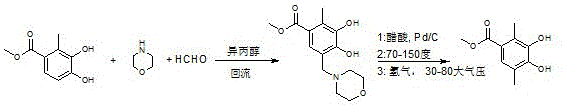
High purity 2,5-dimethyl-3,4-dihydroxy methylbenzoate synthesis method
A technology of methyl dihydroxybenzoate and methyl methylbenzoate, applied in the field of high-purity 2, can solve the problems of difficult preparation of starting materials, unsuitable for large-scale production, hidden dangers of production safety, etc., and meets the requirements of production equipment The effect of not high, short reaction steps and short production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In the embodiment of the present invention, 3,4-dihydroxy-2-methyl-5-morpholine methyl benzoate: 2L of isopropanol and 478g of morpholine were added to a 10L three-necked bottle, and 37% formaldehyde was added under mechanical stirring Solution 478g, then reflux reaction 20min. The temperature was lowered to 50° C., and 5 L of isopropanol and 1000 g of methyl 3,4-dihydroxy-2-methylbenzoate solution were quickly added. Then heated to reflux for 2.5h, the raw material disappeared. Cool down to 10°C and filter, and wash the filter cake with 500ml of isopropanol. It was sucked dry and dried at 50°C to obtain 1253 g of off-white solid with a yield of 81%.
[0020] Synthesis of 2,5-dimethyl-3,4-dihydroxybenzoic acid methyl ester: 5L autoclave, add 3.5L acetic acid, 3,4-dihydroxy-2-methyl-5-morpholine methylbenzoic acid Methyl ester 700g, palladium carbon 5%, hydrogen replacement 3 times, 80MPa pressure, 100°C reaction for 24h, hydrogen needs to be added during the reaction...
Embodiment 2
[0022] In the embodiment of the present invention, 3,4-dihydroxy-2-methyl-5-morpholine methyl benzoate: 2L of isopropanol and 478g of morpholine were added to a 10L three-necked bottle, and 37% formaldehyde was added under mechanical stirring Solution 478g, then reflux reaction 20min. Cool down to 20°C, quickly add 5L of isopropanol and 1000g of 3,4-dihydroxy-2-methylbenzoic acid methyl ester solution. Then heated to reflux for 2.5h, the raw material disappeared. Cool down to 20°C and filter, and wash the filter cake with 500ml of isopropanol. Drained and dried at 50°C to obtain 1235 g of off-white solid with a yield of 80%.
[0023] Synthesis of 2,5-dimethyl-3,4-dihydroxybenzoic acid methyl ester: 5L autoclave, add 3.5L acetic acid, 3,4-dihydroxy-2-methyl-5-morpholine methylbenzoic acid Methyl ester 700g, palladium hydroxide 5%, hydrogen replacement 3 times, 30MPa pressure, 100°C reaction for 24h, hydrogen needs to be added during the reaction. There is little raw materia...
Embodiment 3
[0025] In the embodiment of the present invention, 3,4-dihydroxy-2-methyl-5-morpholine methyl benzoate: 2L of isopropanol and 478g of morpholine were added to a 10L three-necked bottle, and 37% formaldehyde was added under mechanical stirring Solution 478g, then reflux reaction 20min. The temperature was lowered to 50° C., and 5 L of isopropanol and 1000 g of methyl 3,4-dihydroxy-2-methylbenzoate solution were quickly added. Then heated to reflux for 2.5h, the raw material disappeared. Cool down to -5°C and filter, and wash the filter cake with 500ml of isopropanol. It was sucked dry and dried at 50°C to obtain 1275 g of off-white solid with a yield of 82.5%.
[0026] Synthesis of 2,5-dimethyl-3,4-dihydroxybenzoic acid methyl ester: 5L autoclave, add 3.5L acetic acid, 3,4-dihydroxy-2-methyl-5-morpholine methylbenzoic acid Methyl ester 700g, palladium carbon 5%, hydrogen replacement 3 times, 30MPa pressure, 150°C reaction for 24h, hydrogen needs to be added during the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com