Process for producing 5-iodo-2-methylbenzoic acid
A technology of methylbenzoic acid and iodo, which is applied in the field of preparation of 5-iodo-2-methylbenzoic acid, can solve the selectivity of 5-iodo-2-methylbenzoic acid and reaction that has not been developed. Issues not always satisfactorily achieved, rarely reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
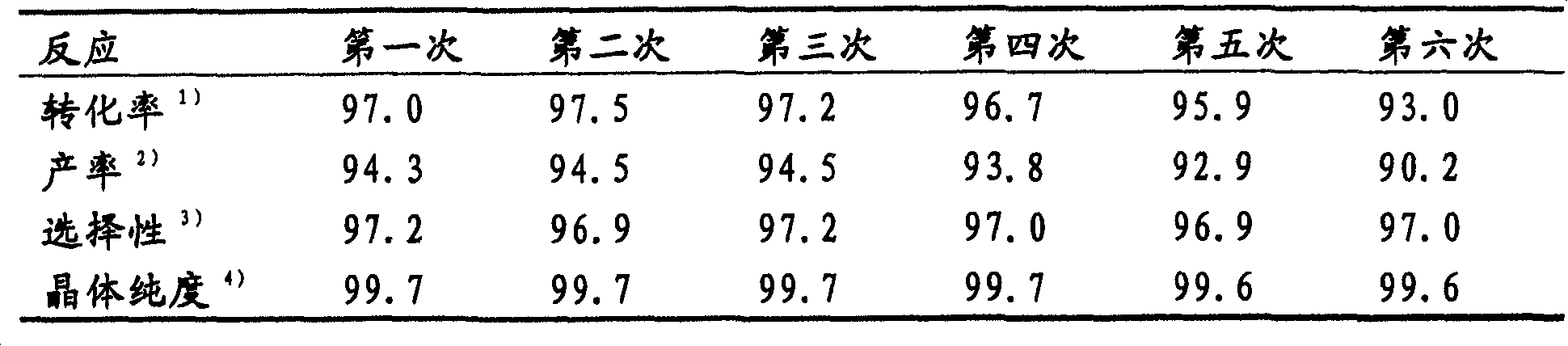
[0054] Add acetic acid (76.5 grams), acetic anhydride (23.5 grams), 2-methylbenzoic acid (20.0 grams), iodine (14.4 grams), 70% iodic acid aqueous solution in the 200 milliliters three-neck flasks that reflux condenser is housed (8.6 g) and H-beta-type zeolite (4.6 g). The resulting mixture was reacted at a reflux temperature of 122°C for 4 hours. After the reaction was completed, the H-β-type zeolite was removed from the reaction mixture by filtration, and the filtrate was cooled to room temperature. Precipitated crystals were collected by filtration, whereby 29.3 g (after drying) of the product were obtained. The collected crystals and mother liquor were analyzed by HPLC (High Performance Liquid Chromatography) to obtain the following reaction results:
[0055] 2-Methylbenzoic acid: 97.0% (conversion rate)
[0056] 5-iodo-2-methylbenzoic acid: 92.0% (yield)
[0057] 94.8% (selective)
[0058] 3-iodo-2-methylbenzoic acid: 0.7% (yield)
[0059] ...
Embodiment 2
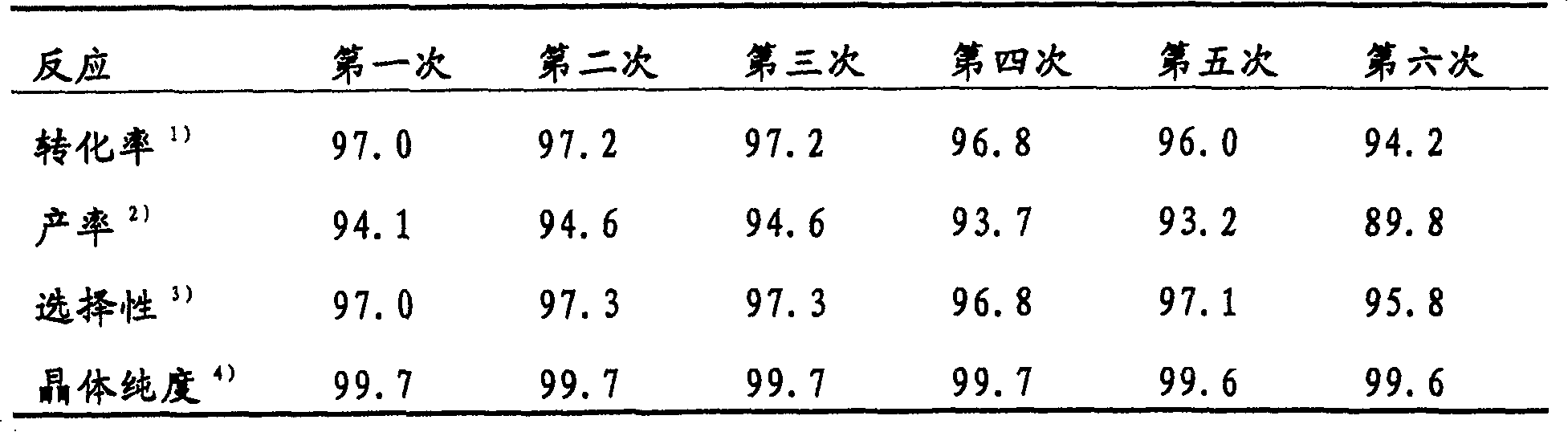
[0064] The procedure in Example 1 was repeated except that acetic acid (96.0 g), acetic anhydride (9.2 g), iodine (15.6 g), and periodic acid (5.5 g) was used instead of iodic acid, whereby 28.5 g of product were obtained. The following reaction analysis results were obtained:
