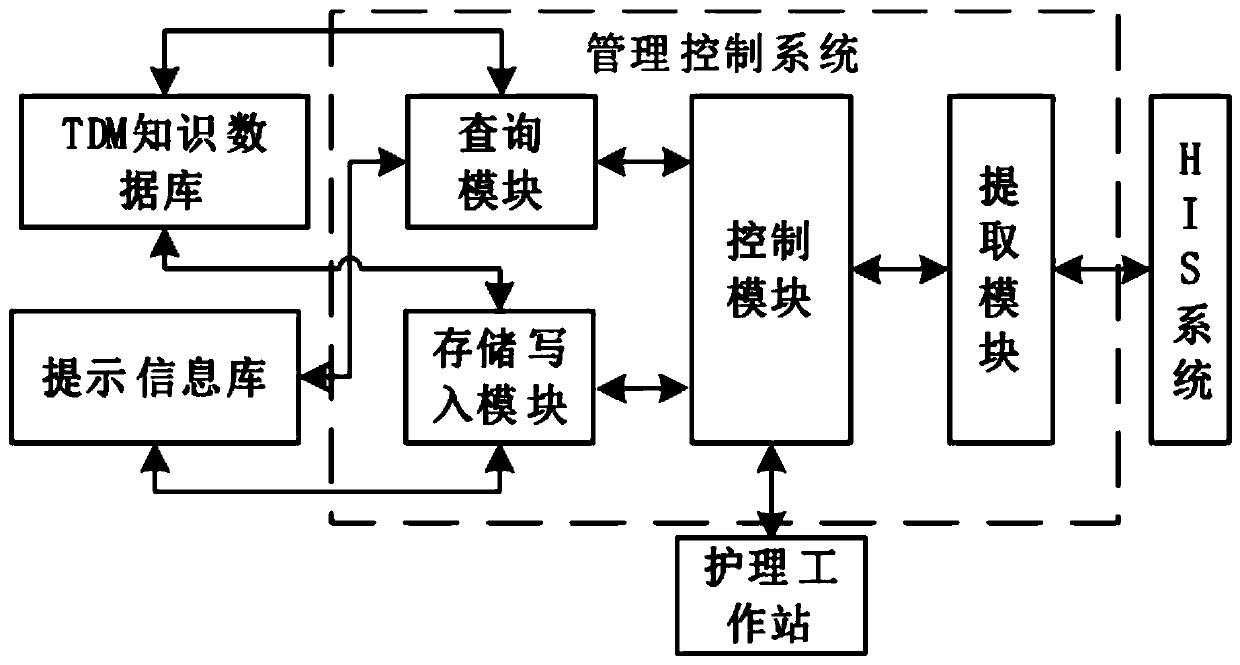
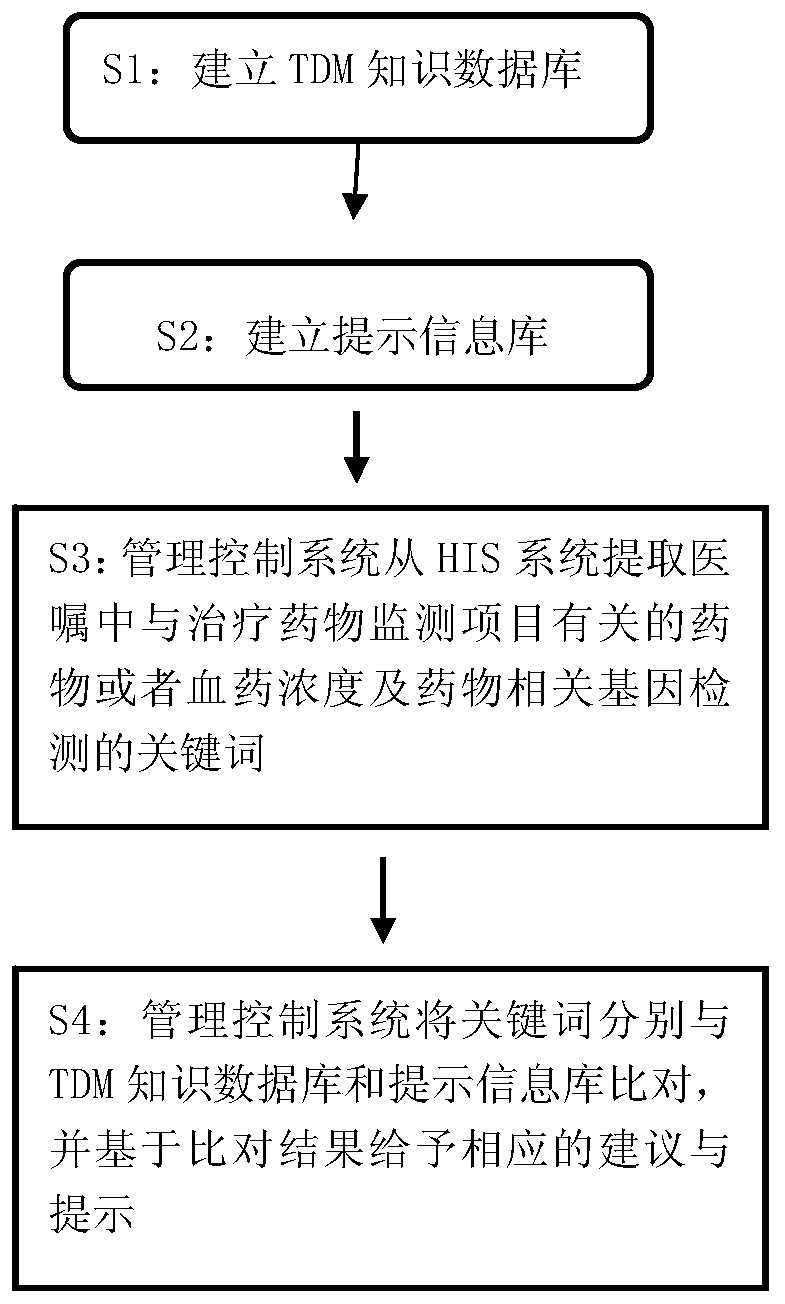
HIS based intelligent therapeutic drug monitoring whole process management system and method thereof
ActiveCN107133456AAvoid duplicate detectionGuaranteed pass rateSpecial data processing applicationsInformation repositoryControl system
The invention discloses an HIS based intelligent therapeutic drug monitoring whole process management system and a method thereof. The therapeutic drug monitoring whole process management system comprises a management control system, an HIS system, a TDM knowledge database, a prompt information base and a nursing workstation, wherein the management control system comprises a control module as well as an extraction module, a query module and a storage writing module connected with the control module, the output end of the HIS system is connected with the input end of the extraction module, the query module is connected with the TDM knowledge database and the prompt information base, the storage writing module is connected with the TDM knowledge database and the prompt information base, and the nursing workstation is connected with the control module. The system can effectively avoid the repeated detection of drug related genes, ensure the qualified rate of plasma concentration specimens, improve the working efficiency of the staff, and reduce the waste of medical resources fundamentally.
Owner:175TH HOSPITAL OF PEOPLES LIBERATION ARMY
Blood amitriptyline drug concentration monitoring kit and detection method thereof
InactiveCN110361489AReduce operating errorsImprove accuracyComponent separationAmitriptylineMedication monitoring
The invention relates to the field of medical detection and in particular to a blood amitriptyline therapeutic drug monitoring kit developed based on multi-dimensional online solid-phase extraction liquid chromatography analytical technology, more specifically to a method for accurately and quantitatively detecting amitriptyline drug concentration in blood by online solid-phase extraction and multi-dimensional liquid chromatography analytical technology. The kit includes a calibration reagent, a quality control reagent, a processing liquid, an extract liquid, a washing liquid, and an eluent. The therapeutic drug monitoring kit of the invention only needs to centrifuge a blood sample, achieves low repeated detection variability, high accuracy and stability, and low detection cost, has various methodological indicators that satisfy the requirement for monitoring the blood amitriptyline therapeutic drugs, and is easy to generalize.
