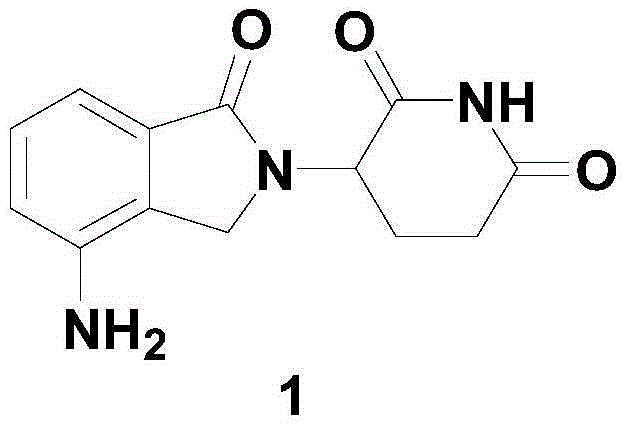
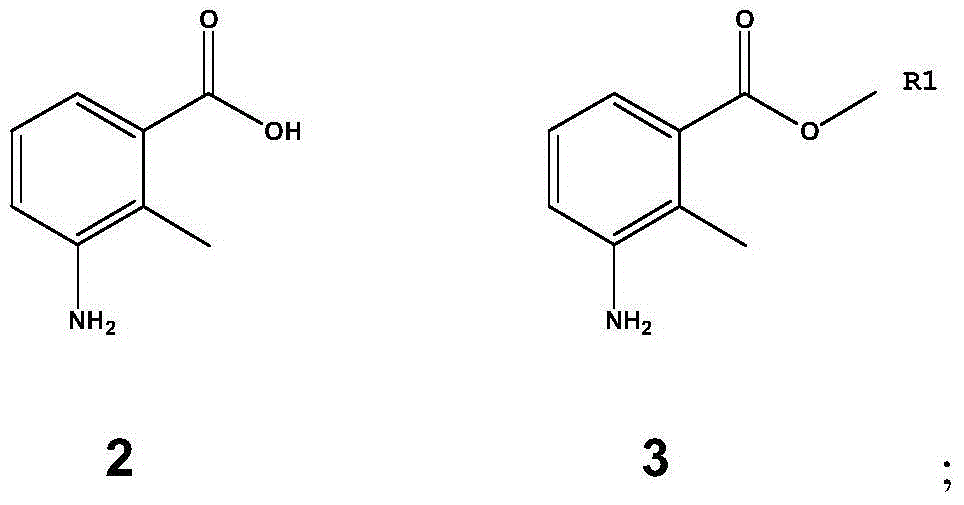
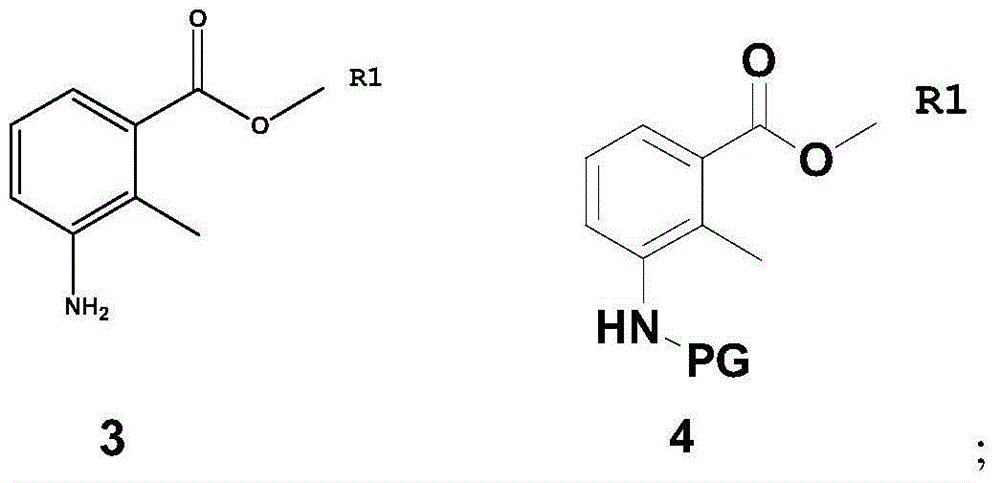
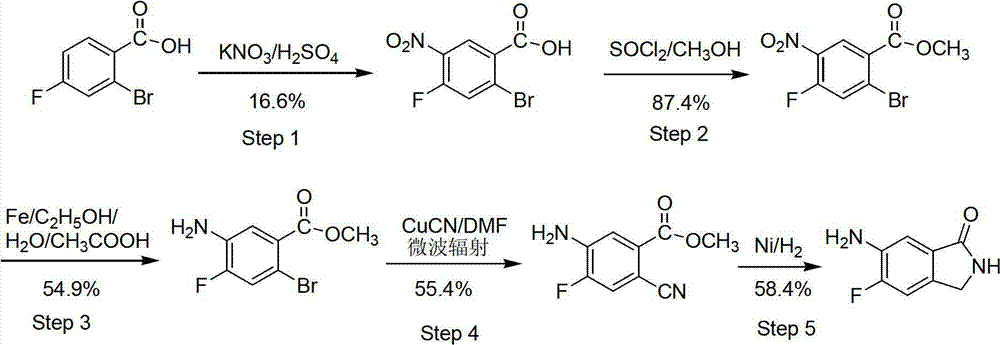
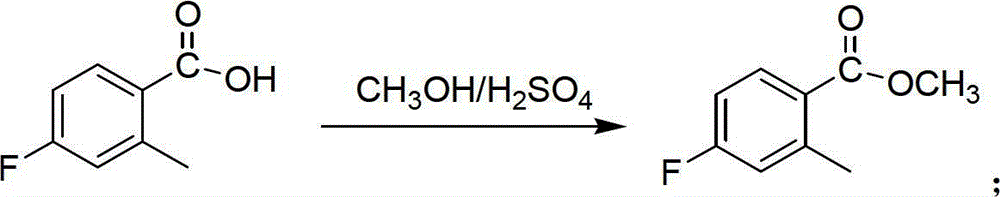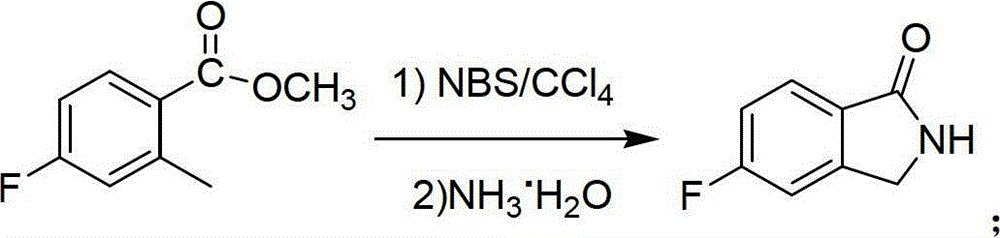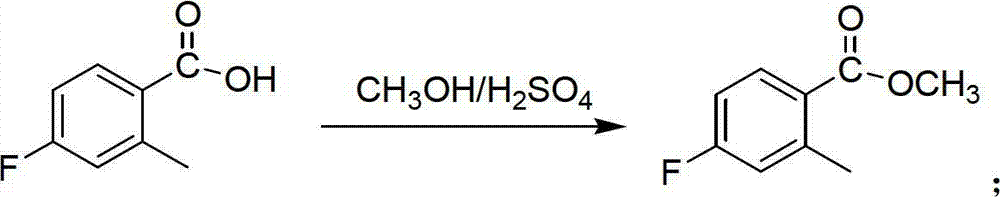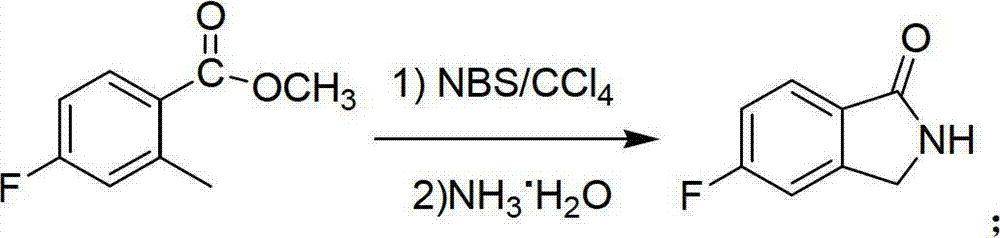Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "2-methylbenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
O-Toluic acid, also 2-methylbenzoic acid, is an aromatic carboxylic acid, with formula (CH3)C6H4(COOH). It is an isomer of p-toluic acid and m-toluic acid.
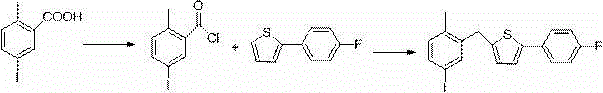
New synthesis process of canagliflozin
The present invention discloses a new synthesis process of canagliflozin. The new synthesis process comprises: adopting 2-methyl benzoic acid as a starting raw material, and adopting a self-made catalyst, iodic acid and iodine to carry out a reaction to produce an intermediate 1, or adopting 2-methyl benzoic acid as a starting raw material, and adding liquid bromine under effects of a metal reagent and a catalyst to synthesize an intermediate 2; optionally selecting the intermediate 1 or 2 to carry out an acylation reaction with thionyl chloride, and then carrying out a Friedel-Crafts reaction to produce an intermediate 3; adopting ALPHA-D-glucose as a raw material, carrying out a reaction with pivaloyl chloride to protect all hydroxyl, and carrying out a reaction with zinc bromide and bromotrimethylsilane to produce an intermediate 4; linking the intermediate 3 and the intermediate 4 to produce an intermediate 5; and finally under an acid condition, removing the pivaloyl to produce the target compound. The new synthesis process has characteristics of high yield, mild condition, safety, reliability, cheap and easily available raw material, and easy production cost control, and is suitable for industrial production.
Owner:HAIMEN RUIYI MEDICAL TECH
Soldering tin paste
InactiveCN1422723AGood spreadabilityPrinted circuit assemblingWelding/cutting media/materialsCarboxylic acidSolder paste
A lead-free solder paste comprises an Sn-Zn based lead-free solder powder mixed with a flux. The flux contains at least one aromatic hydroxycarboxylic acid selected from the group consisting of aromatic carboxylic acids having one hydroxyl group in a meta position (such as 3-hydroxy-2-methylbenzoic acid) and aromatic carboxylic acids having at least two hydroxyl groups (such as dihydroxynaphthoic acid or dihydroxybenzoic acid) in an amount of 0.1-10.0 mass %. The flux may further include 0.5-20 mass % of an aliphatic hydroxy carboxylic acid (such as hydroxyoleic acid).
