Blood amiodarone drug concentration monitoring kit and detection method thereof
An amiodarone and kit technology, which is applied to the field of amiodarone therapeutic drug monitoring kits in blood, can solve the problems of high technical requirements for experimenters, inaccurate qualitative and quantitative analysis, long analysis time, etc., so as to reduce errors and personnel operations. The effect of error, simple and fast detection process, and reduced experimental cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
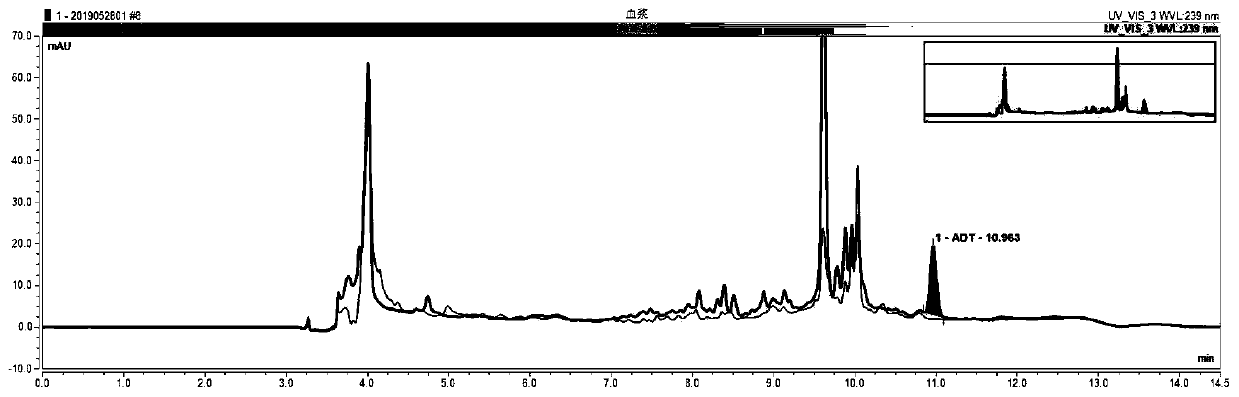
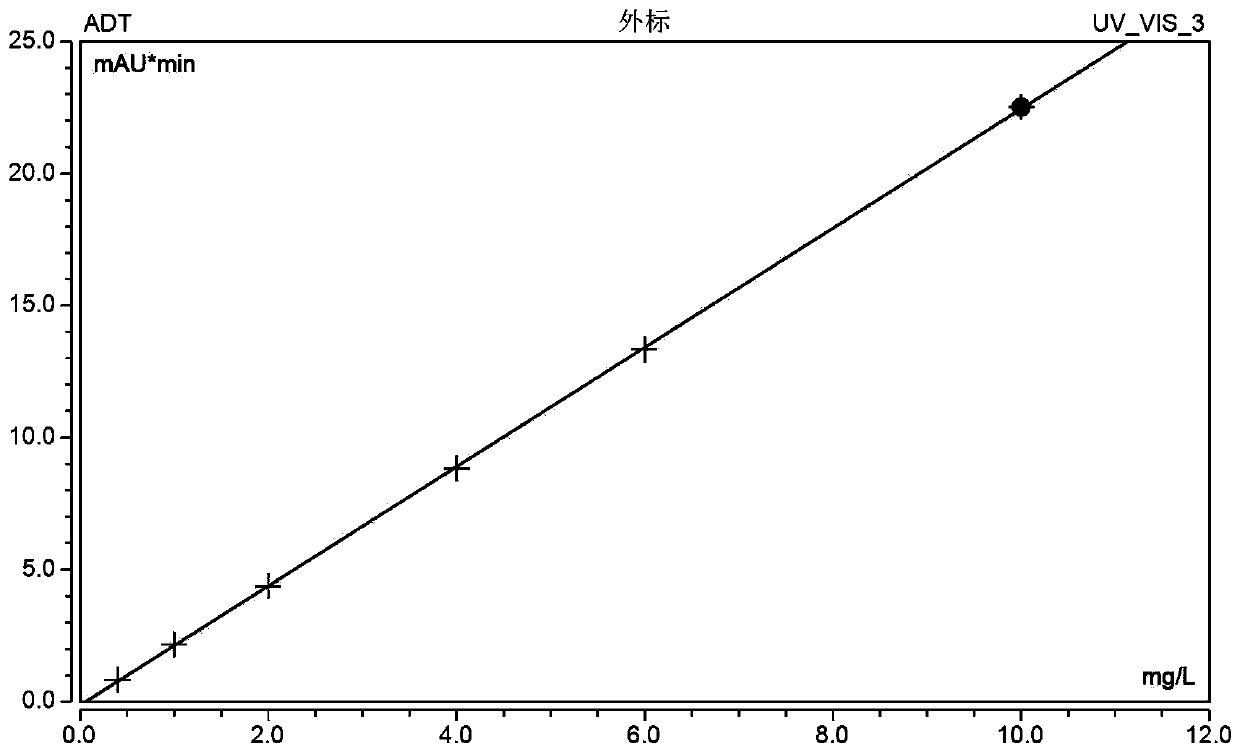
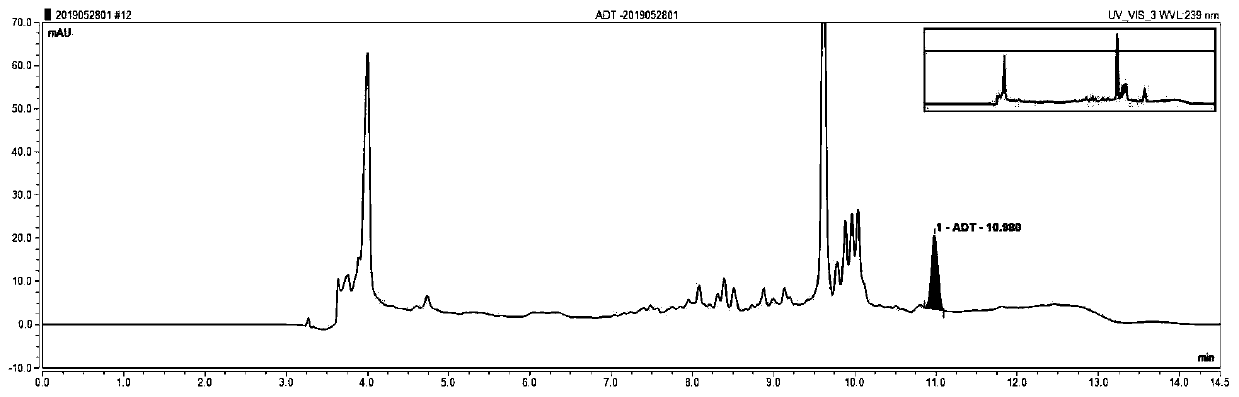
[0035] In order to make the technical means, creative features, goals and effects achieved by the present invention easy to understand, the present invention will be further elaborated below in conjunction with illustrations and specific embodiments.
[0036] One, the preparation of amiodarone therapeutic drug monitoring kit:
[0037] Standard curve reagents: amiodarone is prepared into a series of concentrations with animal serum containing 2% stabilizer and 2% plasticizer, respectively 0.4mg / L, 1.0mg / L, 2.0mg / L, 4.0mg / L, 6.0mg / L, 10.0mg / L, 1.0ml per bottle. The above components were freeze-dried and stored at the bottom of the assay bottle.
[0038] Quality control reagent: amiodarone is formulated with animal serum containing 2% stabilizer and 2% plasticizer to three concentrations of high, medium and low, respectively 0.5mg / L, 3.5mg / L, and 8.0mg / L. The bottle is filled with 1.0ml. The above components were freeze-dried and stored at the bottom of the assay bottle.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration gradient | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com