Owner:苏州艾迪迈医疗科技有限公司
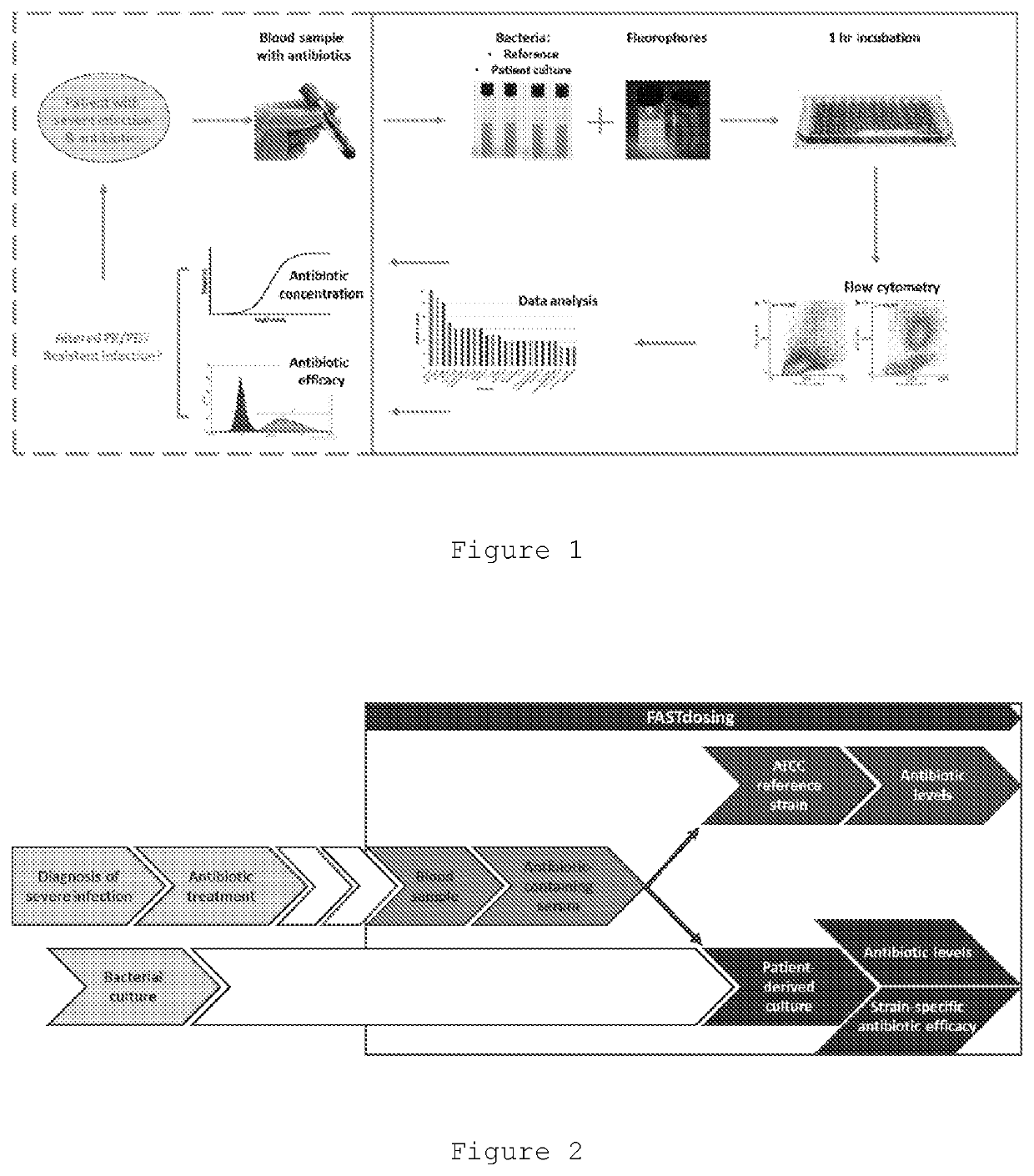
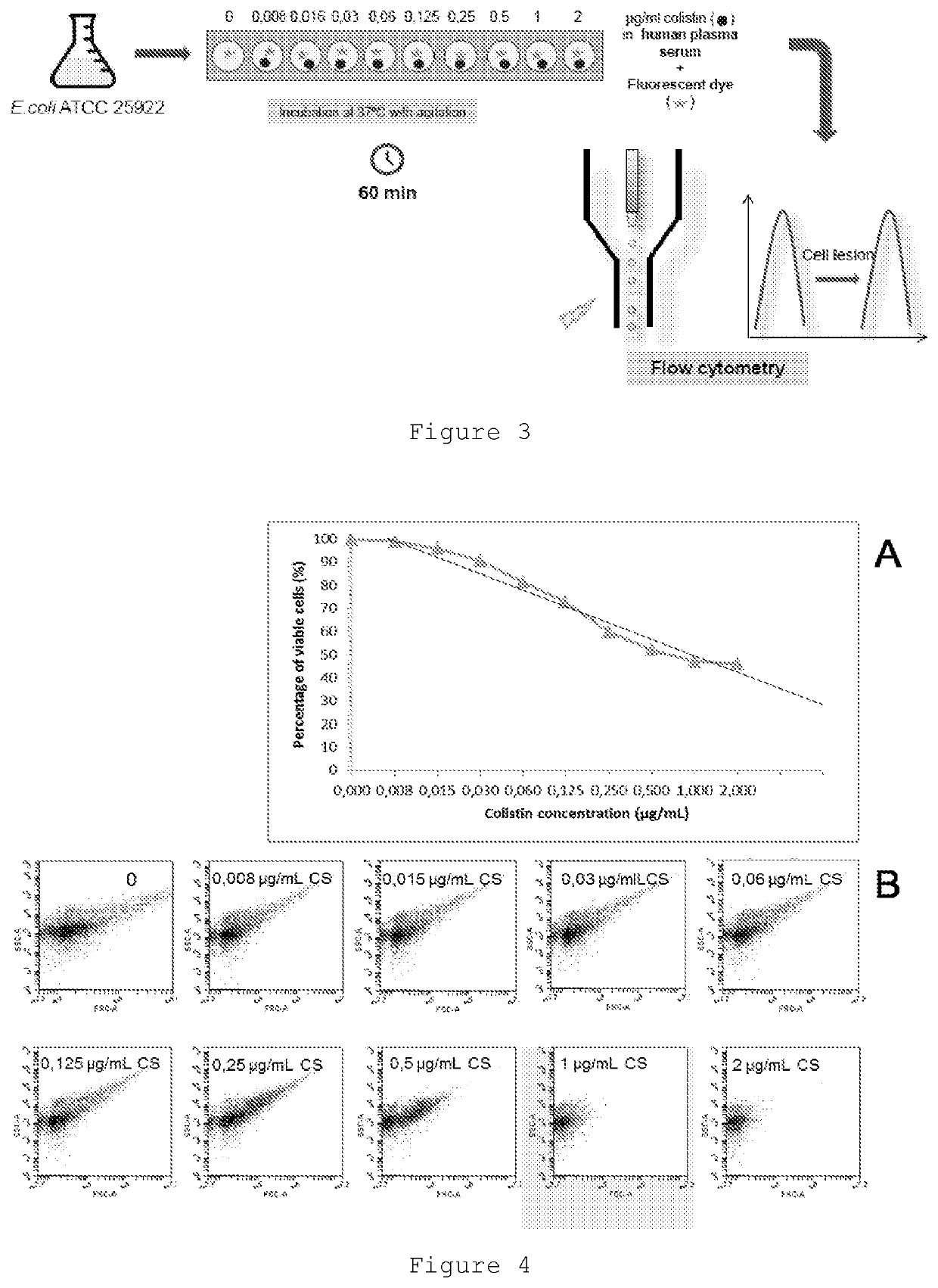
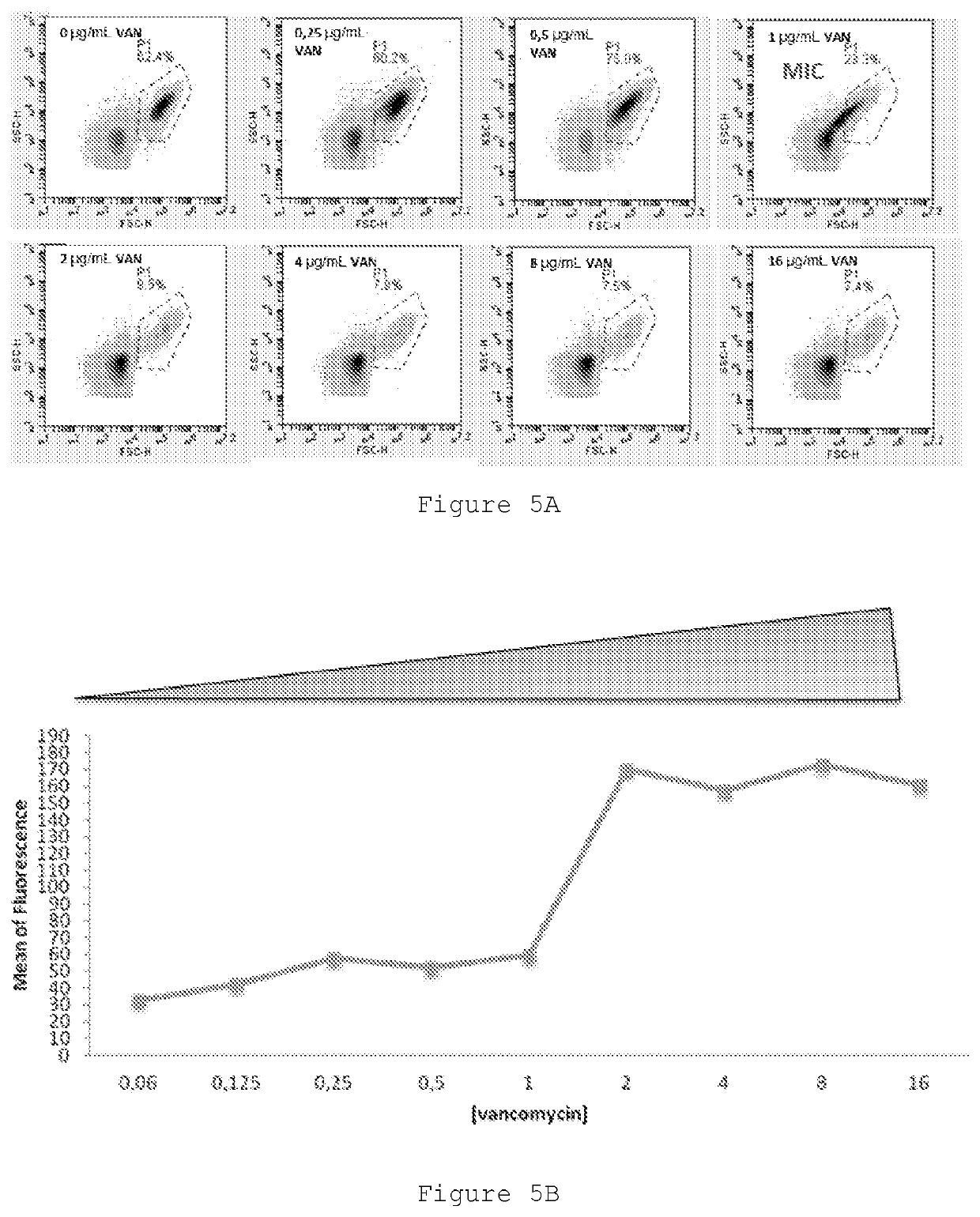
Therapeutic drug monitoring
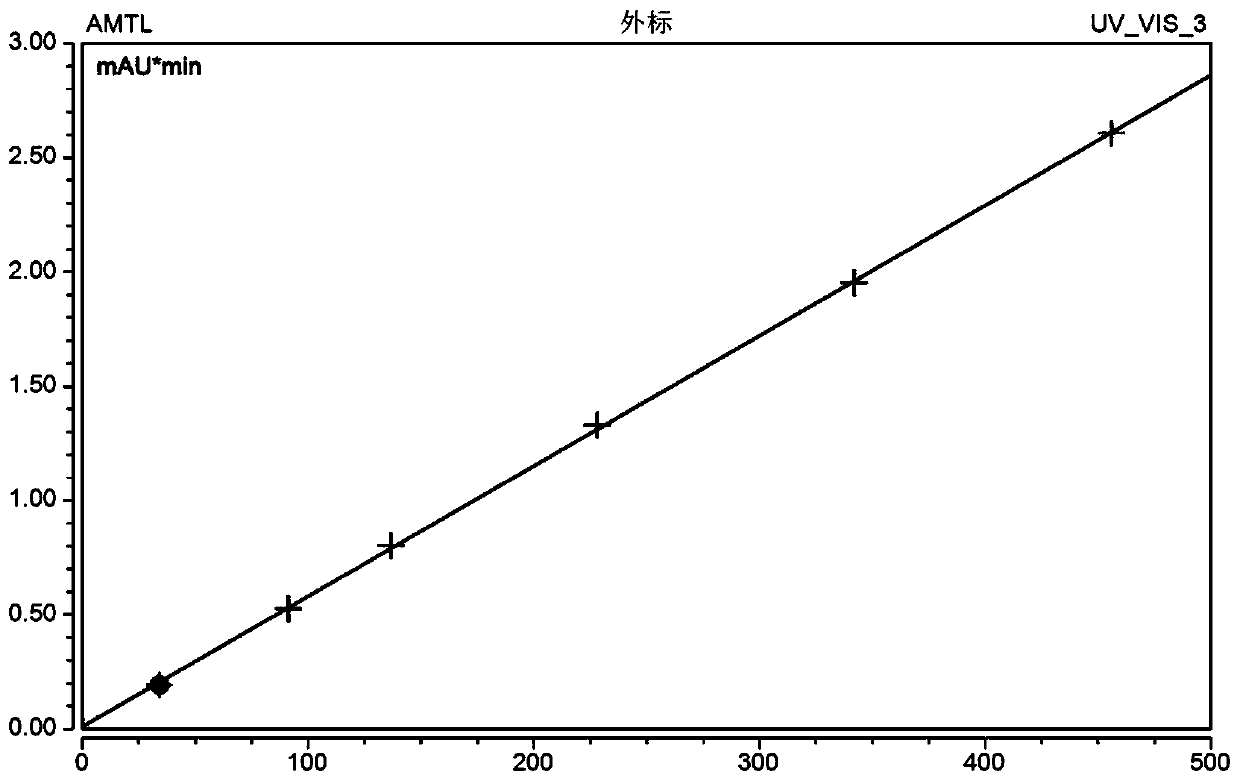
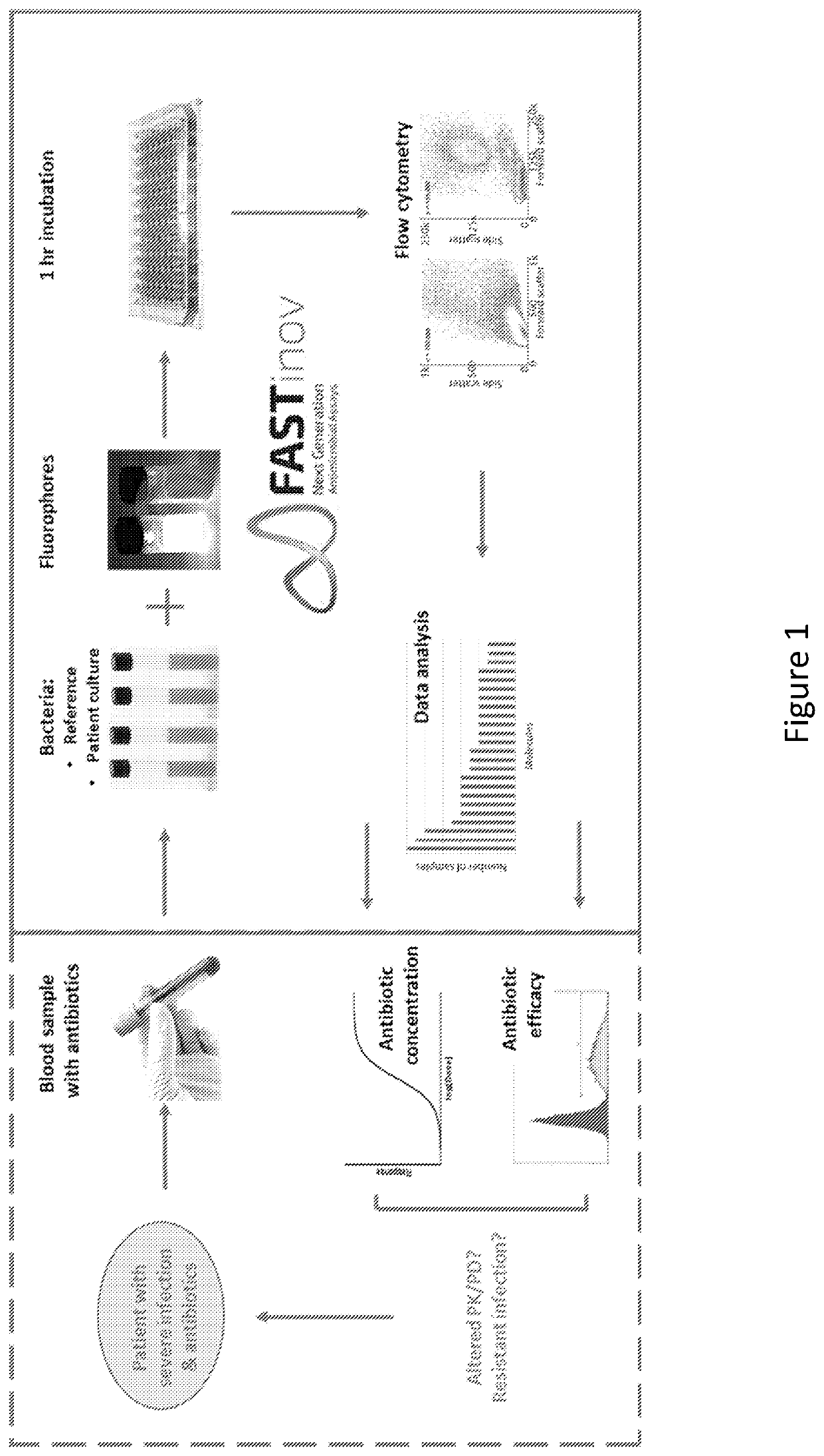
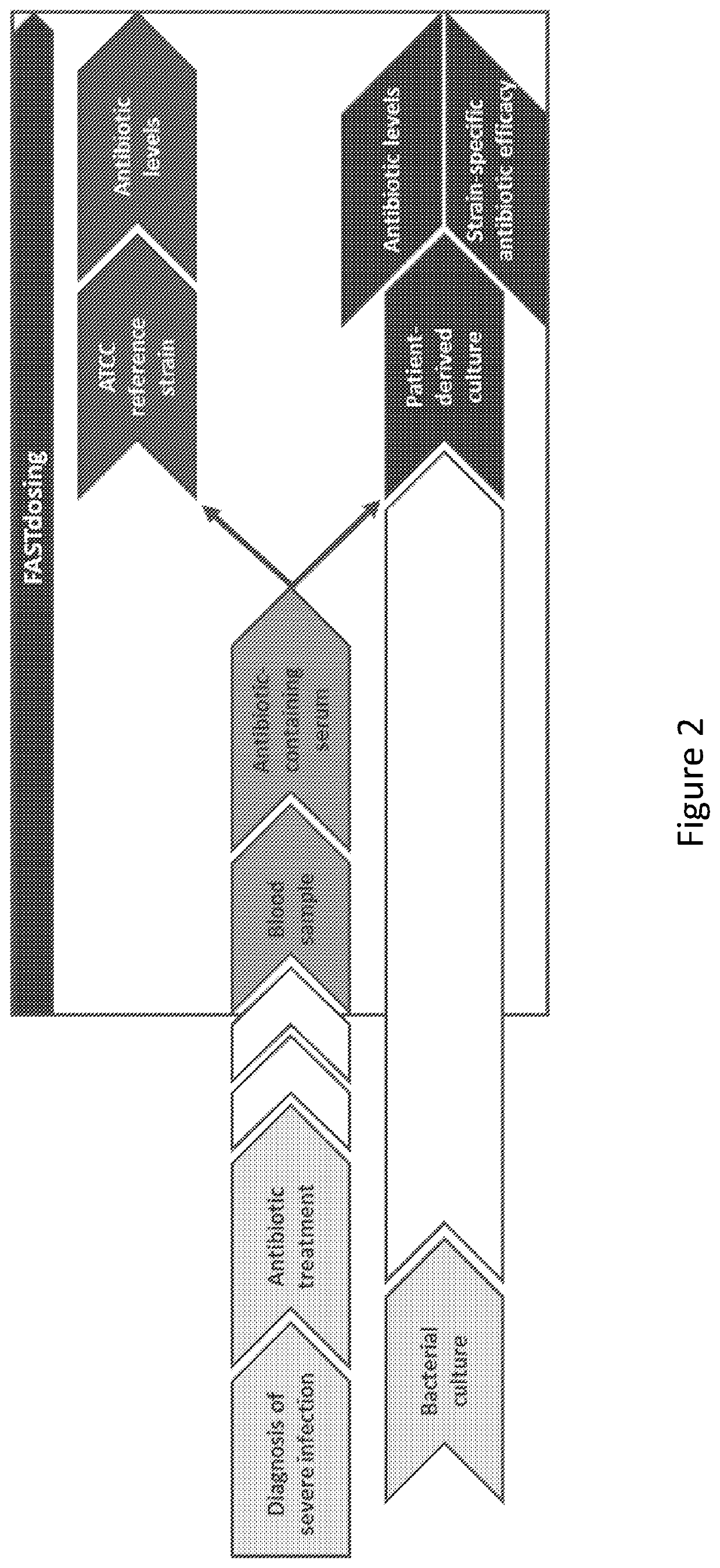
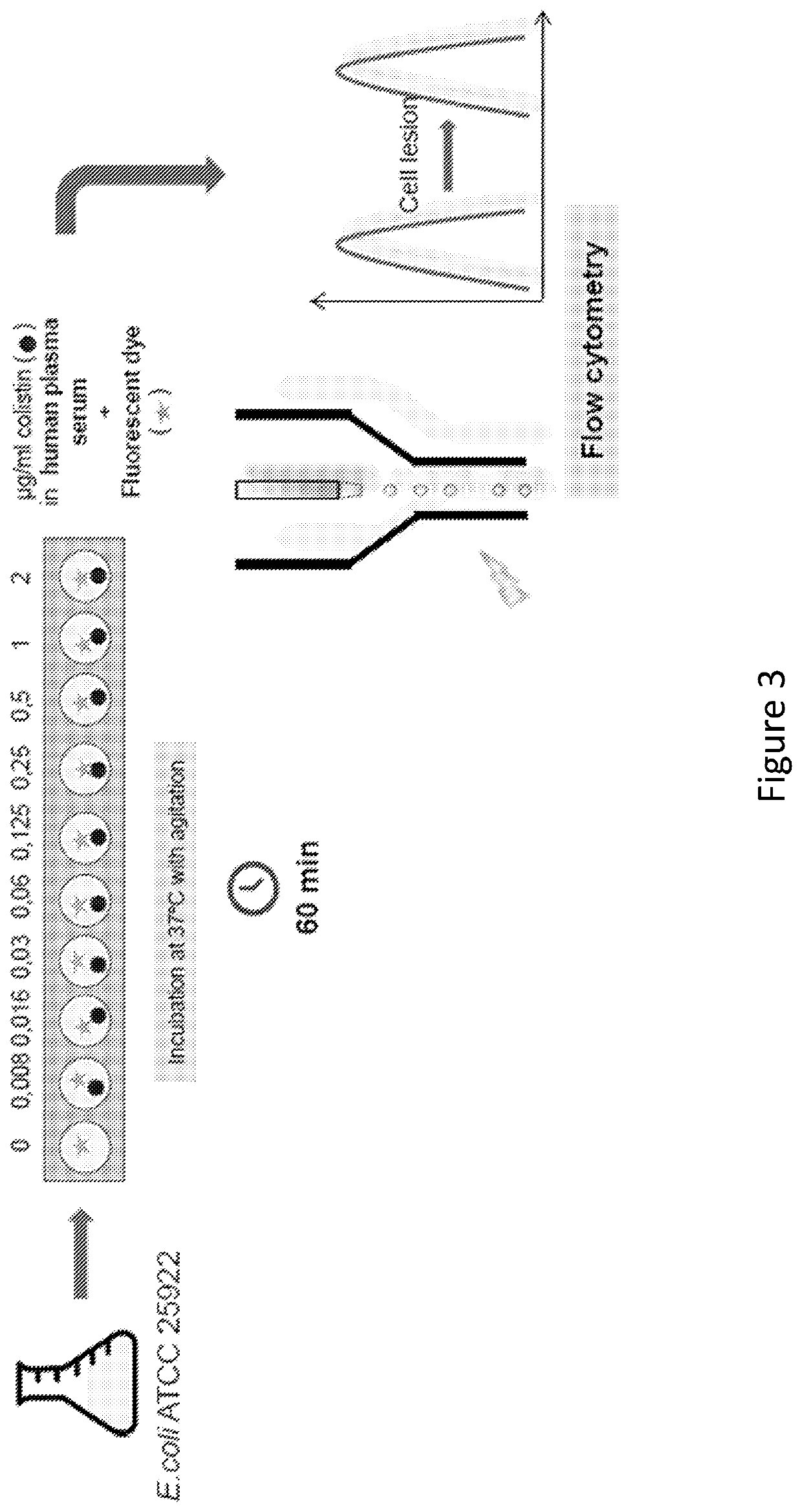
ActiveUS11485993B2Microbiological testing/measurementIndividual particle analysisMicrobial agentFluorophore
Disclosed herein are methods and compositions for antimicrobial quantification and functional measurement. In one aspect, a method for quantifying antimicrobial comprises: obtaining a biological sample