Owner:SENJU METAL IND CO LTD +1
High shear system and method for the production of acids
InactiveUS20100324308A1Physical/chemical process catalystsOrganic compound preparationBenzoic acidMean diameter
Herein disclosed is a method, comprising: forming a dispersion under high shear comprising gas bubbles of an oxidant dispersed in a liquid phase, wherein the bubbles have a mean diameter of less than 1.5 micron; and contacting the dispersion with an oxidation catalyst to produce a product stream, wherein the product stream comprises a substance selected from the group consisting of dicarboxylic acid, benzoic acid, 2-methylbenzoic acid, 3-methylbenzoic acid, 4-methylbenzoic acid, and phthalic anhydride. In some cases, forming the dispersion under high shear comprises introducing the oxidant and the liquid phase into a high shear device comprising at least one rotor and at least one complementarily-shaped stator. Herein also disclosed is a system for producing a substance selected from the group consisting of dicarboxylic acid, benzoic acid, 2-methylbenzoic acid, 3-methylbenzoic acid, 4-methylbenzoic acid, and phthalic anhydride.
Owner:HRD CORP
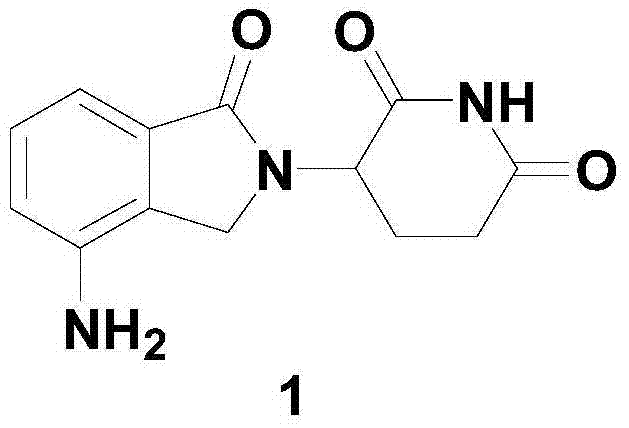
Method for preparing lenalidomide
ActiveCN103497175AHigh purityEfficient manufacturingOrganic chemistryBulk chemical productionPhotochemistry2-methylbenzoic acid
The invention discloses a method for preparing lenalidomide. The method comprises the step of synthesizing lenalidomide by taking 3-amino-2-methylbenzoic acid as an initial raw material. By utilizing the method for preparing lenalidomide, lenalidomide can be effectively prepared, the method is simple in technology, and high in synthesis efficiency, and the purity of prepared lenalidomide is higher. In addition, the preparation method has the advantages of being easily available for the initial raw material, simple for technological operation and mild in reaction conditions, dispensing with special reaction equipment, and having no difficultly separable compounds in the preparation process and the like, thus being more suitable for producing lenalidomide industrially on a large scale.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
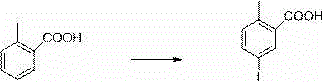
Process for producing 5-iodo-2-methylbenzoic acid
InactiveCN1812954AImprove efficiencyExtended service lifeOrganic compound preparationOrganic chemistry methodsAcetic anhydrideDistillation
The present invention provides a process for producing 5-iodo-2-methylbenzoic acid by iodizing 2-methylbenzoic acid, which comprises, as essential steps, a reaction step in which 2-methylbenzoic acid is iodized in the presence of a microporous compound, iodine, an oxidizing agent, and acetic anhydride and a purification step in which sublimation, distillation, crystallization, or a combination of two or more of these is conducted. By the process, 5-iodo-2-methylbenzoic acid, which is useful in functional chemicals such as medicines, can be easily obtained as a high-purity compound in a high yield. The production steps comprising reaction and separation / purification are simple from the standpoint of process operation and the purification load is small. Furthermore, the microporous compound, e.g., a zeolite catalyst, separated and recovered from the liquid resulting from the reaction can be repeatedly used after a simple treatment. Consequently, the catalyst has a long life and the target compound can be produced by the efficient process.
Owner:MITSUBISHI GAS CHEM CO INC
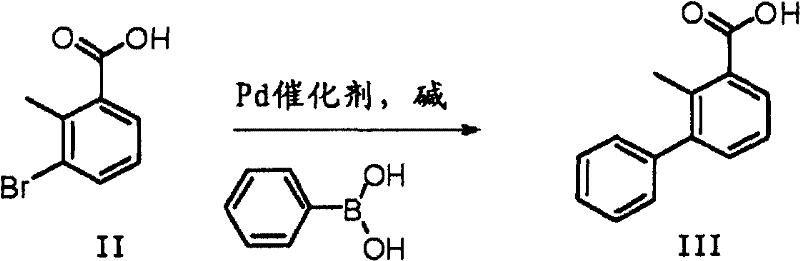
Preparation method of 2-methyl-3-biphenylmethanol
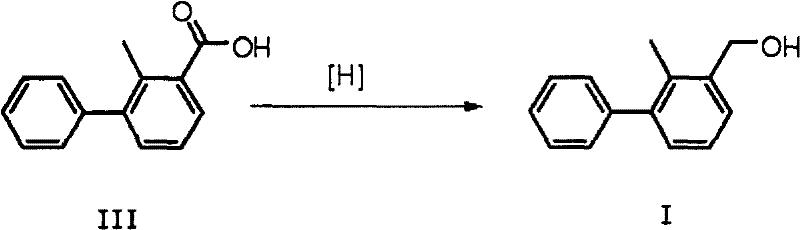
InactiveCN102603485ASimple processRelaxed reaction conditionsOrganic compound preparationHydroxy compound preparationBromineBoric acid
The invention discloses a preparation method of 2-methyl-3-biphenylmethanol, which comprises the following steps: carrying out a Suzuki coupling reaction between 3-bromo-2-methylbenzoic acid and phenyl substituted boric acid or phenyl substituted borate to obtain 3-phenyl-2-methylbenzoic acid, and then carrying out a reduction reaction to obtain 2-methyl-3-biphenylmethanol, wherein the Suzuki coupling reaction is carried out at 10-150 DEG C for 1-12 hours under the action of an alkali. 3-phenyl-2-methylbenzoic acid can be directly reduced with a reducing agent such as borane and lithium aluminium hydride to obtain 2-methyl-3-biphenylmethanol.
Owner:NUTRICHEM LAB CO LTD
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveUS20060161028A1High purityCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:MITSUBISHI GAS CHEM CO INC
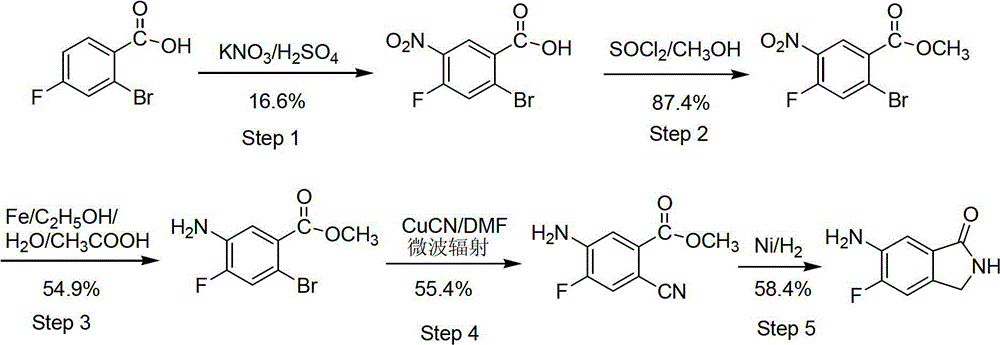
Preparation method of 6-amino-5-fluorine-1-isoindolinone
ActiveCN102911109ARaw materials are cheap and easy to getLow costOrganic chemistryAntibiosisBenzoic acid
The invention provides a preparation method of 6-amino-5-fluorine-1-isoindolinone, which comprises the steps of taking 4-fluorine-2-methyl benzoic acid as a raw material, obtaining 5-fluorine-1-isoindolinone through esterification, bromination and cyclization, and obtaining 6-amino-5-fluorine-1-isoindolinone by nitrating and reducing the 5-fluorine-1-isoindolinone. According to the preparation method, the synthetic route is simple, the operation is easy, raw materials are cheap and easy to obtain, the reaction condition is mild, intermediates and products are easy to separate, the productivity is higher, and the preparation method is suitable for industrialized production. The prepared 6-amino-5-fluorine-1-isoindolinone pharmaceutical intermediate has a wide application value in pharmaceutical chemicals, biological a cancer fighting and antibiosis, pesticides and the like.
Owner:SHANXI MEDICAL UNIV
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveCN1747910AHigh purity productHigh purityCarboxylic acid nitrile preparationMolecular sieve catalystsOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:日本精细化工株式会社
Preparation method of canagliflozin hemihydrate and monocrystal thereof
InactiveCN104744449ASynthetic operation is simpleImprove securityOrganic chemistryBenzoic acidMethanol water
The invention relates to a preparation method of canagliflozin hemihydrate and monocrystal thereof, belonging to the technical field of canagliflozin hemihydrate. The method comprises the following steps: crystallizing the raw material 5-iodo-2-methyl benzoic acid by using canagliflozin hemihydrate monocrystal as a crystal seed to prepare the canagliflozin hemihydrate, and carrying out solvent volatilization in a methanol-water solvent environment to prepare the canagliflozin hemihydrate monocrystal. The preparation method of the canagliflozin hemihydrate is simple, has the advantages of high yield and low cost, and is suitable for industrial production. The preparation method of the canagliflozin hemihydrate monocrystal is simple and convenient, and can obtain the high-quality monocrystal compound.
