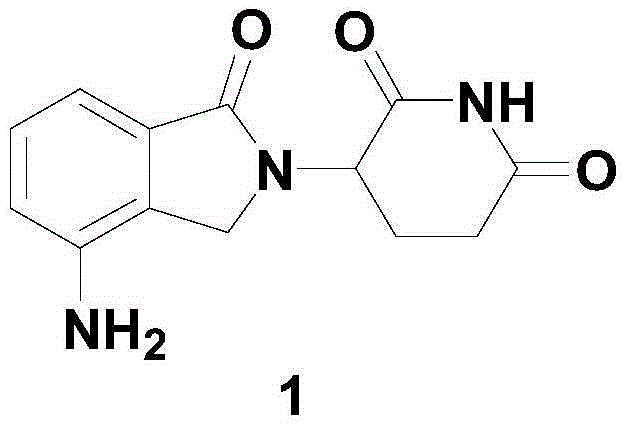
Process for preparing lenalidomide
A compound and selected technology, applied in the field of medicine, can solve the problems of low production safety, difficult industrialized production, complex preparation process, etc., and achieve the effects of high synthesis efficiency, short production cycle and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Esterification reaction
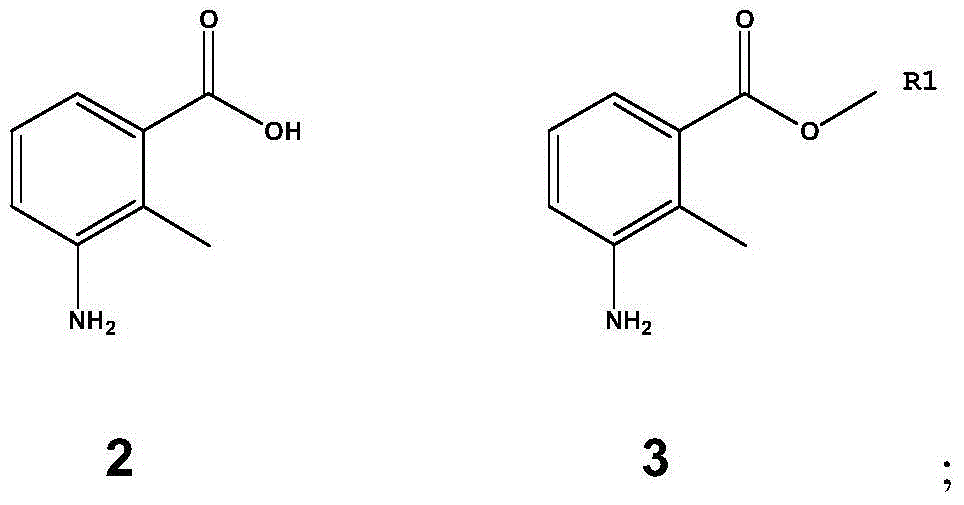
[0056] 3-Amino-2-methylbenzoic acid (100 g, 0.662 mol) and p-toluenesulfonic acid (200 g, 1.162 mol), add 3000 ml of methanol, stir, reflux for 10 hours, add ethyl acetate after cooling to room temperature 3000 ml and 3000 ml of 1M potassium carbonate aqueous solution were stirred, filtered, the filtrate was extracted with ethyl acetate, washed with 1M potassium carbonate aqueous solution and saturated brine, the organic layer was dried and concentrated under reduced pressure to obtain 3-amino-2-methylbenzoic acid Methyl ester (Compound 3, 93.0 g, 0.564 mol), yield 85%. 1 H NMR (CDCl 3 ):δ2.36(s,3H),3.82(s,3H),6.71-6.77(m,1H),6.99-7.01(m,1H),7.16-7.20(m,1H).MS(m / z ):165[M+H] + .
Embodiment 2
[0057] Embodiment 2: Esterification reaction
[0058] 3-amino-2-methylbenzoic acid (100 grams, 0.662 moles) and sulfuric acid (63 milliliters, 1.162 moles), add 3000 milliliters of methanol, stir, reflux reaction for 10 hours, after cooling to room temperature, add 3000 milliliters of ethyl acetate and 3000 ml of 1M potassium carbonate aqueous solution was stirred, filtered, and the filtrate was extracted with ethyl acetate, washed with 1M potassium carbonate aqueous solution and saturated brine, and the organic layer was dried and concentrated under reduced pressure to obtain 3-amino-2-methylbenzoic acid methyl ester ( Compound 3, 79.0 g, 0.483 mol), yield 73%. MS(m / z):165[M+H] + .
Embodiment 3
[0059] Embodiment 3: Amino protection reaction
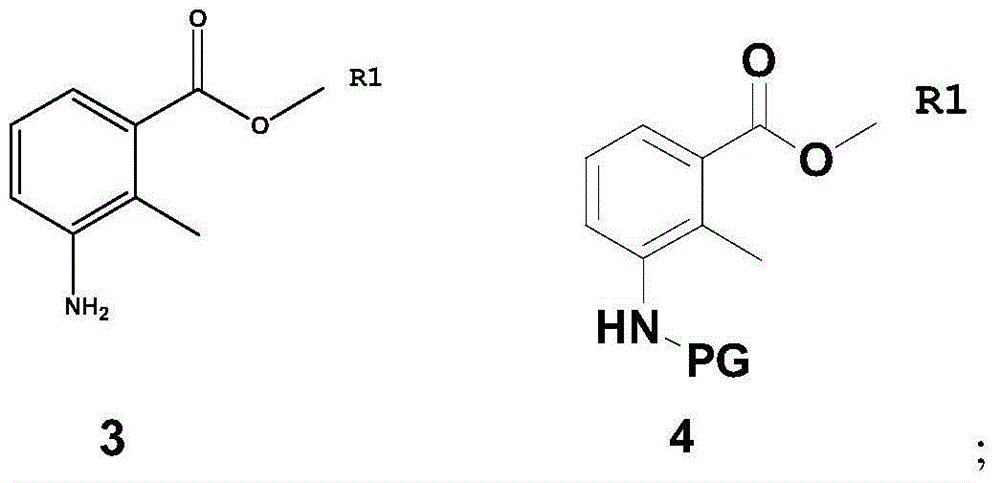
[0060] Dissolve compound 3 (93.0 g, 0.564 mol) in 1000 ml of dichloromethane, add di-tert-butyl dicarbonate (147.5 g, 0.677 mol), control the temperature at 25 degrees Celsius, stir for 5 hours, extract with dichloromethane, and use Wash with saturated aqueous sodium bicarbonate and saturated brine to obtain methyl 3-tert-butoxyformamide-2-methylbenzoate (compound 4(a), 143.5 g, 0.541 mol), with a yield of 96%. 1 H NMR (CDCl 3 ):δ1.41(s,9H),2.35(s,3H),3.87(s,3H),6.92-6.97(m,1H),7.05-7.11(m,1H),7.56-7.59(m,1H ).MS(m / z):265[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com