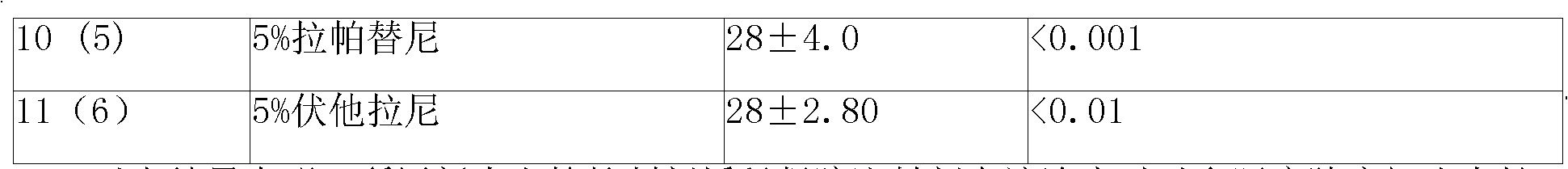Anticancer sustained-release gel injection
A slow-release gel injection and slow-release microsphere technology, which is applied in the direction of anti-tumor drugs, medical preparations of non-active ingredients, pharmaceutical formulas, etc., can solve the problem of tumor cell planting or dissemination, tissue trauma, and tumor cell ineffectiveness. Clear etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
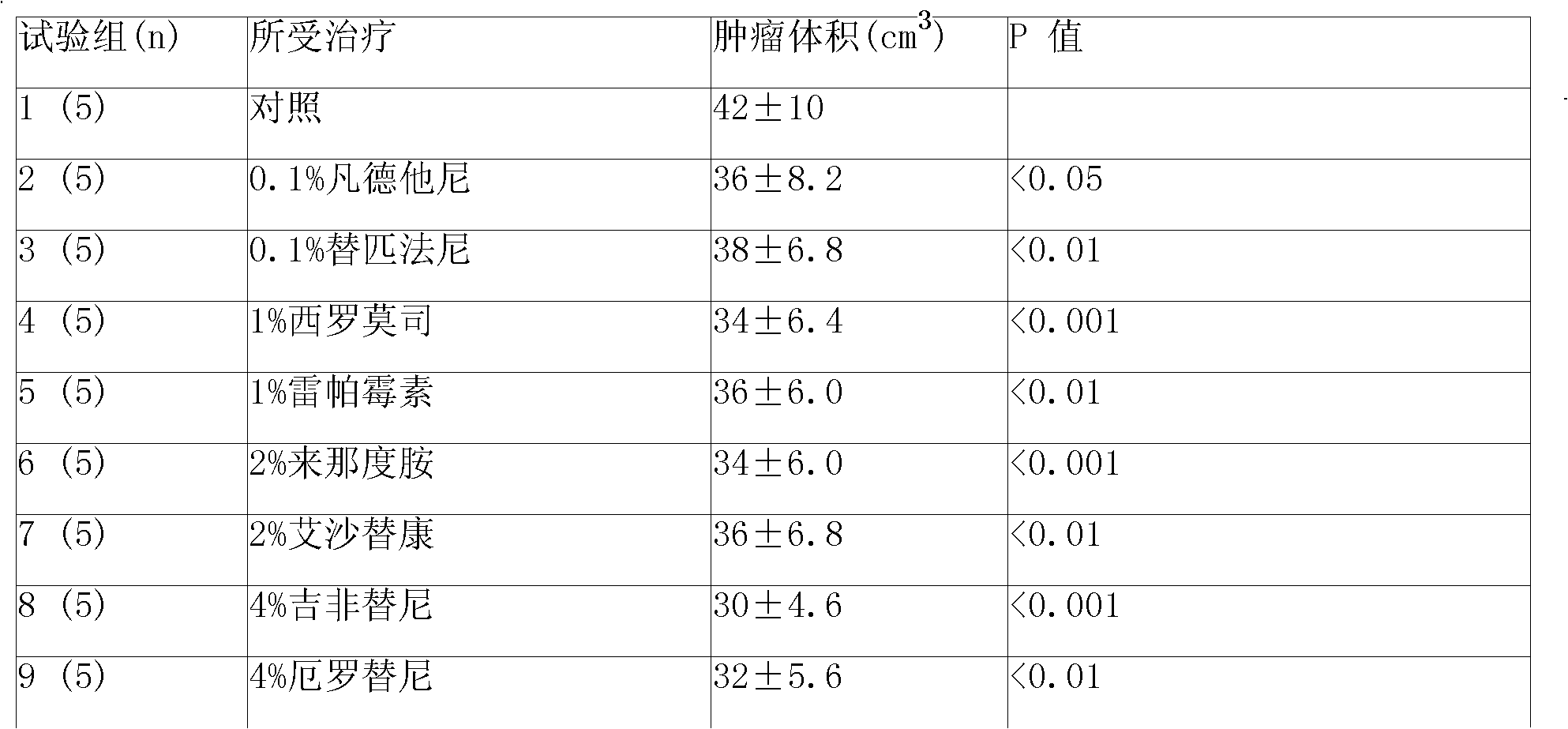
[0073] Put 4, 2, 1 and 0.5g of amphiphilic block copolymers (PLGA-PEG-PLGA) into four containers of A, B, C and D respectively, and then pour them into four containers of A, B, C and D respectively Add 6, 8, 9 and 9.5 milliliters of water for injection into the container to prepare 40%, 20%, 10% and 5% hydrogels.