Owner:BEIJING UNIV OF TECH
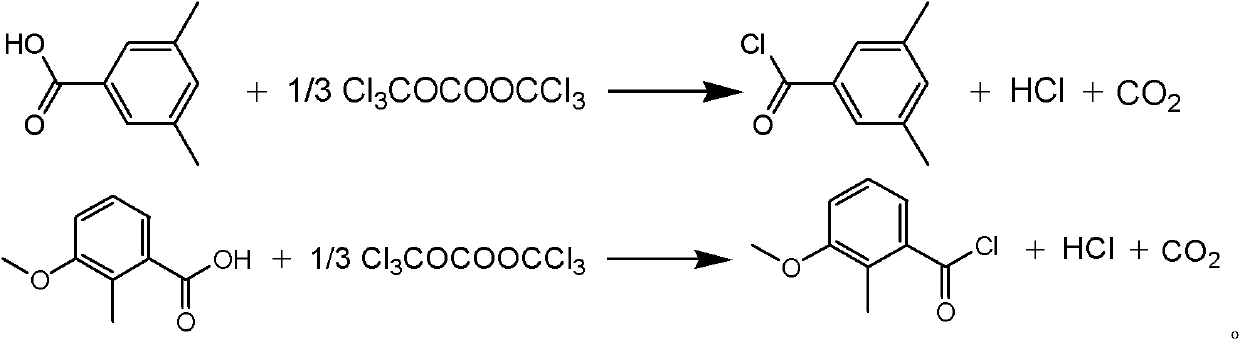
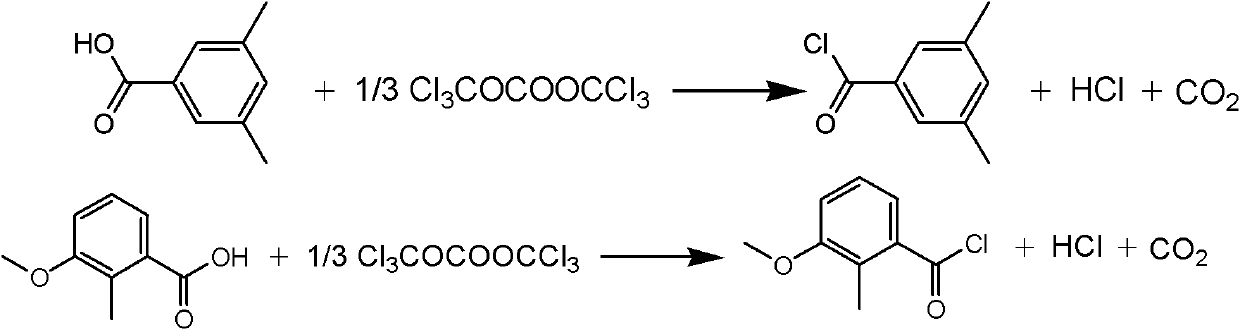
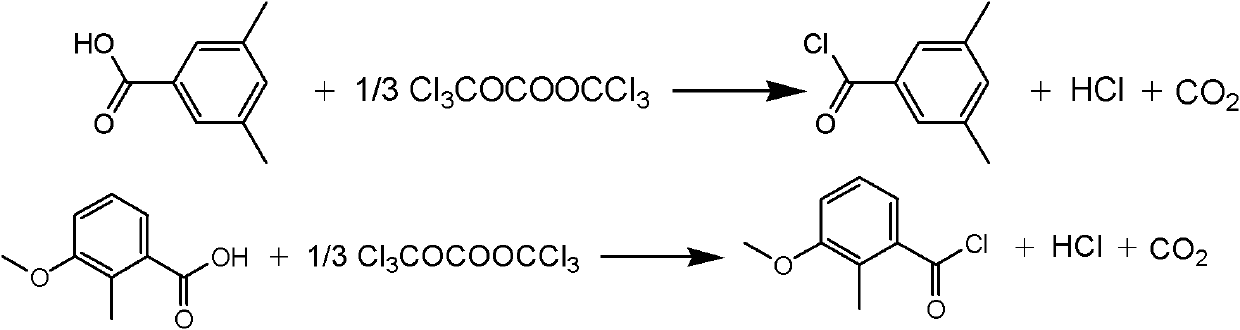
Synthesis method of methoxyfenozide key intermediate-substituted methyl benzoyl chloride
InactiveCN102584573AEmission reductionHigh yieldOrganic compound preparationCarboxylic compound preparationBenzoic acidOrganic solvent
The invention discloses a synthesis method of methoxyfenozide key intermediate-substituted methyl benzoyl chloride. The corresponding substituted methyl benzoyl chloride is obtained by reacting 3,5-mesitylenic acid or 3-methoxyl-2-methyl benzoic acid with di(trichloromethyl)carbonic ester under the action of an organic amine catalyst in an organic solvent according to a chemical equation. The method has the advantages of mild reaction condition, safe and reliable operating process, high product yield, elimination of potential safety hazard from a process source, reduction in three waste generating sources, and high industrial implementation value.
Owner:HANGZHOU VOCATIONAL & TECHN COLLEGE
Co-production method of 3-nitro-2-methylbenzoic acid and 3-nitrophthalic acid
ActiveCN111362806ALow toxicityReduce pollutionOrganic chemistryOrganic compound preparationO-XyleneWastewater
The invention discloses a co-production method of 3-nitro-2-methylbenzoic acid and 3-nitrophthalic acid. The co-production method comprises the following steps: (1) oxidizing 3-nitro-o-xylene into 3-nitro-2-methylbenzoic acid in nitric acid in the presence of a catalyst and an initiator by using oxygen as an oxidant; (2) after the reaction is finished, filtering the reaction product when the reaction product is still hot to obtain a 3-nitro-2-methylbenzoic acid crude product and mother liquor; (3) re-crystallizing the 3-nitro-2-methylbenzoic acid crude product to obtain a 3-nitro-2-methylbenzoic acid finished product; and (4) further oxidizing the mother liquor obtained in the step (2) to prepare 3-nitro-phthalic acid, cooling and filtering to obtain a 3-nitrophthalic acid crude product after the reaction is finished, applying the obtained filtrate to the step of oxidizing 3-nitrophthalic acid, and re-crystallizing the obtained crude product to obtain the 3-nitrophthalic acid finishedproduct. The problems of large amount of waste water and waste salt, difficult post-treatment and serious environmental pollution in the existing process are solved.
Owner:JIANGSU YONGAN CHEM CO LTD
Process for preparing lenalidomide
InactiveCN103497175BHigh purityHigh synthesis efficiencyOrganic chemistryBulk chemical productionPhotochemistry2-methylbenzoic acid
The invention discloses a method for preparing lenalidomide. The method uses 3-amino-2-methylbenzoic acid as a starting material to synthesize lenalidomide. The method for preparing lenalidomide of the present invention can effectively prepare lenalidomide, and the process is simple, the synthesis efficiency is high, and the purity of the prepared lenalidomide is high. In addition, the preparation method also has the advantages of easy availability of starting materials, simple process operation, mild reaction conditions, no need for special reaction equipment, and no difficult-to-separate compounds in the preparation process, so it is more suitable for large-scale and industrial production of lenalidomide .
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Process for producing 5-iodo-2-methylbenzoic acid
InactiveUS7642374B2High selectivityOrganic compound preparationCarboxylic compound separation/purificationAcetic anhydrideDistillation
The present invention provides a process for producing 5-iodo-2-methylbenzoic acid through iodination of 2-methylbenzoic acid, the process including, as essential steps, a reaction step of iodinating 2-methylbenzoic acid in the presence of a microporous compound, iodine, an oxidizing agent, and acetic anhydride, and a purification step including sublimation, distillation, crystallization, or a combination of two or more of these. According to the present invention, 5-iodo-2-methylbenzoic acid, which is useful for producing functional chemicals such as drugs, can be produced at high purity and high yield in a simple manner. Since the production process includes a simple reaction step and a simple separation / purification step, the load of purification is mitigated. In addition, the microporous compound such as a zeolite catalyst which has been separated and recovered from the reaction mixture can be repeatedly employed after performing of a simple treatment. Thus, the production process ensures a long service life of catalysts and high efficiency.
Owner:MITSUBISHI GAS CHEM CO INC
Process for producing 5-iodo-2-methylbenzoic acid
InactiveUS20060167312A1High selectivityHigh yieldOrganic compound preparationCarboxylic compound separation/purificationAcetic anhydrideDistillation
The present invention provides a process for producing 5-iodo-2-methylbenzoic acid through iodination of 2-methylbenzoic acid, the process including, as essential steps, a reaction step of iodinating 2-methylbenzoic acid in the presence of a microporous compound, iodine, an oxidizing agent, and acetic anhydride, and a purification step including sublimation, distillation, crystallization, or a combination of two or more of these. According to the present invention, 5-iodo-2-methylbenzoic acid, which is useful for producing functional chemicals such as drugs, can be produced at high purity and high yield in a simple manner. Since the production process includes a simple reaction step and a simple separation / purification step, the load of purification is mitigated. In addition, the microporous compound such as a zeolite catalyst which has been separated and recovered from the reaction mixture can be repeatedly employed after performing of a simple treatment. Thus, the production process ensures a long service life of catalysts and high efficiency.
Owner:MITSUBISHI GAS CHEM CO INC
Soldering tin paste
InactiveCN1267243CGood spreadabilityPrinted circuit assemblingWelding/cutting media/materialsCarboxylic acidSolder paste
A lead-free solder paste comprises an Sn-Zn based lead-free solder powder mixed with a flux. The flux contains at least one aromatic hydroxycarboxylic acid selected from the group consisting of aromatic carboxylic acids having one hydroxyl group in a meta position (such as 3-hydroxy-2-methylbenzoic acid) and aromatic carboxylic acids having at least two hydroxyl groups (such as dihydroxynaphthoic acid or dihydroxybenzoic acid) in an amount of 0.1-10.0 mass %. The flux may further include 0.5-20 mass % of an aliphatic hydroxy carboxylic acid (such as hydroxyoleic acid).
Owner:SENJU METAL IND CO LTD +1
Preparation method of 6-amino-5-fluorine-1-isoindolinone
ActiveCN102911109BRaw materials are cheap and easy to getLow costOrganic chemistryBenzoic acidMedicinal chemistry
The invention provides a preparation method of 6-amino-5-fluorine-1-isoindolinone, which comprises the steps of taking 4-fluorine-2-methyl benzoic acid as a raw material, obtaining 5-fluorine-1-isoindolinone through esterification, bromination and cyclization, and obtaining 6-amino-5-fluorine-1-isoindolinone by nitrating and reducing the 5-fluorine-1-isoindolinone. According to the preparation method, the synthetic route is simple, the operation is easy, raw materials are cheap and easy to obtain, the reaction condition is mild, intermediates and products are easy to separate, the productivity is higher, and the preparation method is suitable for industrialized production. The prepared 6-amino-5-fluorine-1-isoindolinone pharmaceutical intermediate has a wide application value in pharmaceutical chemicals, biological a cancer fighting and antibiosis, pesticides and the like.
