Method for synthesizing 3,4-difluoro-2-methylbenzoic acid
A technique for the synthesis of toluic acid and its synthesis method, which is applied in the fields of chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate, etc., and can solve the problems of multiple synthesis steps, high price of bromine, unfavorable atom economy, etc. problem, to achieve the effect of simple synthesis process and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
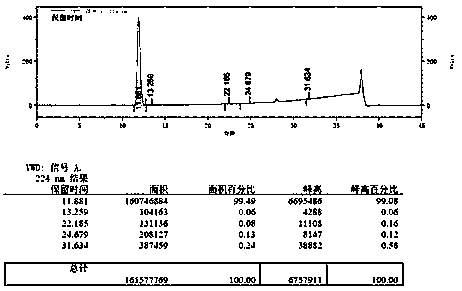
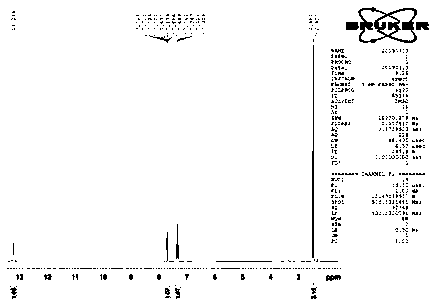
Image
Examples
Embodiment 1
[0008] Embodiment 1: in the three-necked flask that electric stirrer is housed, thermometer and airway tube, add 12.8g (0.1mol) 2,3-difluorotoluene and 13.1g (0.1mol) anhydrous aluminum trichloride, pass into Carbon dioxide gas, turn on the stirring device. Observe the temperature change, when the reaction temperature rises to the highest (about 28 °C) and then falls back, place the three-necked flask in a water bath, and the temperature of the water bath is stabilized at 28 °C. The reaction was stopped after 10 h. The mixture in the three-necked flask was slowly poured into ice water with constant stirring. The obtained white precipitate was suction filtered. After suction filtration, the white solid was dispersed in 50 mL of petroleum ether, stirred, and suction filtered. The solid was taken, and the filtrate was recovered for later use. Suspend the above white powder in 20 mL of methanol, add 2 mL of concentrated HCl, stir at 45 °C for 30 min, then filter with suction, ...
Embodiment 2
[0009] Embodiment 2: Add 8.96g (0.07mol) 2,3-difluorotoluene and 13.1g (0.1mol) anhydrous aluminum trichloride in the three-necked flask that electric stirring device is housed, thermometer and airway, pass into Carbon dioxide gas, turn on the stirring device. Observe the temperature change. When the reaction temperature rises to the highest level (about 30 °C) and then falls back, place the three-necked flask in a water bath, and the temperature of the water bath is stabilized at 30 °C. After the reaction was carried out for 10 h, the reaction was stopped. The mixture in the three-necked flask was slowly poured into ice water with constant stirring. The obtained white precipitate was suction filtered. After suction filtration, the white solid was dispersed in 50 mL of petroleum ether, stirred, and suction filtered. The solid was taken, and the filtrate was recovered for later use. Suspend the above white powder in 20 mL of methanol, add 2 mL of concentrated HCl, stir at 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com