from a patient receiving an antimicrobial; incubating the biological sample with a reference microbial strain and a fluorophore for detecting cell lesion; measuring a first signal of fluorescent intensity in the incubated biological sample using flow cytometry; and comparing the first signal to a calibrating curve previously generated for the antimicrobial, thereby quantifying the antimicrobial present in the biological sample.
Owner:FASTINOV SA
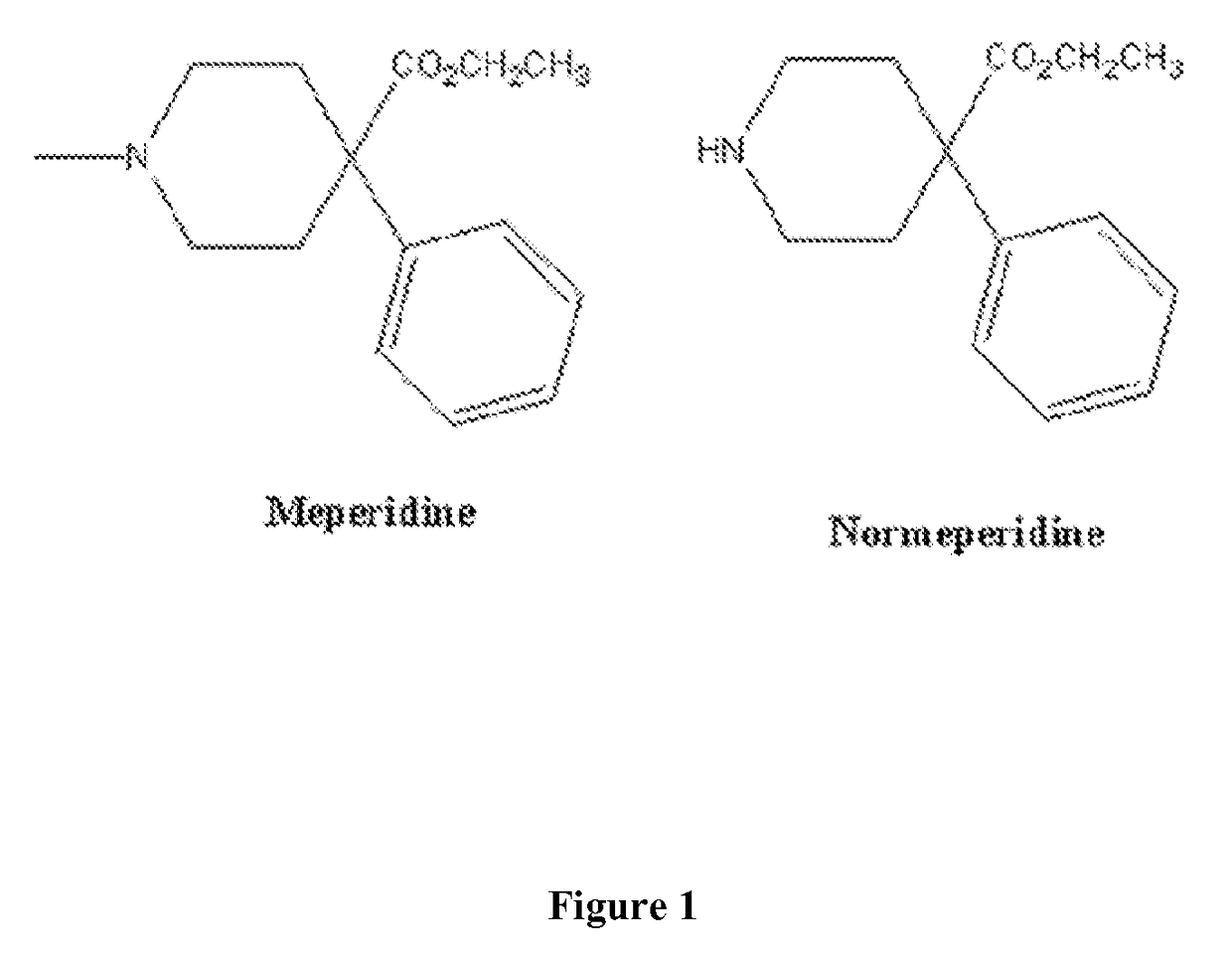
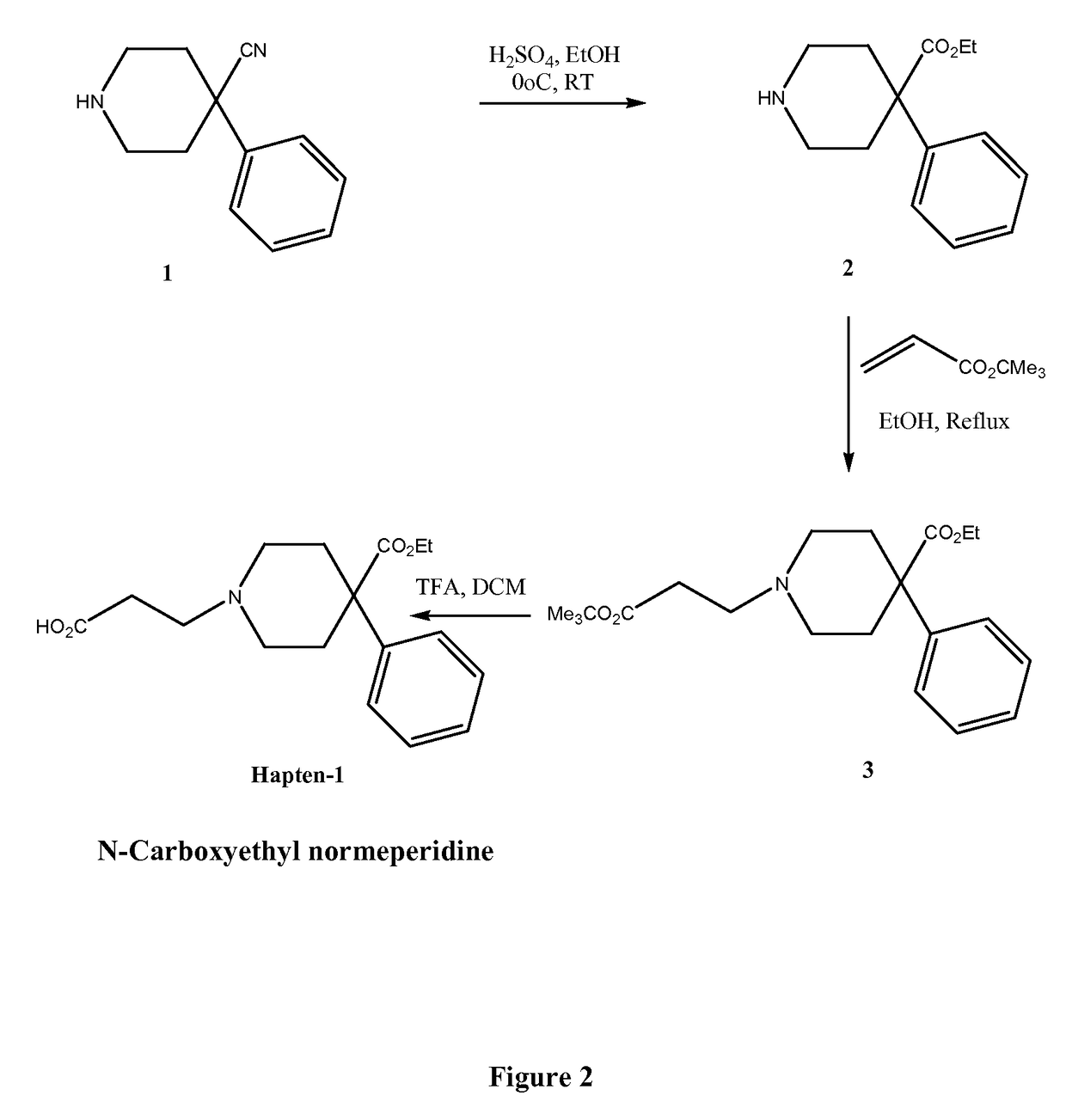
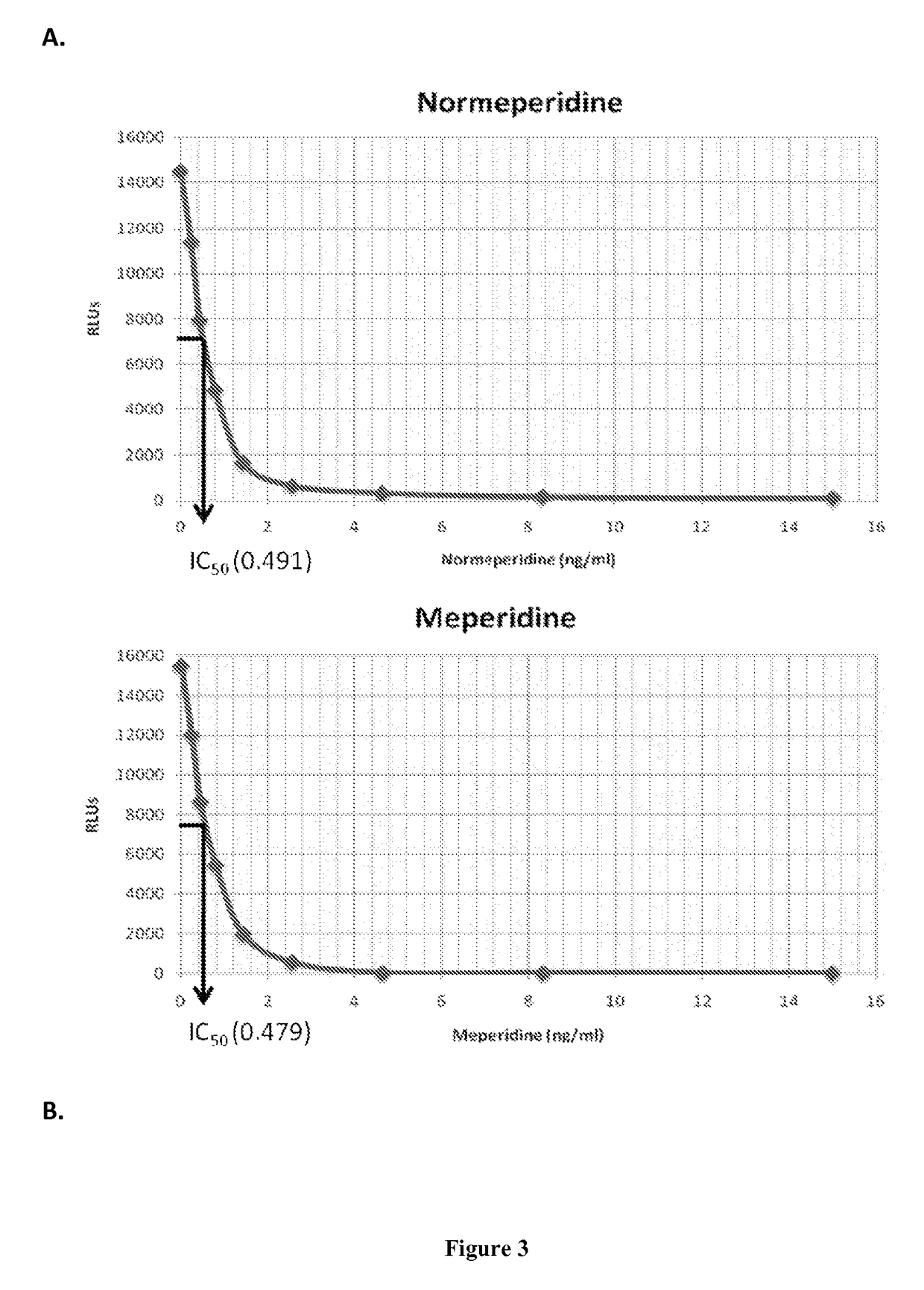
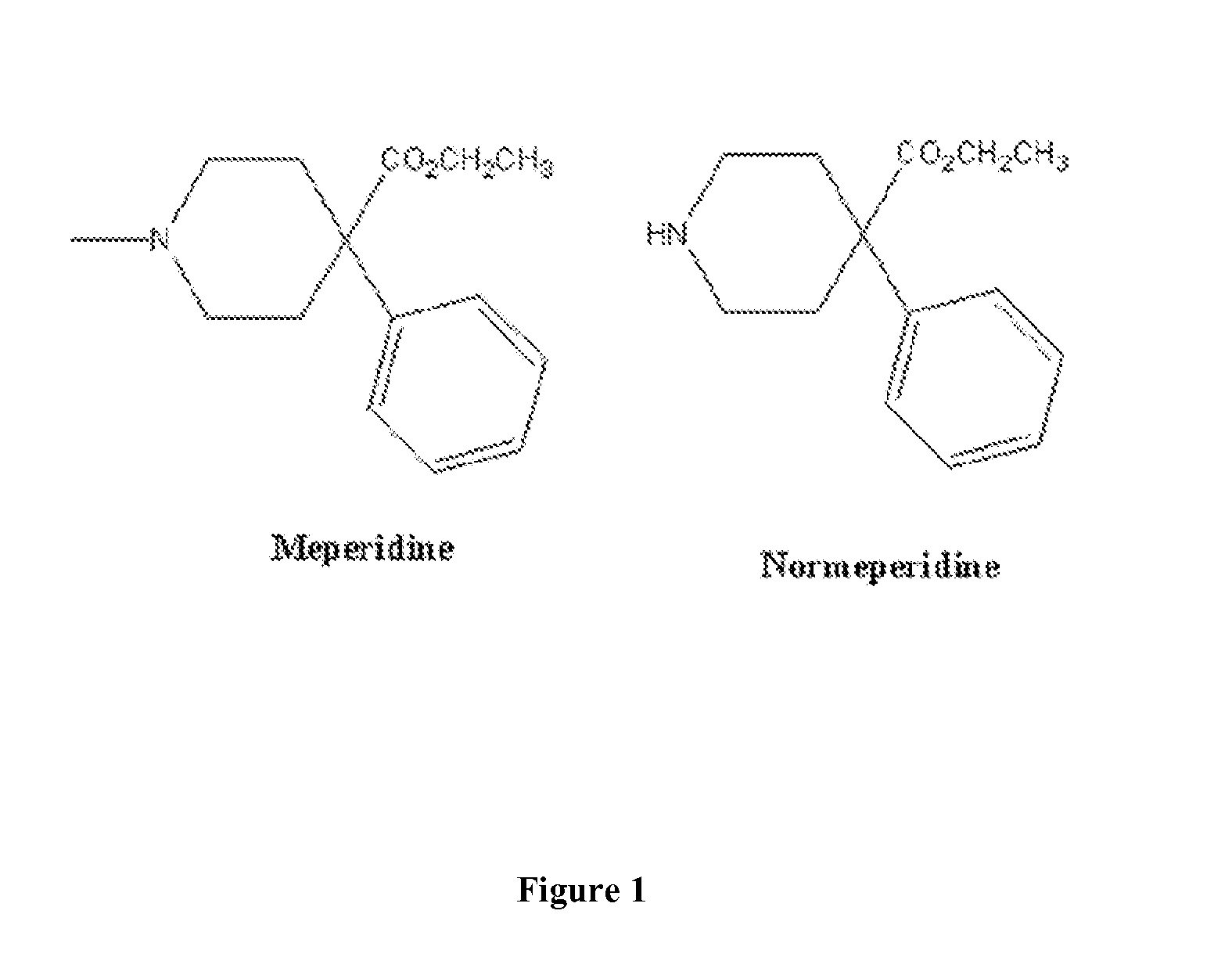
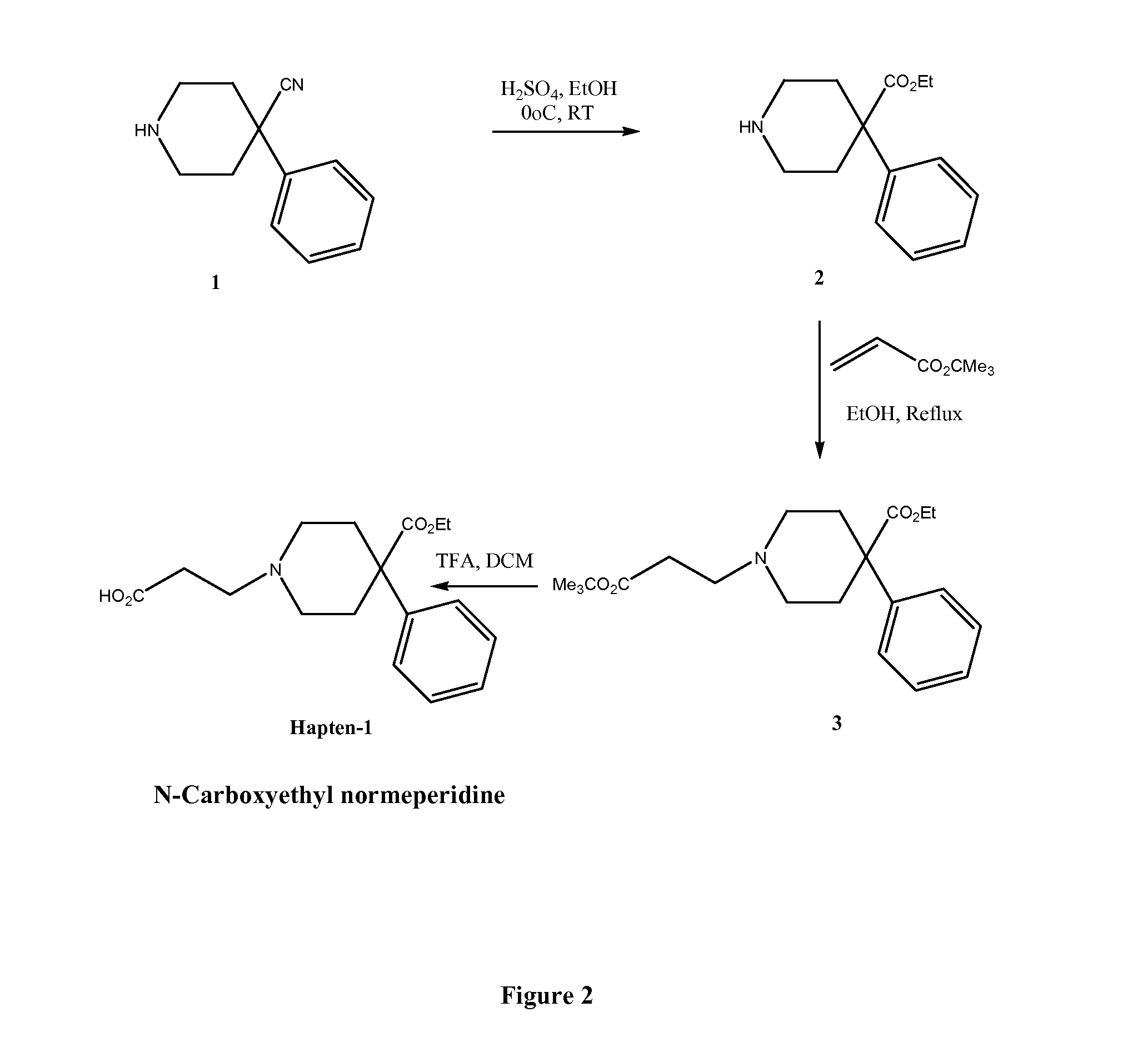