Owner:SHANXI MEDICAL UNIV
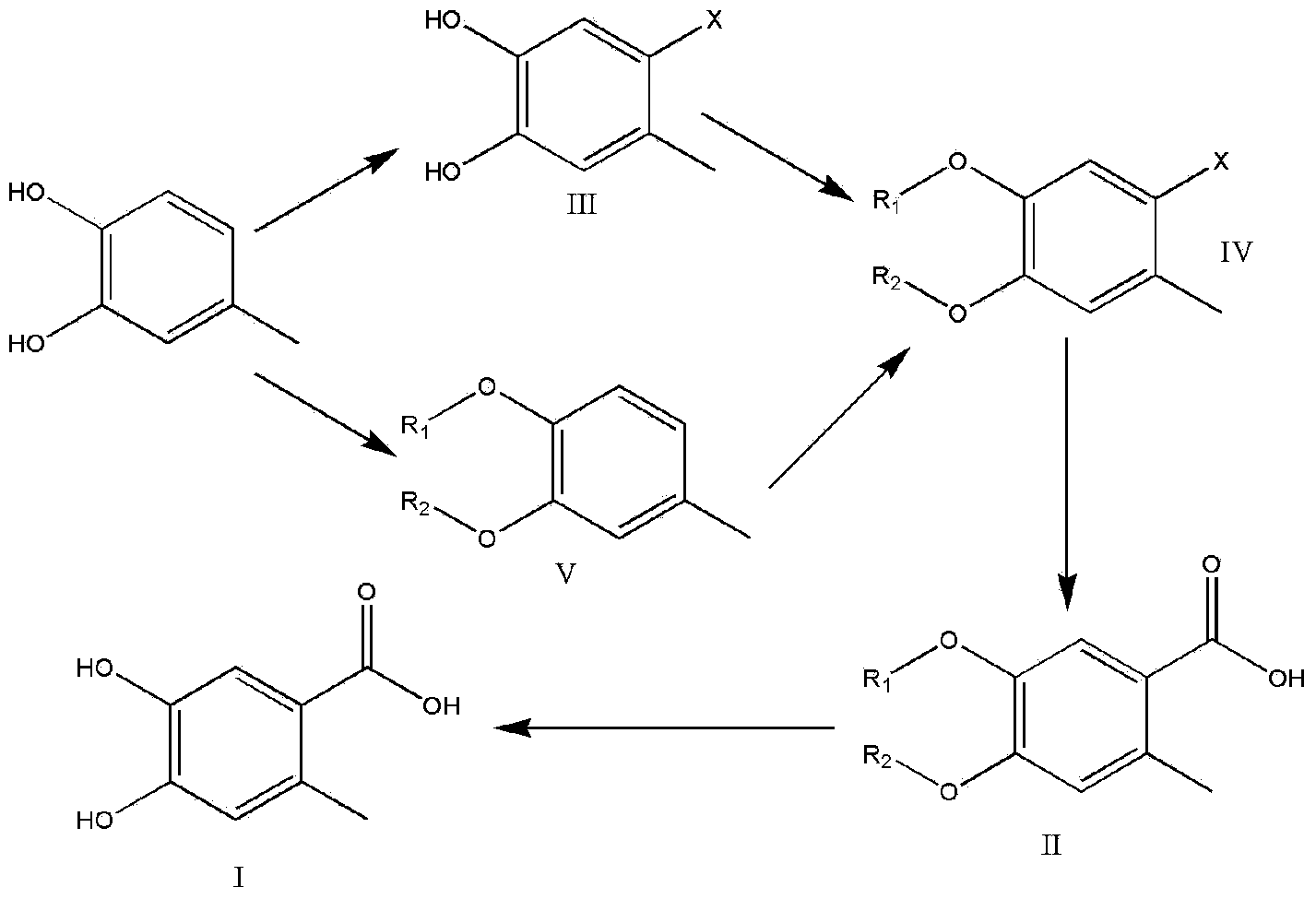
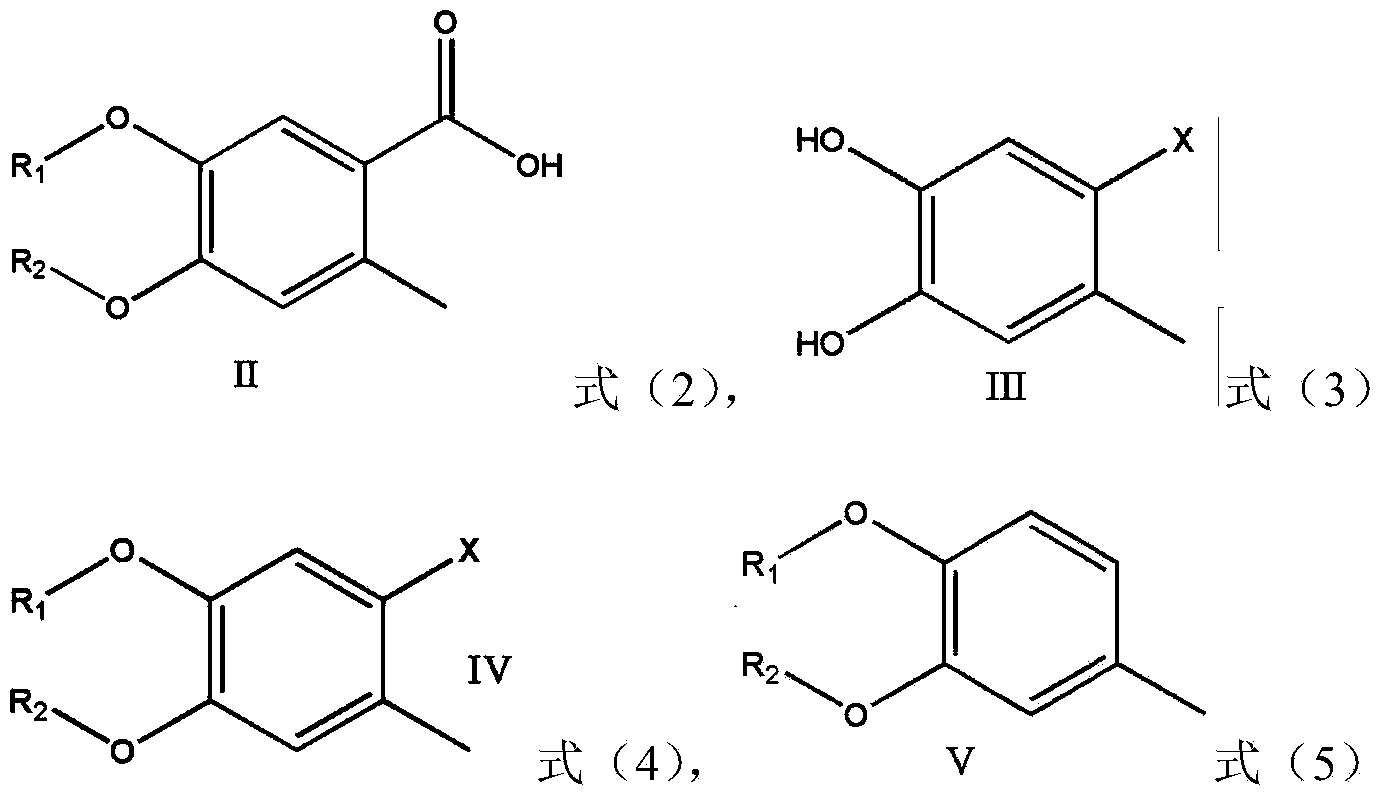
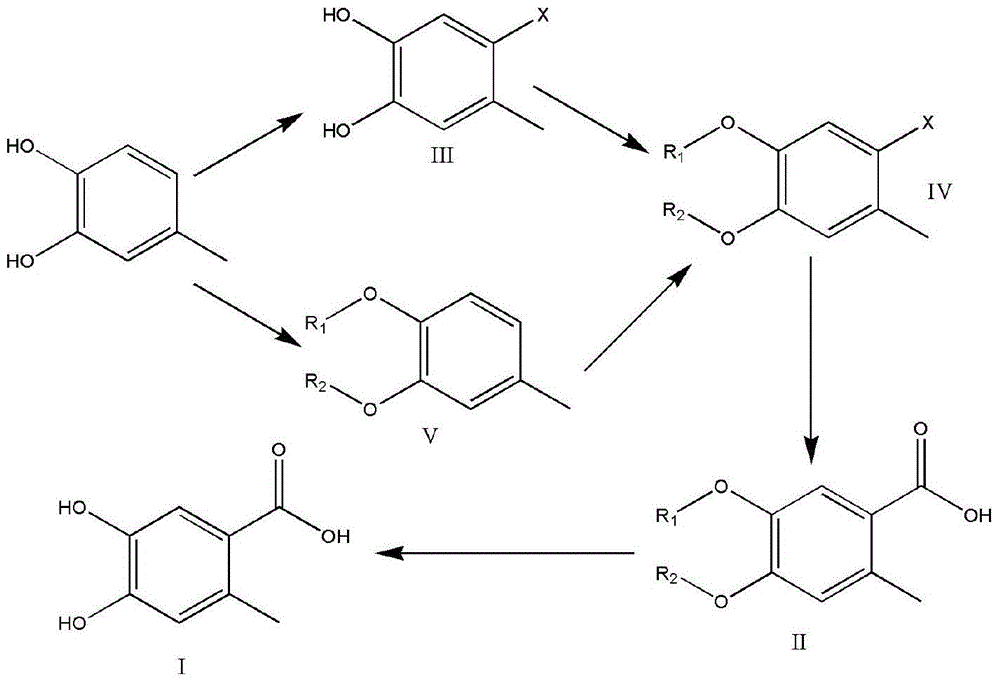
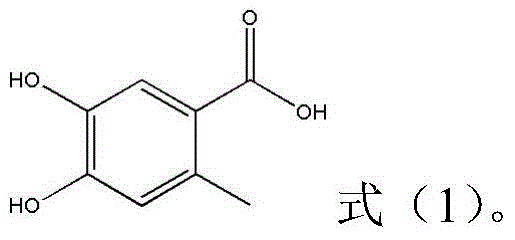
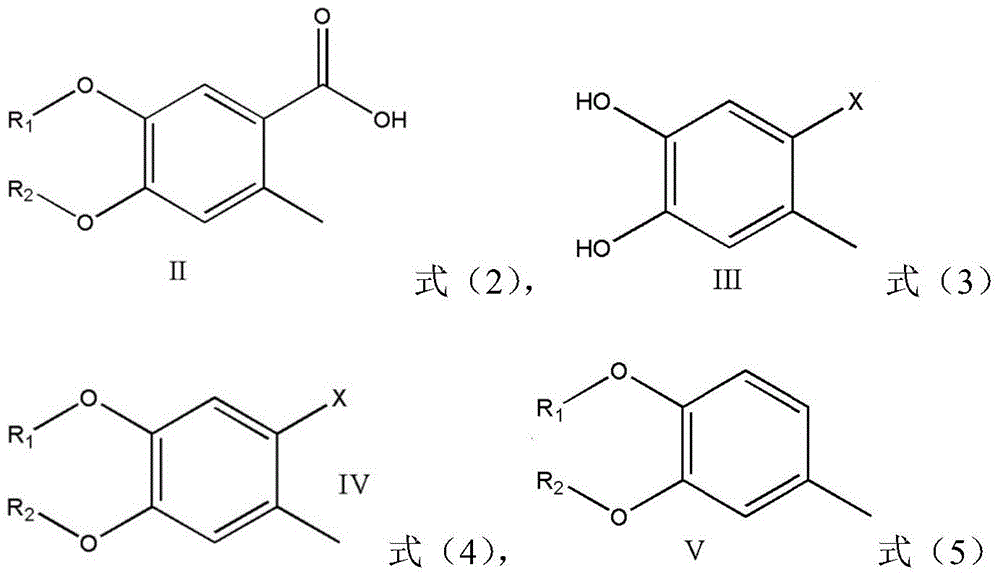
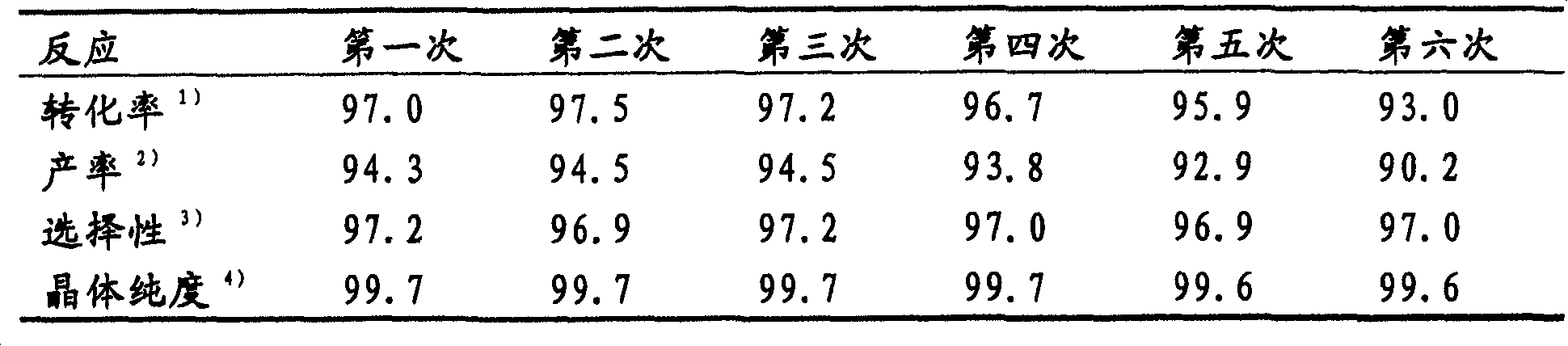
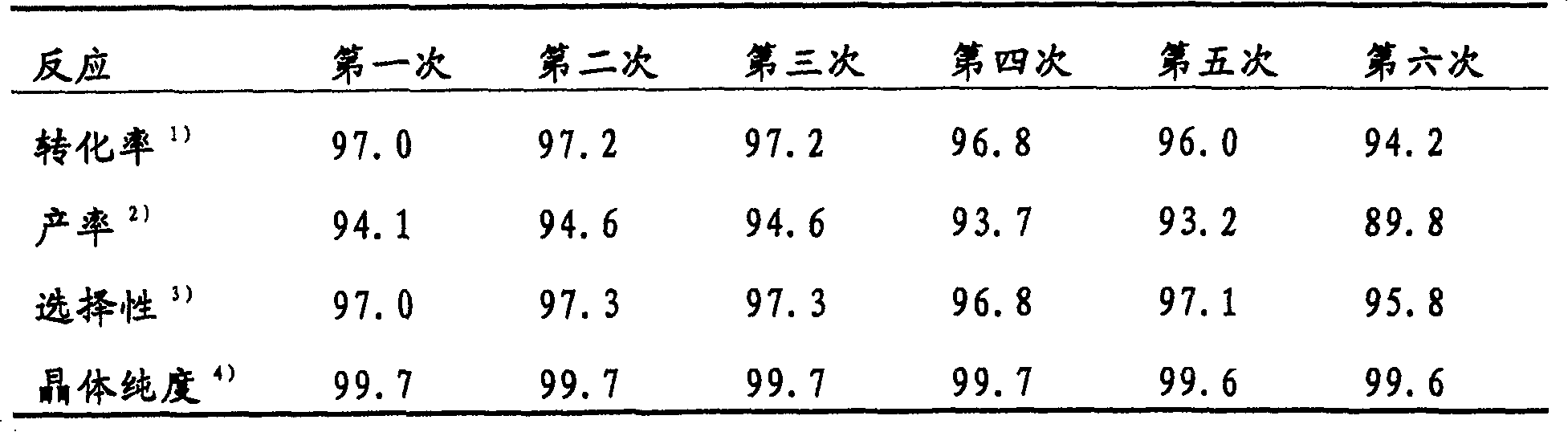
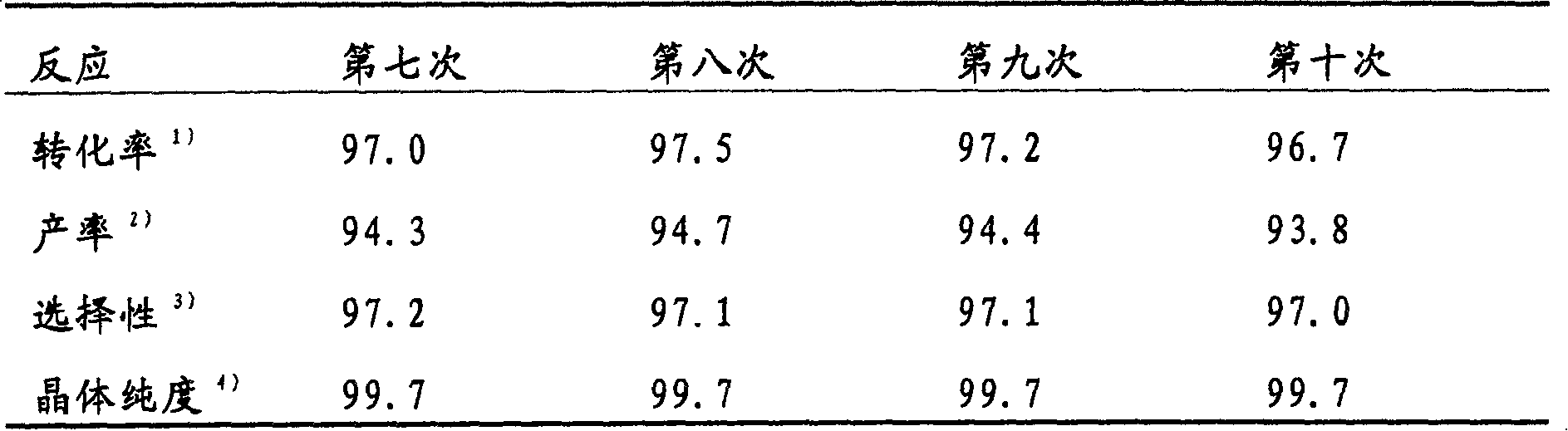
Preparation method of 4,5-dihydroxyl-2-methyl benzoic acid
ActiveCN104387265ASimple and safe operationRaw materials are easy to getOrganic compound preparationCarboxylic compound preparationBenzoic acidMedicinal chemistry
The invention discloses a preparation method of 4,5-dihydroxyl-2-methyl benzoic acid and belongs to the fields of fine chemical engineering and medicine intermediates. The preparation method of 4,5-dihydroxyl-2-methyl benzoic acid comprises the following steps: preparing an intermediate III or V by taking 4-methylbenze-1,2-diphenol as a raw material, then preparing an intermediate IV by virtue of the intermediate III or V, then transforming the intermediate IV into an intermediate II, and finally preparing 4,5-dihydroxyl-2-methyl benzoic acid by virtue of the intermediate II. The preparation method of 4,5-dihydroxyl-2-methyl benzoic acid has the advantage that the problems that raw materials are difficult to acquire, byproducts are many, yield is low, cost is high, steps are miscellaneous, safety can not be guaranteed and serious pollution is produced in the prior art are overcome.
Owner:YANCHENG TEACHERS UNIV
Preparation method of methyl 4-bromoacetyl-2-methylbenzoate
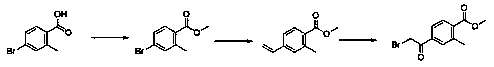
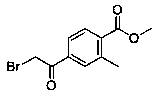
ActiveCN109553532ALow costShort routeOrganic compound preparationCarboxylic acid esters preparationBoronic acidPotassium