[0074] The molecular weight of polyethylene glycol in the amphiphilic block copolymer is 800-1200, accounting for 20% of the weight of the amphiphilic block copolymer; in the glycolide-lactide copolymer, the ratio of glycolide and lactide The molar ratio is 6:1.
[0075] The preparation of microspheres is prepared by double emulsion method or O / W method. The auxiliary material in the sustained-release microspheres is polylactic acid / glycolic acid copolymer, wherein the blending ratio of lactic acid (LA) and glycolic acid (GA) can be 75 / 25 (W / W), the molecular weight of the copolymer of lactic acid and glycolic acid (PLGA) can be 15,000-38,000, and the weight ratio...
Embodiment 2
[0077] Measure the gelation temperature of four kinds of hydrogels in embodiment 1, the result shows that the gelation temperature of 40% and 20% hydrogel is respectively 31 ℃ (40%) and 35 ℃ (20%), and 10 The gelation temperatures of the % and 5% hydrogels were not determined at 10°C-38°C. After adding 5% slow-release microspheres (W / W) to the hydrogel, measure the gelation temperature of four kinds of hydrogels in Example 1, the results show that the gelation temperature of 40% and 20% hydrogels 33°C (40%) and 37.5°C (20%), respectively, while the gelation temperatures of 10% and 5% hydrogels were not detected at 10°C-39°C.
Embodiment 3
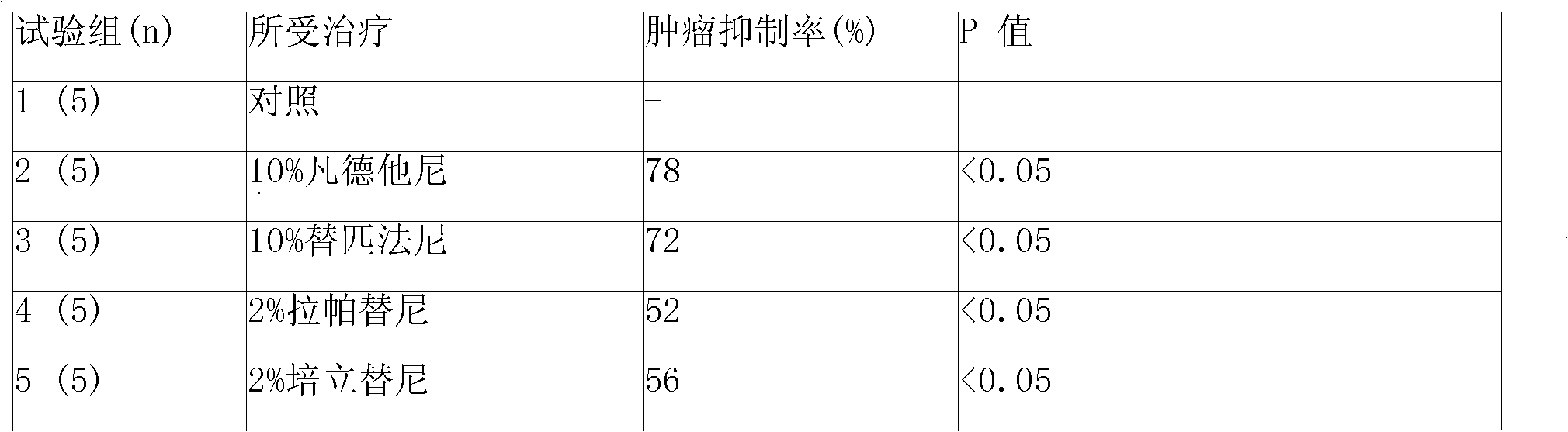
[0079] Put 4, 2, 1 and 0.5g of amphiphilic block copolymers (PLGA-PEG-PLGA) into four containers of A, B, C and D respectively, and then pour them into four containers of A, B, C and D respectively Add 6, 8, 9 and 9.5 milliliters of water for injection into the container to prepare 40%, 20%, 10% and 5% hydrogels.
[0080] The molecular weight of polyethylene glycol in the amphiphilic block copolymer is 1200-1600, accounting for 15% of the weight of the amphiphilic block copolymer; in the glycolide-lactide copolymer, the ratio of glycolide and lactide The molar ratio is 4:1.
[0081] The preparation of microspheres is prepared by double emulsion method or O / W method. The auxiliary material in the sustained-release microspheres is polylactic acid / glycolic acid copolymer, wherein the blending ratio of lactic acid (LA) and glycolic acid (GA) can be 75 / 25 (W / W), the molecular weight of the copolymer of lactic acid and glycolic acid (PLGA) can be 25,000-35,000, and the weight rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com