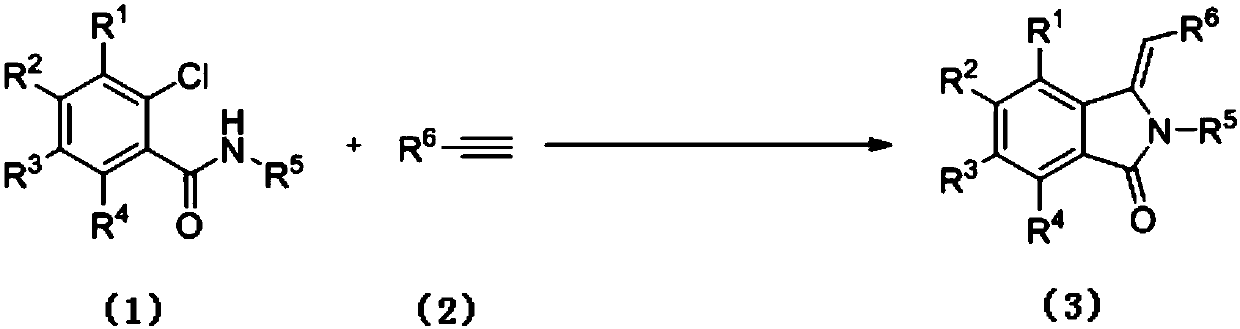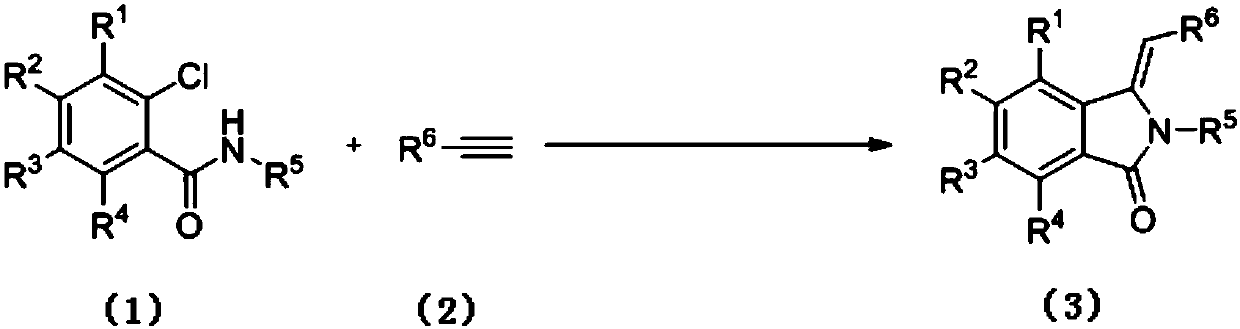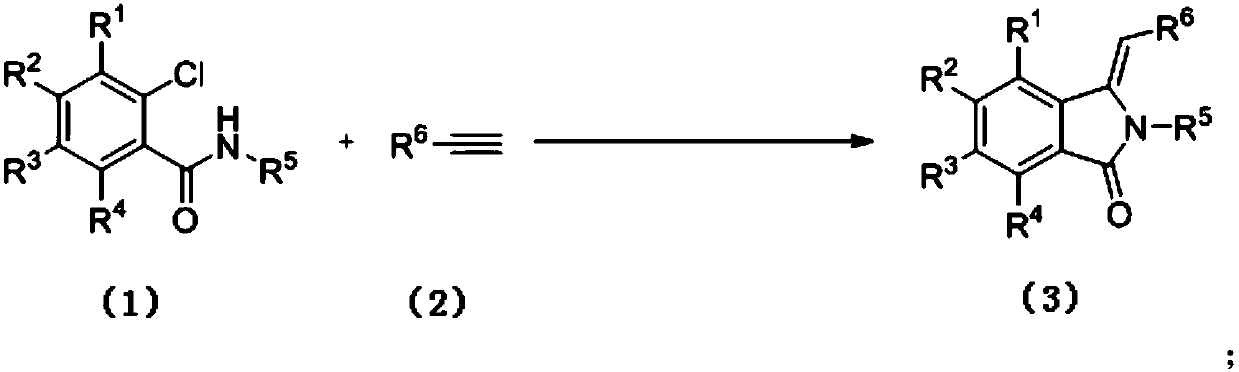Preparation method of 3-methylene isoindole-1-one derivatives
A technology of methylene isoindoles and derivatives, applied in the field of preparation of organic compounds, achieving the effects of high yield, mild reaction conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of 3-benzylidene 2-phenylisoindol-1-ketone (3a), the reaction formula is as follows:
[0037]
[0038] (1) Weigh o-chlorobenzanilide 1a (0.116g, 0.5mmol), phenylacetylene 2 (0.061g, 0.6mmol), 1,10-phylloline (0.009g, 0.05mmol) and dissolve in 2mL acetonitrile , potassium carbonate (0.138 g, 1 mmol), copper oxide (0.004 g, 0.05 mmol) were added. The mixture was heated to 90° C. under the protection of argon, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain compound 3a. Isolated yield was 48%.
[0039] (2) Weigh o-chlorobenzanilide 1a (0.116g, 0.5mmol), phenylacetylene 2 (0.061g, 0.6mmol), 1,10-phylloline (0.009g, 0.05mmol) and dissolve in 2mL acetonitrile , adding potassium carbonate (0.138g, 1mmol), nano copper oxide (0.004g, 0.05mmol). The mixture was heated to 90° C. under the prot...
Embodiment 2
[0063] The synthesis of 3-benzylidene-2-(o-tolyl)isoindol-1-one (3b), the reaction formula is as follows:
[0064]