The invention provides a preparation method of methyl 4-bromoacetyl-2-methylbenzoate. The preparation method comprises the following steps: (1) dissolving 4-bromo-2-methylbenzoic acid in methanol, andperforming an esterification reaction under the catalytic action of sulfuric acid so as to produce a first intermediate compound; (2) performing a reaction between the first intermediate compound with potassium vinyl fluoroborate or vinyl boronic acid under the catalytic action of palladium so as to obtain a second intermediate compound; and (3) performing an alpha-halogenated ketone synthesis reaction on the second intermediate compound under the action of a halogenating reagent so as to obtain the methyl 4-bromoacetyl-2-methylbenzoate. The preparation method has the advantages of low raw material cost, a short route, mild reaction conditions, simple requirements for equipment and experimental conditions, large-scale amplification synthesis and a high application value.
Owner:荆门医药工业技术研究院 +1
Method for synthesizing roxadustat intermediate
InactiveCN110498783ALow costReaction is easy to controlOrganic chemistryN-BromosuccinimideChemical preparation
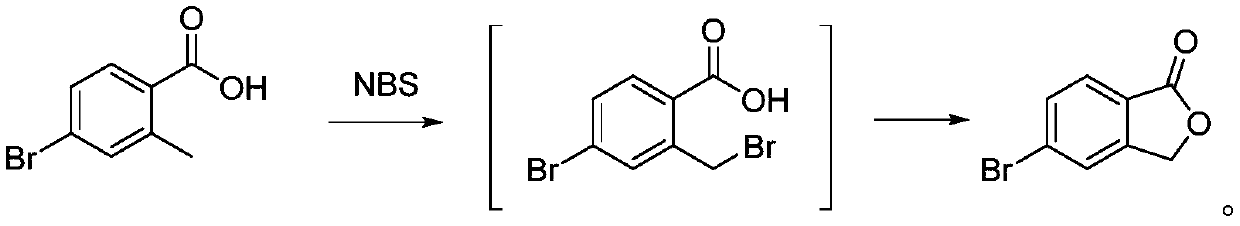
The invention belongs to the technical field of chemical preparation, and discloses a method for synthesizing a roxadustat intermediate. The method comprises the following steps: mixing 4-bromo-2-methylbenzoic acid and a solvent to obtain a mixture 1, adding N-bromosuccinimide and an initiator into the mixture 1, performing uniform mixing to obtain a mixture 2, and performing a reaction to obtainthe roxadustat intermediate 5-bromophthalide. The method provided by the invention has the following advantages: the method for synthesizing the roxadustat intermediate provided by the invention adopts the 4-bromo-2-methylbenzoic acid as the reaction raw material, the raw material is simple and easy to obtain, the costs are low, the reaction is easy to control, and the final product 5-bromophthalide has a yield of 96.5% or more and purity of 98.5% or more.