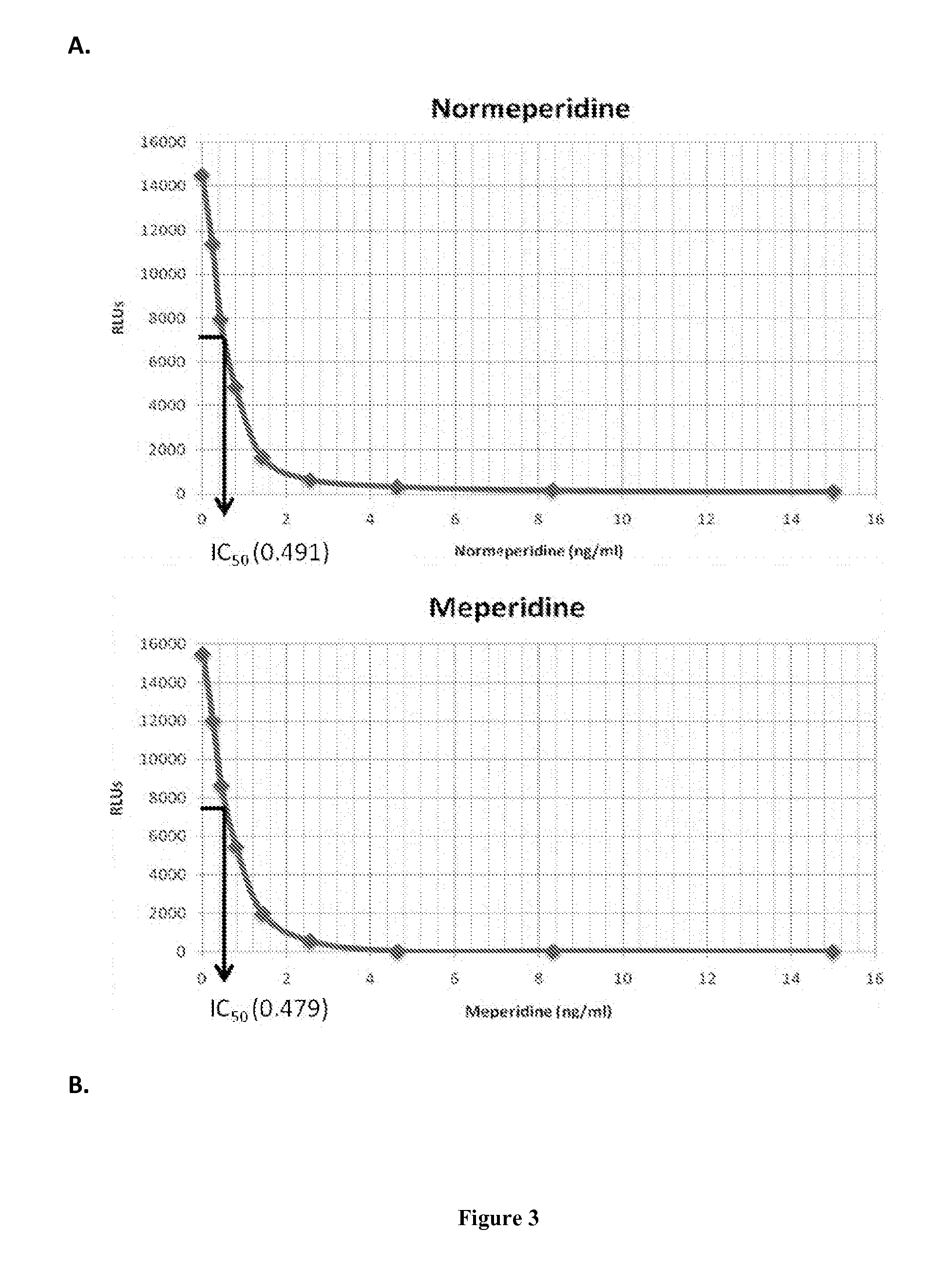
Immunoassays for meperidine and metabolites
The invention provides novel haptens and immunogens for the preparation of novel monoclonal antibodies, which detect the synthetic opioid meperidine and its active metabolite normeperidine. These antibodies enable methods and kits, which are useful in an immunoassay for therapeutic drug monitoring (TDM) and in extending the window of detection for cases of abuse and drug-facilitated sexual assault (DFSA).
Owner:NORTHERN BANK LTD
Immunoassays for meperidine and metabolites