[0065] 2-Methylbenzoic acid: 93.0% (conversion rate)
[0066] 5-iodo-2-methylbenzoic acid: 89.3% (yield)
[0067] 96.0% (selective)
[0068] 3-iodo-2-methylbenzoic acid: 0.2% (yield)
[0069] 0.2% (optional)
[0070] 5-iodo-2-methylbenzoic acid crystals: 73.7% (yield)
[0071] 5-iodo-2-methylbenzoic acid: 99.5% (purity in crystal form)
[0072] By analyzing the crystals thus obtained, it was found that the free iodine content was 5 ppm, and Li, Na, K, Mg, Ca, Sr, Ba, Sc, Y, Ti, Zr, V, Nb, Cr, Mo, W, Mn were not detected , Fe, Ru, Co, Rh, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Al, In, Si, Sn, Pb, P, Sb or S. Both Group 1 and Group 2 element contents are 1 ppm or...
Embodiment 3
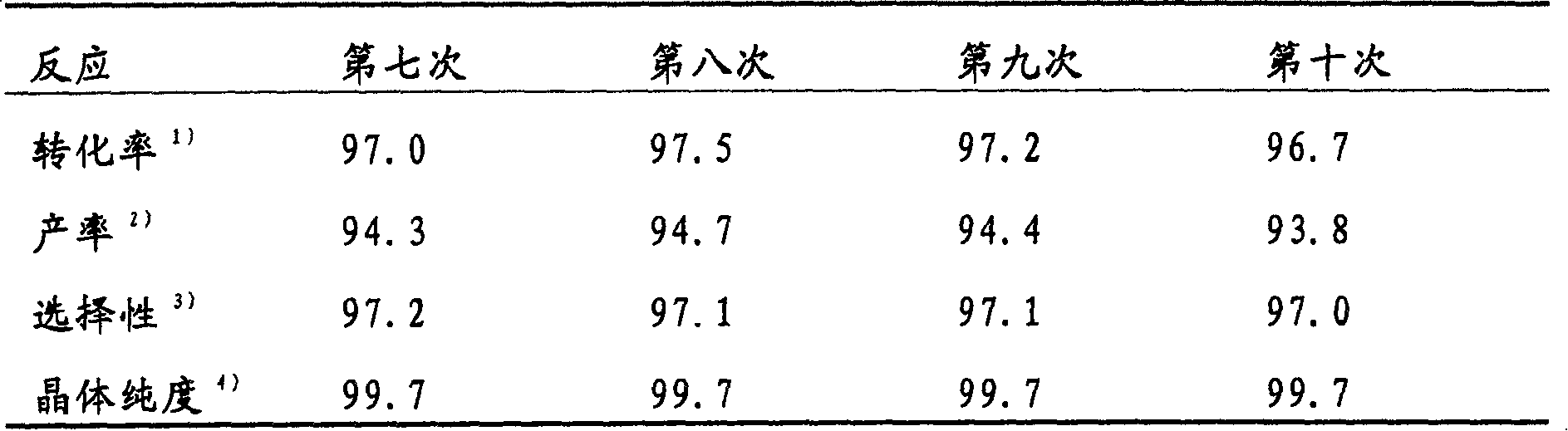
[0074] The reaction of Example 1 was repeated under the same conditions, and H-β-type zeolite was removed from the reaction mixture by filtration. Water (50.0 g) was added to the filtrate, followed by cooling to room temperature. Precipitated crystals were collected by filtration, whereby 33.0 g of a product were obtained. The following reaction analysis results were obtained:
[0075] 2-Methylbenzoic acid: 97.0% (conversion rate)
[0076] 5-iodo-2-methylbenzoic acid: 92.0% (yield)
[0077] 94.8% (selective)
[0078] 3-iodo-2-methylbenzoic acid: 0.7% (yield)
[0079] 0.7% (optional)
[0080] 5-iodo-2-methylbenzoic acid crystals: 85.6% (yield)
[0081] 5-iodo-2-methylbenzoic acid: 99.8% (purity in crystal form)
[0082] By analyzing the crystals thus obtained, it was found that the free iodine content was 5 ppm, and Li, Na, K, Mg, Ca, Sr, Ba, Sc, Y, Ti, Zr, V, Nb, Cr, Mo, W, Mn were not detected , Fe, Ru, Co, Rh, Ni, Pd, Pt, Cu,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com