Owner:成都蓝蜻蜓生物技术有限公司
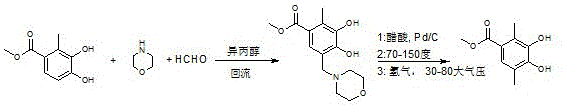
High purity 2,5-dimethyl-3,4-dihydroxy methylbenzoate synthesis method
ActiveCN105130808ARaw material stabilityEasy to synthesizeOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsMannich reaction
The present invention discloses a high purity 2,5-dimethyl-3,4-dihydroxy methylbenzoate synthesis method, which comprises: adopting 3,4-dihydroxy-2-methyl methylbenzoate as a raw material, carrying out a Mannich reaction to obtain a first-step product, dissolving the first-step product in an acetic acid solution, adding a catalyst, carrying out morpholine removing at a temperature of 70-150 DEG C under a hydrogen pressure of 20-80 MPa to obtain a crude product, and carrying out recrystallization on the obtained crude product with an acetic acid aqueous solution to obtain the high purity product. According to the present invention, the raw materials are stable and easy to synthesize; the reaction steps are short, the operation is simple, and the requirement on the production equipment is not high; the required production time is short, and the production cost is saved; and the method is suitable for large-scale industrial production and small batch preparation in the laboratory.
Owner:上海睿腾医药科技有限公司
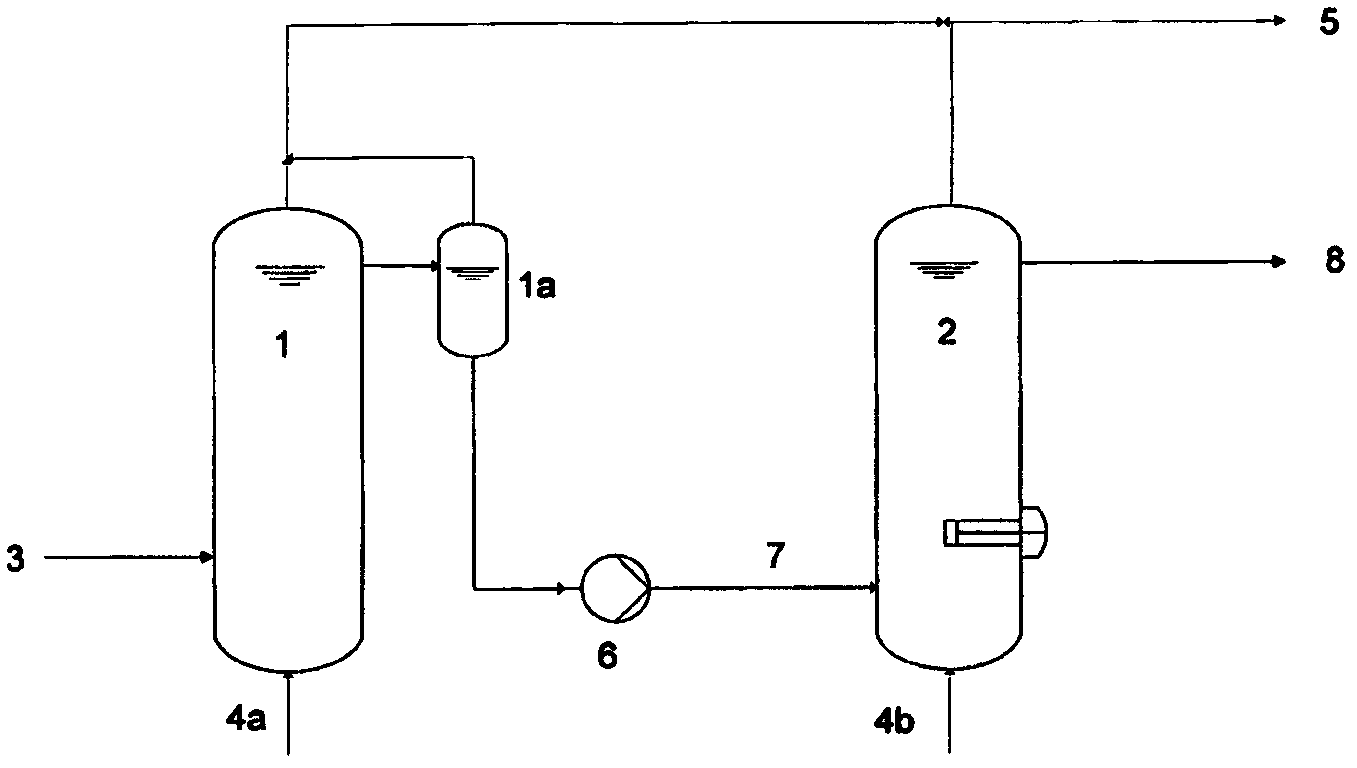
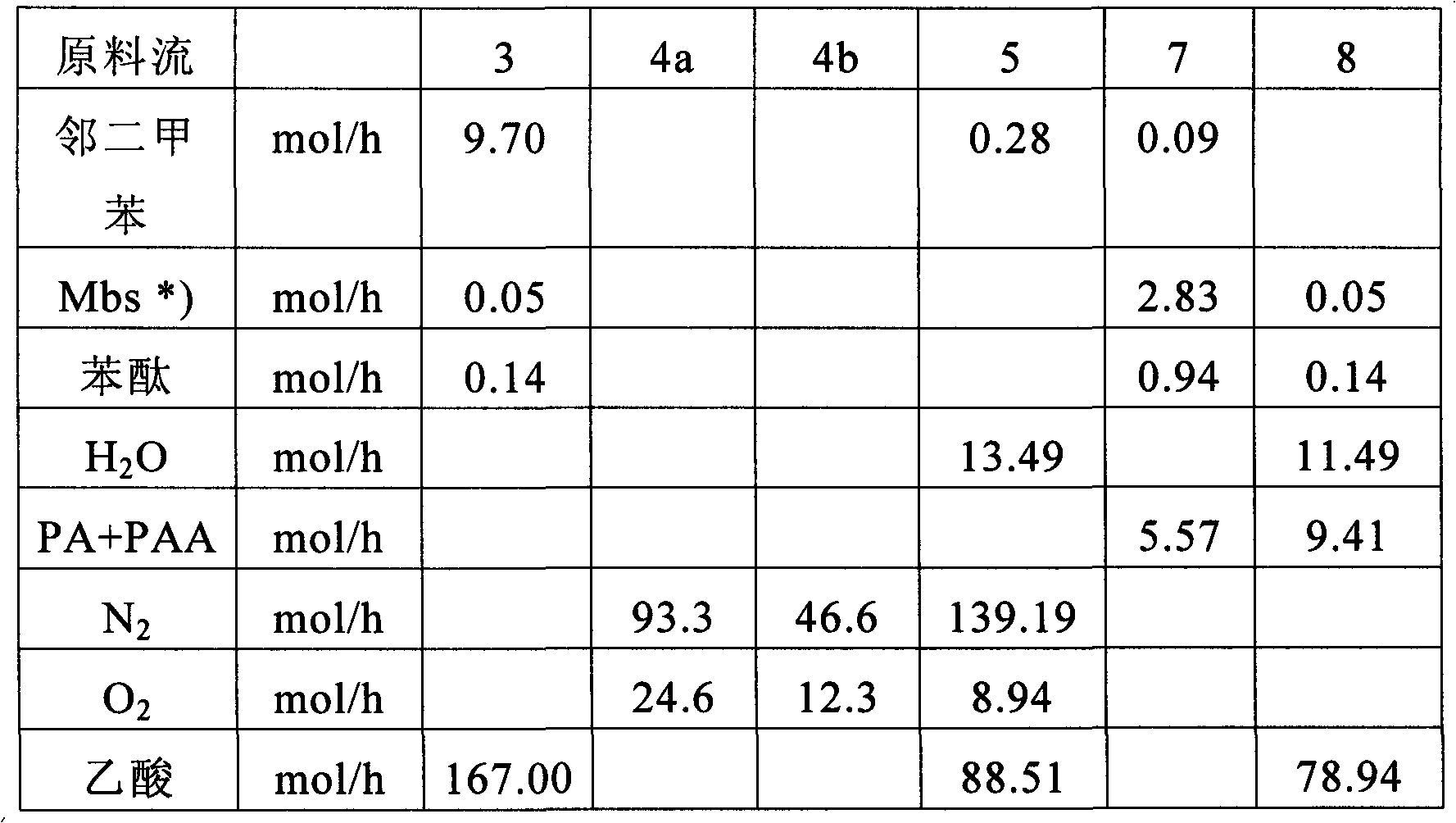
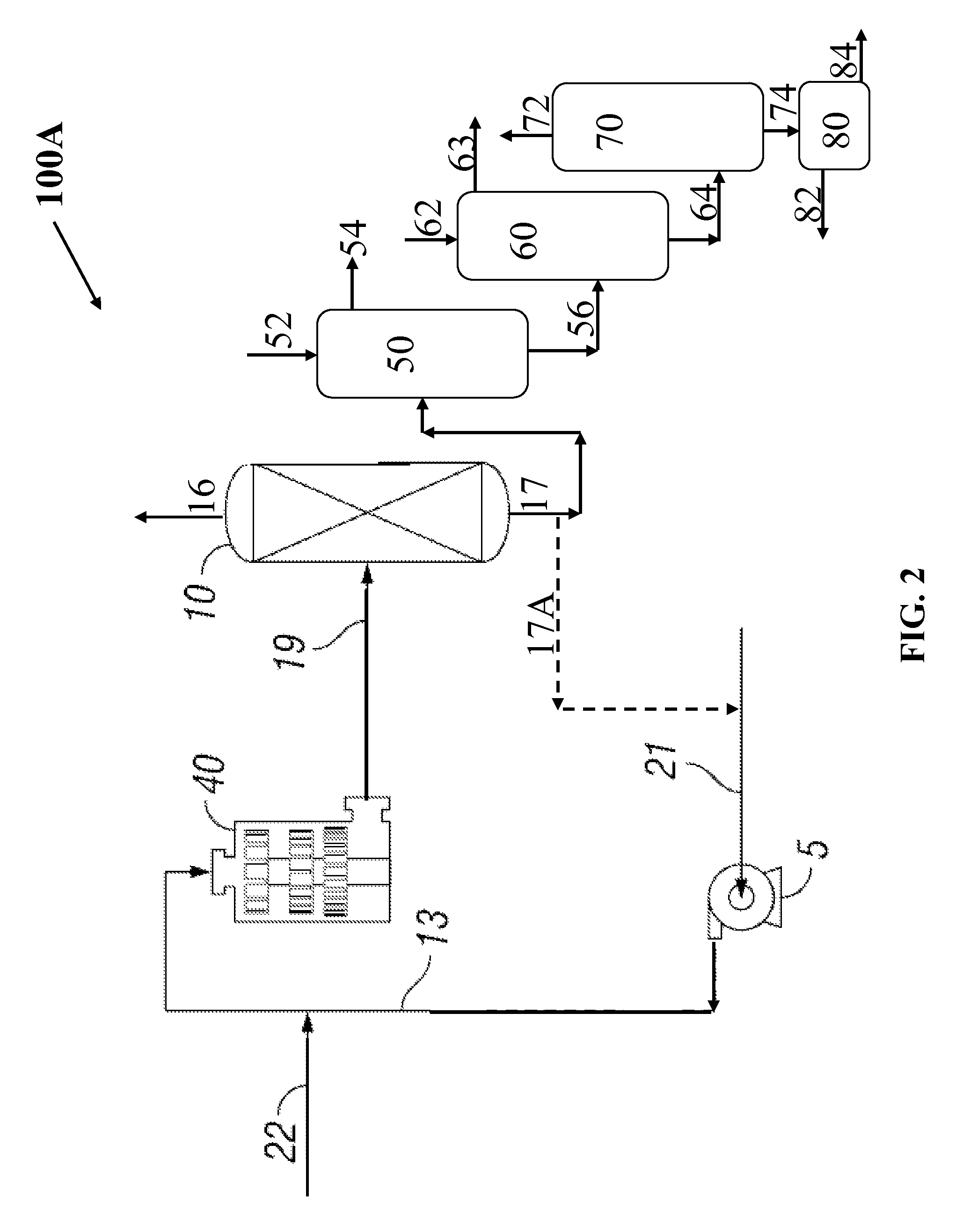
Method for manufacturing phthalic acid/phthalic acid hydride
InactiveCN102471210AReduce lossesSmall sizeOrganic compound preparationCarboxylic acid anhydrides preparationAcetic acidBenzaldehyde
Owner:LURGI
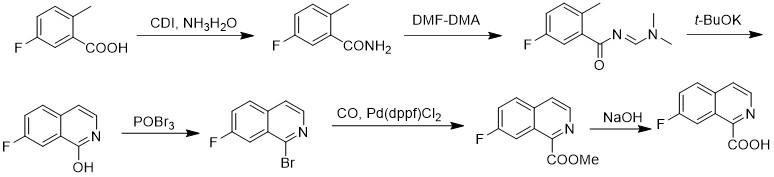
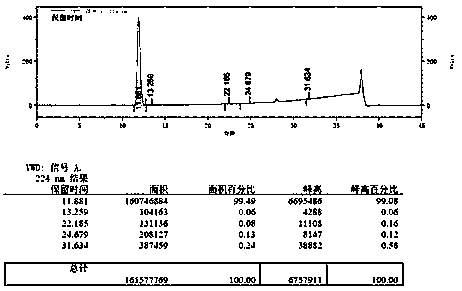
The synthetic method of 7-fluoroisoquinoline-1-carboxylic acid
The invention discloses a method for synthesizing 7-fluoroisoquinoline-1-carboxylic acid. The method uses 5-fluoro-2-methylbenzoic acid as a raw material to obtain 5-fluoroisoquinoline-1-carboxylic acid through condensation with N,N-carbonyldiimidazole ‑Fluoro‑2‑methylbenzamide reacted with N,N‑dimethylformamide dimethyl acetal (E) ‑N‑((dimethylamino)methylene)‑5‑fluoro‑2‑methylbenzamide, followed by cyclization in potassium tert-butoxide to give 7‑fluoroisoquinoline‑1‑alcohol, which is then reacted with tribromo Oxon reaction generates 1-bromo 7-fluoroisoquinoline, and then under carbon monoxide conditions, 7-fluoroisoquinoline-1-carboxylate methyl ester is obtained, and finally hydrolyzed in aqueous sodium hydroxide solution to obtain 7-fluoroisoquinoline- 1‑Carboxylic acid. The method has simple synthesis route, reasonable process selection, low cost of raw materials, simple and easy-to-obtain raw materials, simple and safe operation, no use of highly toxic reagents, convenient post-treatment, high total yield, easy scale-up, and large-scale production.
Owner:SUZHOU KANGRUN PHARMA
Method for synthesizing 3,4-difluoro-2-methylbenzoic acid
InactiveCN110204433AAvoid dangerHigh purityOrganic compound preparationCarboxylic compound separation/purificationSynthesis methodsWarm water