The invention provides novel haptens and immunogens for the preparation of novel monoclonal antibodies, which detect the synthetic opioid meperidine and its active metabolite normeperidine. These antibodies enable methods and kits, which are useful in an immunoassay for therapeutic drug monitoring (TDM) and in extending the window of detection for cases of abuse and drug-facilitated sexual assault (DFSA).
Owner:NORTHERN BANK LTD
His-based intelligent therapeutic drug monitoring whole process management system and its method
ActiveCN107133456BAvoid duplicate detectionGuaranteed pass rateDrug and medicationsInformation repositoryBlood drug concentration
The invention discloses an HIS based intelligent therapeutic drug monitoring whole process management system and a method thereof. The therapeutic drug monitoring whole process management system comprises a management control system, an HIS system, a TDM knowledge database, a prompt information base and a nursing workstation, wherein the management control system comprises a control module as well as an extraction module, a query module and a storage writing module connected with the control module, the output end of the HIS system is connected with the input end of the extraction module, the query module is connected with the TDM knowledge database and the prompt information base, the storage writing module is connected with the TDM knowledge database and the prompt information base, and the nursing workstation is connected with the control module. The system can effectively avoid the repeated detection of drug related genes, ensure the qualified rate of plasma concentration specimens, improve the working efficiency of the staff, and reduce the waste of medical resources fundamentally.
Owner:175TH HOSPITAL OF PEOPLES LIBERATION ARMY
Improved Therapeutic Drug Monitoring
ActiveUS20200190554A1Microbiological testing/measurementIndividual particle analysisMicrobial agentFluorophore
Disclosed herein are methods and compositions for antimicrobial quantification and functional measurement. In one aspect, a method for quantifying antimicrobial comprises: obtaining a biological sample from a patient receiving an antimicrobial; incubating the biological sample with a reference microbial strain and a fluorophore for detecting cell lesion; measuring a first signal of fluorescent intensity in the incubated biological sample using flow cytometry; and comparing the first signal to a calibrating curve previously generated for the antimicrobial, thereby quantifying the antimicrobial present in the biological sample.
Owner:FASTINOV SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com