Method for synthesizing Imatinib
A synthetic method, imatinib technology, applied in the field of organic synthesis, can solve problems such as difficult purification, easy volatility, and great environmental impact, and achieve the effects of industrial production, mild reaction conditions, and clean and complete reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 imatinib,
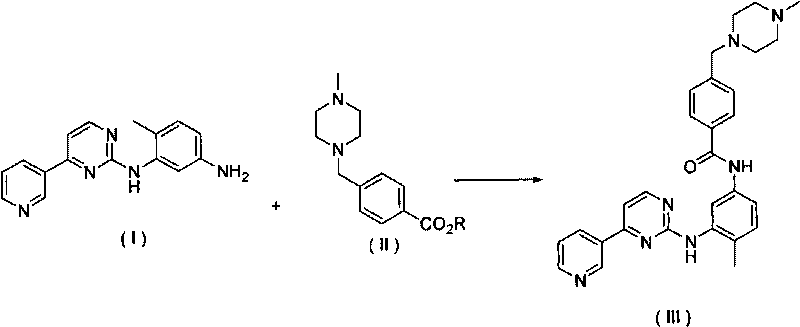
[0036] In a 500ml dry four-necked flask, add 250ml of tetrahydrofuran, 4-methyl-N-3-(4-pyridin-3-yl-pyrimidin-2-yl)-1, 27.7g of 3-phenylenediamine and 4-( 25g of 4-methylpiperazine-1-methyl)-methyl benzoate, stirred and dissolved, added 10g of sodium methoxide, heated at 70°C to reflux for overnight reaction, detected the completion of the reaction and concentrated the tetrahydrofuran, washed the obtained solid with water, and dried to obtain Imatinib 45g, yield 91.0%.
[0037] The spectral data are as follows:
[0038] 1 H NMR (500M, DMSO) δ: 10.2(s, 1H), 9.30(s, 1H), 8.99(s, 1H), 8.72(d, J=4.0Hz, 1H), 8.57(s, 1H), 8.53 (s, 1H), 8.11(s, 1H), 8.00(s, 1H), 7.98(s, 1H), 7.58-7.51(m, 4H), 7.44(d, J=4.3Hz, 1H), 7.22( d, J=8.1Hz, 1H), 3.70(s, 2H), 3.50-3.25(m, 2H), 3.20-2.90(m, 4H), 2.81(s, 3H), 2.40(s, 3H), 2.24 (s, 3H). 13 C NMR (125M, DMSO) δ: 164.9, 161.3, 161.1, 159.4, 150.8, 147.7, 137.7, 137.1, 134.9, 134.3, 132.3, 129.9, 129.1, 127.7, 1...
Embodiment 2
[0041] In a 5000ml dry four-neck flask, add 3000ml of dichloromethane, 4-methyl-N-3-(4-pyridin-3-yl-pyrimidin-2-yl)-1,3-phenylenediamine 277g and 4- (4-Methylpiperazine-1-methyl)-ethyl benzoate 270g, after stirring and dissolving, add 100g of sodium methoxide, heat at 40°C and reflux reaction overnight, after detecting the completion of the reaction, concentrate the toluene, wash the obtained solid with water, and dry 455g of imatinib was obtained, with a yield of 92.0%. Spectral data ditto.
Embodiment 3
[0043]In a 5000ml dry four-necked flask, add toluene 3000ml, 4-methyl-N-3-(4-pyridin-3-yl-pyrimidin-2-yl)-1,3-phenylenediamine 277g and 4-(4 -Methylpiperazine-1-methyl)-benzyl benzoate 450g, after stirring and dissolving, add 200g of sodium ethoxide, heat to 50°C and react overnight, after detecting the completion of the reaction, concentrate the toluene, wash the obtained solid with water, dry to obtain Matinib 445g, yield 90.0%. Spectral data ditto.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com