The invention discloses a method for preparing 3,4-difluoro-2-methylbenzoic acid. 2,3-difluorotoluene used as a starting material reacts with carbon dioxide in the presence of anhydrous aluminum trichloride to obtain the 3,4-difluoro-2-methylbenzoic acid. The synthesis method is simple, economical and practical, allows the desired product to be obtained in one step, and allows the reaction to be carried out at room temperature or in a warm water bath, so the method has the advantages of low energy consumption, easiness in operation, high yield, low cost and great application prospect.
Owner:HUNAN NORMAL UNIVERSITY
High shear system and method for the production of acids
InactiveUS9205388B2Physical/chemical process catalystsOrganic compound preparationBenzoic acidOropheic acid
Herein disclosed is a method, comprising: forming a dispersion under high shear comprising gas bubbles of an oxidant dispersed in a liquid phase, wherein the bubbles have a mean diameter of less than 1.5 micron; and contacting the dispersion with an oxidation catalyst to produce a product stream, wherein the product stream comprises a substance selected from the group consisting of dicarboxylic acid, benzoic acid, 2-methylbenzoic acid, 3-methylbenzoic acid, 4-methylbenzoic acid, and phthalic anhydride. In some cases, forming the dispersion under high shear comprises introducing the oxidant and the liquid phase into a high shear device comprising at least one rotor and at least one complementarily-shaped stator. Herein also disclosed is a system for producing a substance selected from the group consisting of dicarboxylic acid, benzoic acid, 2-methylbenzoic acid, 3-methylbenzoic acid, 4-methylbenzoic acid, and phthalic anhydride.
Owner:HRD CORP
A kind of preparation method of 4,5-dihydroxy-2-methylbenzoic acid
ActiveCN104387265BSimple and safe operationRaw materials are easy to getOrganic compound preparationCarboxylic compound preparationBenzoic acidMedicinal chemistry
Owner:YANCHENG TEACHERS UNIV
Process for producing 5-iodo-2-methylbenzoic acid
InactiveCN100406422CImprove efficiencyExtended service lifeOrganic compound preparationOrganic chemistry methodsAcetic anhydrideDistillation
The present invention provides a process for producing 5-iodo-2-methylbenzoic acid by iodizing 2-methylbenzoic acid, which comprises, as essential steps, a reaction step in which 2-methylbenzoic acid is iodized in the presence of a microporous compound, iodine, an oxidizing agent, and acetic anhydride and a purification step in which sublimation, distillation, crystallization, or a combination of two or more of these is conducted. By the process, 5-iodo-2-methylbenzoic acid, which is useful in functional chemicals such as medicines, can be easily obtained as a high-purity compound in a high yield. The production steps comprising reaction and separation / purification are simple from the standpoint of process operation and the purification load is small. Furthermore, the microporous compound, e.g., a zeolite catalyst, separated and recovered from the liquid resulting from the reaction can be repeatedly used after a simple treatment. Consequently, the catalyst has a long life and the target compound can be produced by the efficient process.