[0065] Weigh o-chlorobenzoyl-o-toluidine 1b (0.123g, 0.5mmol), phenylacetylene 2 (0.061g, 0.6 mmol), and 1,10-phylloline (0.009g, 0.05mmol) were dissolved in 2mL of acetonitrile, Potassium carbonate (1.0 mmol), nano copper oxide (0.004 g, 0.05 mmol) were added. The mixture was heated to 90° C. under the protection of argon, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain compound 3b. The isolated yield was 86%.
[0066] NMR and high-resolution mass spectrometry are performed on the separated product 3b, as follows:
[0067] 1 H NMR (400MHz, CDCl 3 )δ: 7.95(d, J=7.5Hz, 1H), 7.85(d, J=7.8Hz, 1H), 7.67(t, J=7.4Hz, 1H), 7.55(t, J=7.4Hz, 1H) , 7.05–6.85 (m, 7H), 6.80 (d, J=7.7Hz, 1H), 2.12 (...
Embodiment 3
[0071] The synthesis of 3-benzylidene-2-(p-tolyl)isoindol-1-one (3c) has the following reaction formula:
[0072]
[0073] Weigh o-chlorobenzoyl-p-toluidine 1c (0.123g, 0.5mmol), phenylacetylene 2 (0.061g, 0.6mmol), and 1,10-phylloline (0.009g, 0.05mmol) were dissolved in 2mL of acetonitrile, Potassium carbonate (0.138g, 1mmol) and nano copper oxide (0.004g, 0.05mmol) were added. The mixture was heated to 90° C. under the protection of argon, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain compound 3c. The isolated yield was 87%.
[0074] NMR and high-resolution mass spectrometry are performed on the separated product 3c, as follows:
[0075] 1 H NMR (400MHz, DMSO) δ: 7.93(d, J=7.6Hz, 1H), 7.83(d, J=7.7Hz, 1H), 7.65(t, J=7.5Hz, 1H), 7.52(t, J =7.4Hz, 1H), 7.00–6.82(m, 9H), 6.80(s, 1H), 2.21(s, 3H).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com