Synthesis method of methoxyfenozide key intermediate-substituted methyl benzoyl chloride
A technology of methylbenzoyl chloride and methoxyfenozide, which is applied in the field of compound preparation, can solve the problems of difficult treatment of phosphorus-containing wastewater, equipment corrosion, large investment, etc., and achieves reduced discharge, mild reaction conditions, and safe elimination. hidden effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
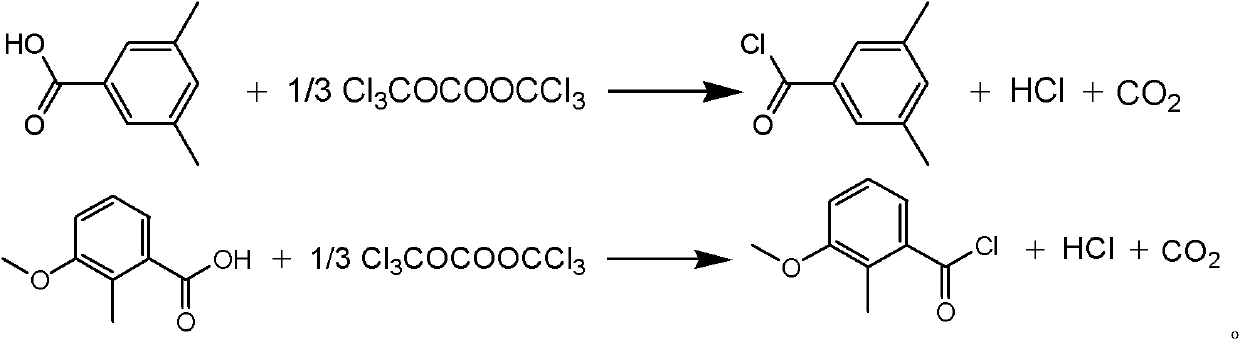
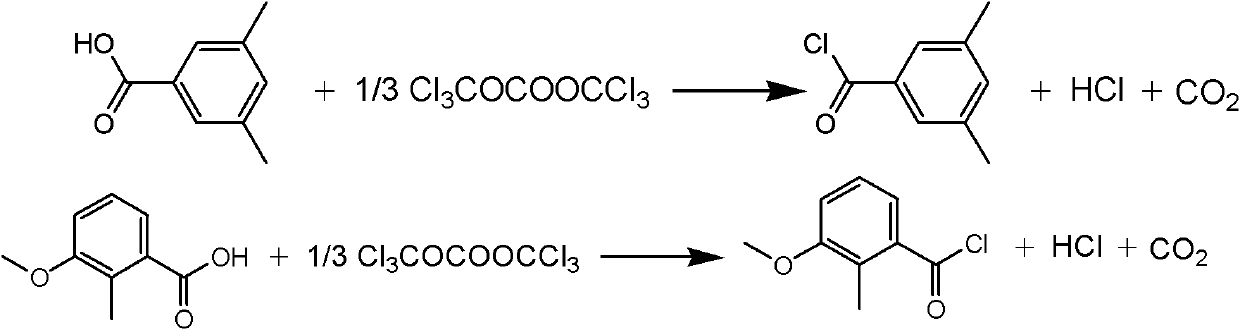
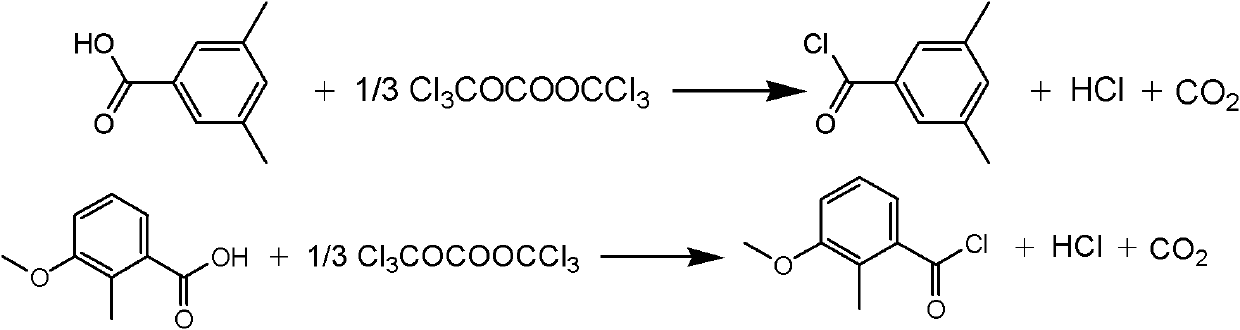
[0021] Example 1 The synthetic method of a methoxyfenozide key intermediate substituted toluyl chloride described in this example is 3,5-dimethylbenzoic acid and bis(trichloromethyl)carbonate in an organic Under the action of an amine catalyst, an acid chlorination reaction occurs in an organic solvent to obtain the corresponding substituted methylbenzoyl chloride. The operation process is: first dissolve the organic amine catalyst in the organic solvent, then add 3,5-dimethylbenzoic acid to dissolve, then slowly add the organic solvent solution dissolved with bis(trichloromethyl) carbonate, control The rate of addition keeps the temperature of the reaction solution within 20°C. After the addition is complete, heat the reaction solution to 50°C-150°C, keep it warm for 2-5 hours, and track and detect it by chromatography. After the reaction is complete, evaporate the solvent under reduced pressure and distill The corresponding substituted methylbenzoyl chloride was collected. ...
Embodiment 2
[0023] Example 2 The synthetic method of a kind of methoxyfenozide key intermediate substituted methylbenzoyl chloride described in this example, the amount ratio of the feed material described in it is 3,5-dimethylbenzoic acid: bis( Trichloromethyl) carbonate: catalyst is 1: 0.4: 0.2,3, the charging capacity of 5-dimethylbenzoic acid is 15g (0.1mol), and bis(trichloromethyl) carbonate charging capacity is 12g (0.04 mol), the catalyst is pyridine, and the consumption is 1.6g (0.02mol), and the organic solvent is toluene, and the consumption is 100ml, which is 5.8 times of the quality of 3,5-dimethylbenzoic acid. Reaction temperature is 100~110 ℃, other operation is the same as embodiment 1, obtains 3,5-dimethylbenzoyl chloride 14.5g, product yield 86%, purity (GC) 98.5%, boiling point 232~135 ℃.
Embodiment 3
[0024] Example 3 The synthetic method of a kind of methoxyfenozide key intermediate substituted toluoyl chloride described in this example, the amount ratio of the feed material described in it is 3,5-dimethylbenzoic acid: bis( Trichloromethyl) carbonate: catalyst is 1: 0.4: 0.05, the charging capacity of 3,5-dimethylbenzoic acid is 15g (0.1mol), and bis(trichloromethyl) carbonate charging capacity is 12g (0.04 mol), the catalyst is triethylamine, and the consumption is 0.5g (0.005mol), and the organic solvent is chlorobenzene, and the consumption is 100ml, which is 7.3 times of 3,5-dimethylbenzoic acid quality. Reaction temperature is 120~130 ℃, other operation is the same as embodiment 1, 3,5-dimethylbenzoyl chloride 14.7g, product yield 87.5%, purity (GC) 99%, boiling point 235~236 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com