Owner:MITSUBISHI GAS CHEM CO INC
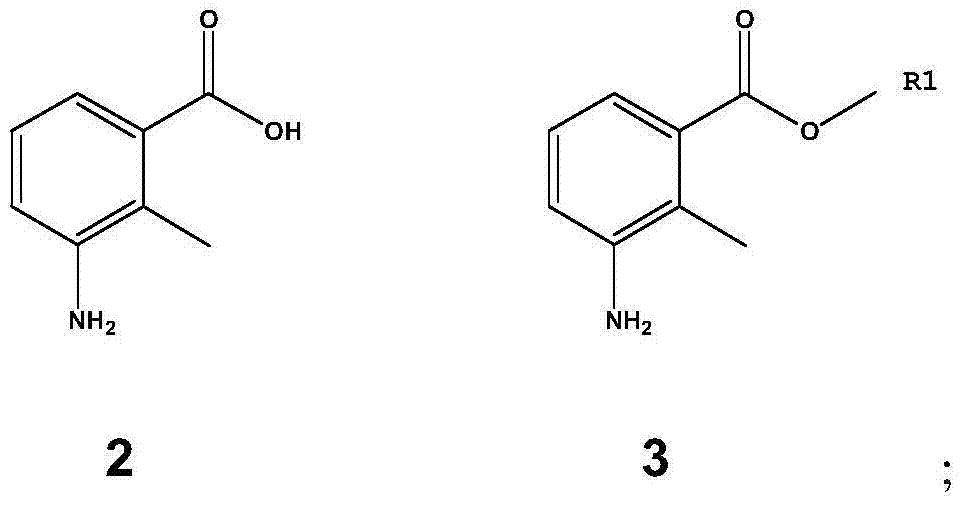
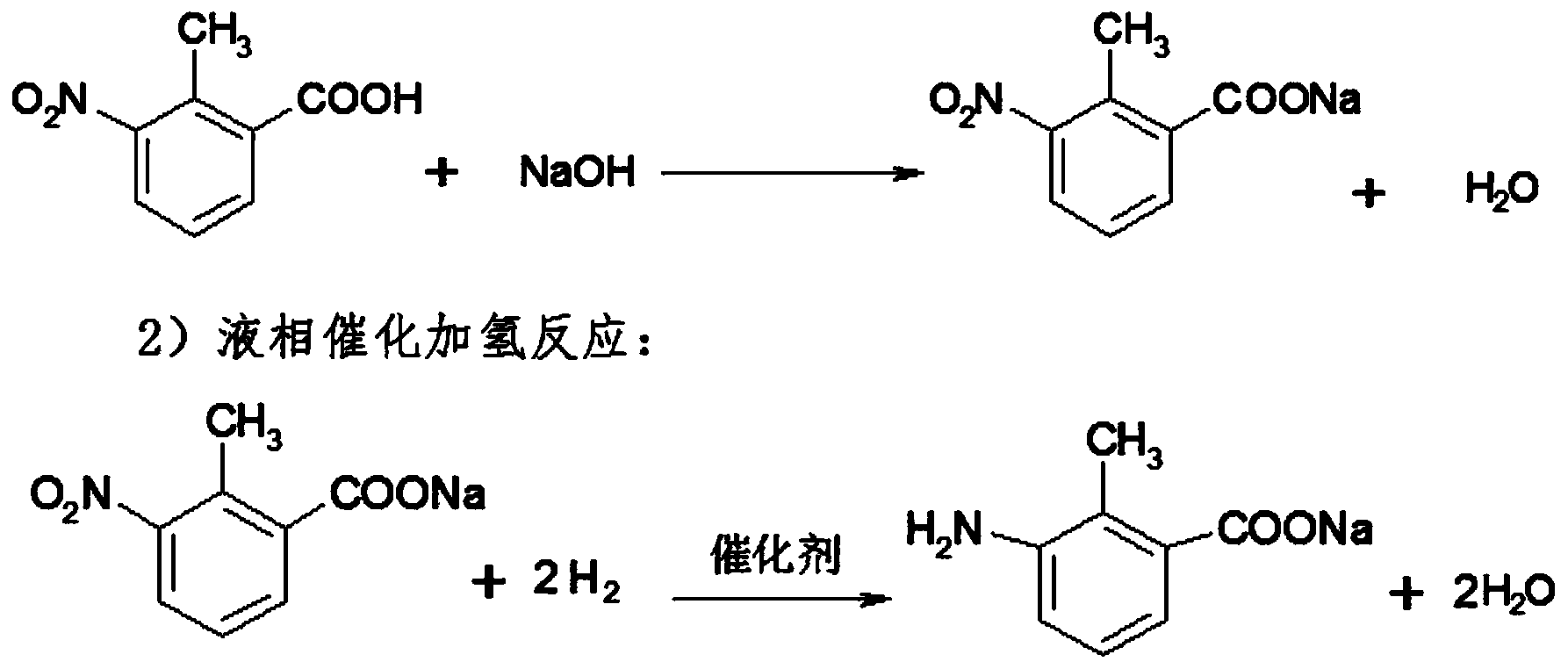
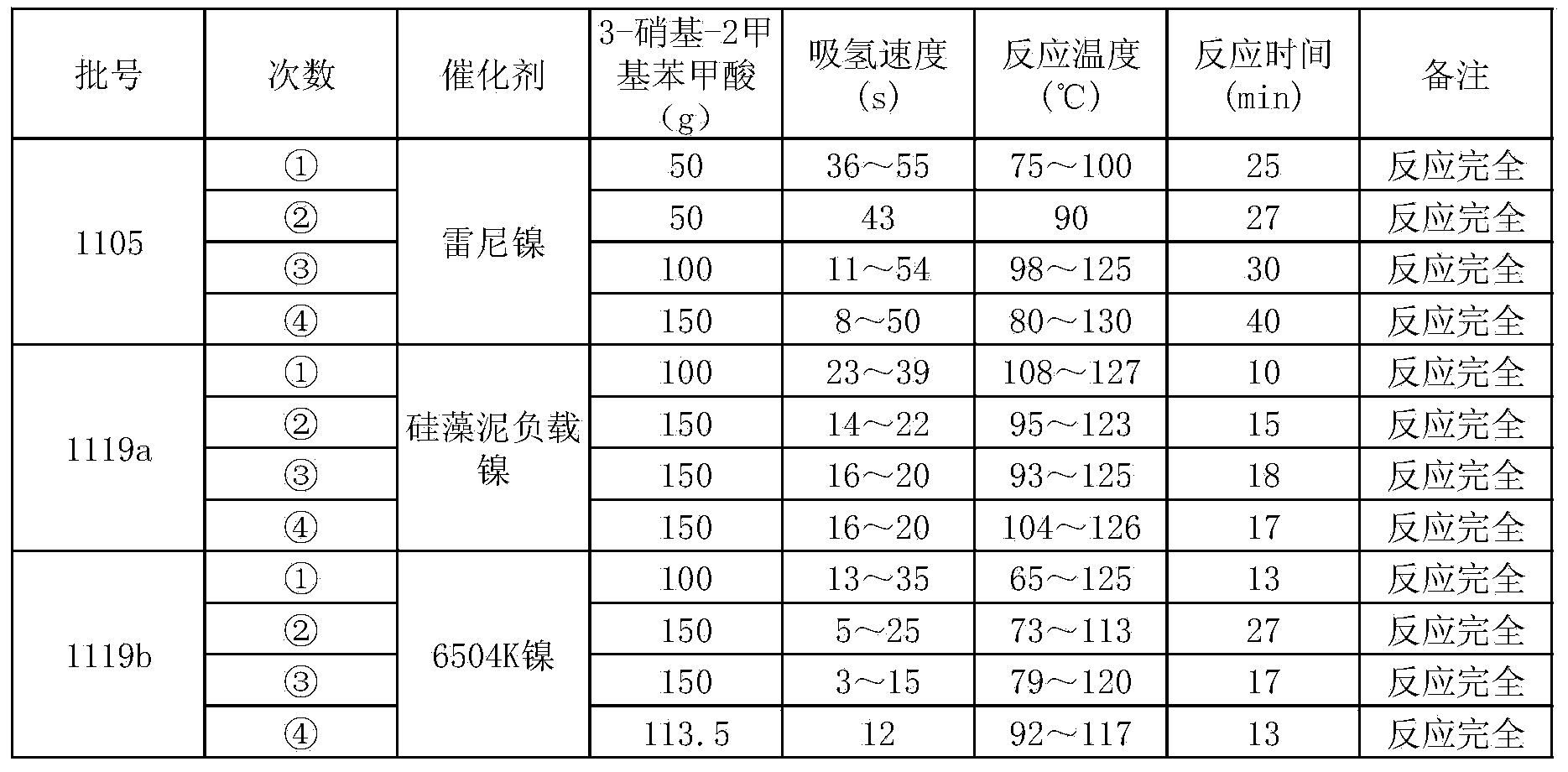
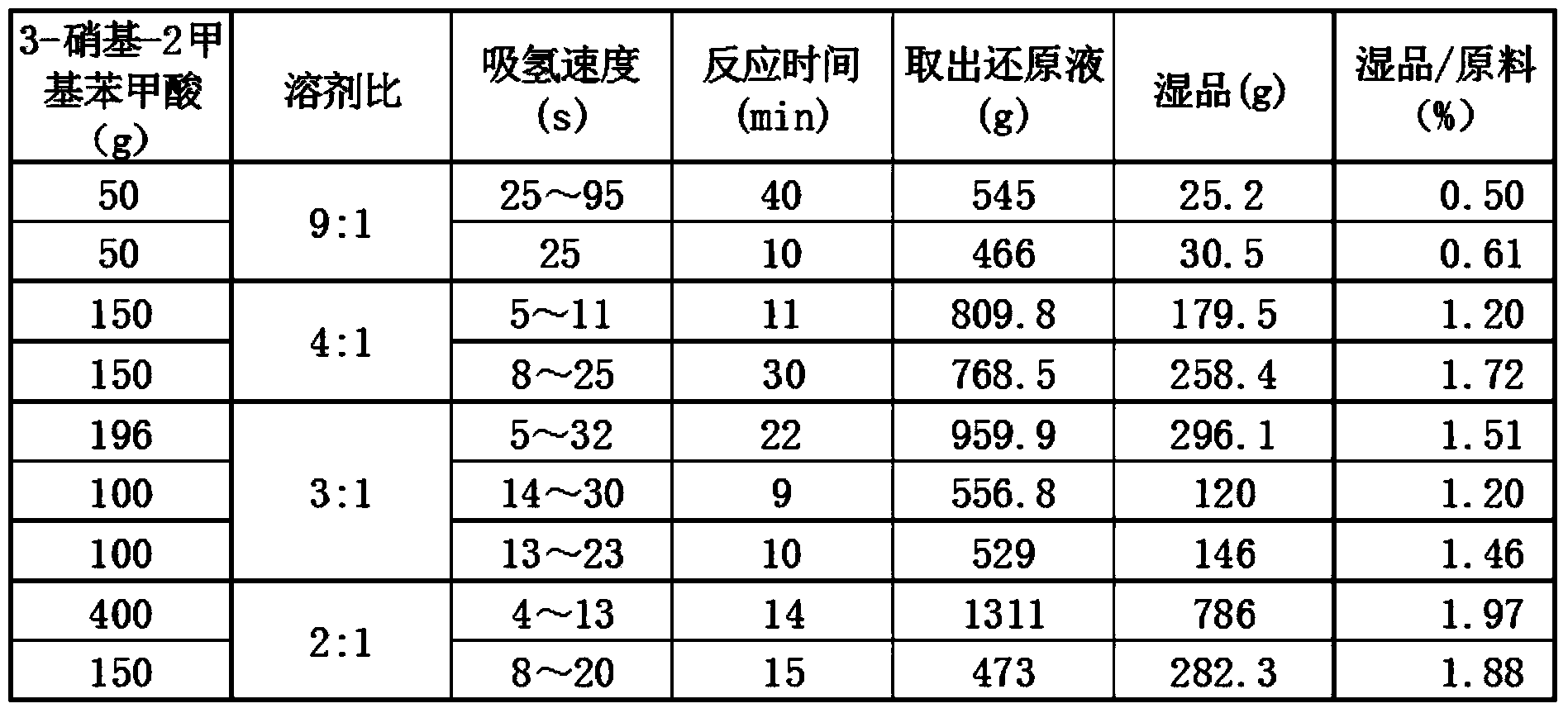
Method for preparing 3-amino-2-methyl benzoic acid
InactiveCN104072383ASimple post-processingHigh yieldOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidSolvent
The invention discloses a method for preparing 3-amino-2-methyl benzoic acid. The method comprises the following steps: by using 3-nitryl-2-methyl benzoic acid as a raw material, salifying; and carrying out liquid phase catalytic hydrogenation to prepare the 3-amino-2-methyl benzoic acid. The method for preparing 3-amino-2-methyl benzoic acid provided by the invention is environmentally friendly and wide in application range, and many different solvents can be selected as liquid phases. The method is simple in post-treatment process and free from purification and is just filtered and dried after acidification. The product purity can reach over 99% and the yield is high (over 95%).
Owner:四川北方红光特种化工有限公司
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveUS7750182B2Carboxylic acid nitrile preparationOrganic compound preparationOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:MITSUBISHI GAS CHEM CO INC
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveCN100436385CHigh purity productHigh purityCarboxylic acid nitrile preparationMolecular sieve catalystsOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:日本精细化工株式会社
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com