Method for the preparation of pure nilotinib and its salt
A technology for forming salts and compounds, which is applied in the field of preparing pure nilotinib and its salts, and can solve problems such as low yield values
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
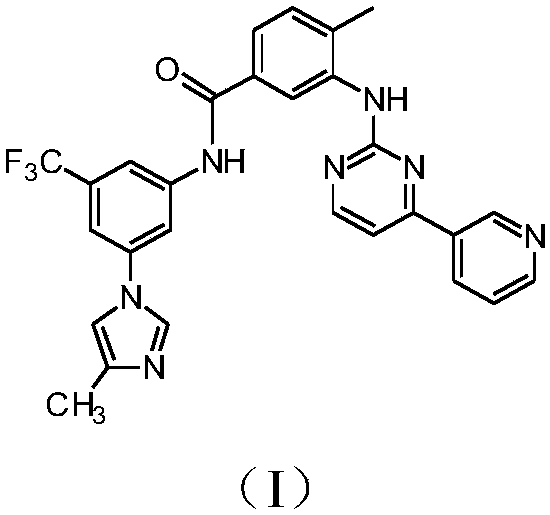
[0138] Example 1: Standard synthesis of compounds of formula (I)
[0139]
[0140] A mixture of compound 5 (530 g, 1.73 mol) in N-methyl-2-pyrrolidone (abbreviated as NMP) (3710 mL) was heated at 60° C., and then thionyl chloride (159 g, 1.34 mol) was added within about 30 min. ). The reaction was then stirred at the same temperature for 1 hour. A solution of compound 6 (417.3 g, 1.73 mol) in NMP (1590 mL) was then added to the reaction mixture. The resulting reaction mixture was heated at 90°C, and the reaction was stirred at the same temperature for 2 hours. The reaction mixture was then cooled to 80 °C and water (4770 mL) was added. The pH was adjusted to pH=11 with 30% sodium hydroxide solution (988.6 mL). The resulting mixture was then cooled to T=40°C and stirred for 3 hours. The mixture was then filtered and the filtrate containing compound (I) was washed with water. The resulting solid was dried in vacuo to give Compound (I) as a white solid (824.6 g, 86% yiel...
Embodiment 2
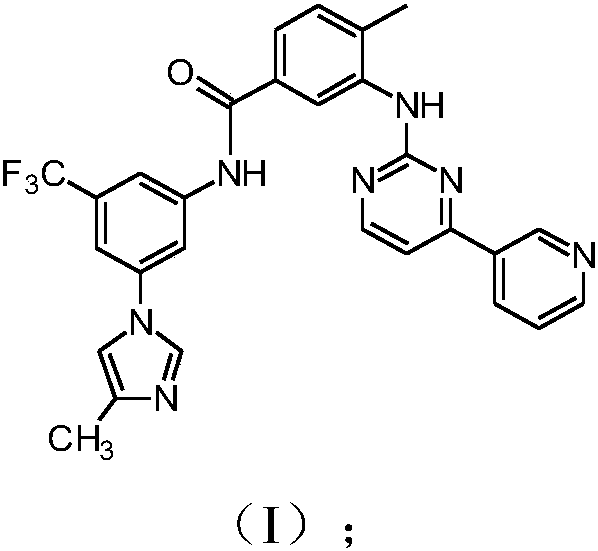
[0141] Example 2: Synthesis of compounds of formula (I)
[0142]
[0143] To a mixture of compound (I) (800 g, 1.51 mol) in methanol (12000 mL) was added 32% aqueous hydrochloric acid until the pH was 2.5-3.5 (indicative amount 148 mL). The resulting mixture was then heated at 60-65°C and stirred at the same temperature for 1 hour until completely dissolved. To the resulting solution was then added 15% aqueous sodium hydroxide until pH 9 (indicative amount 307 mL). The resulting mixture was then cooled to T=40° C. within 1 hour and then filtered at the same temperature. The obtained filtrate containing Compound (I) was washed with methanol (800 mL). The resulting solid was dried under vacuum to give Compound (I) as a white solid (738.4 g, 92% yield, HPLC purity 99.7%, acid (IV) impurity <0.05%).
Embodiment 3
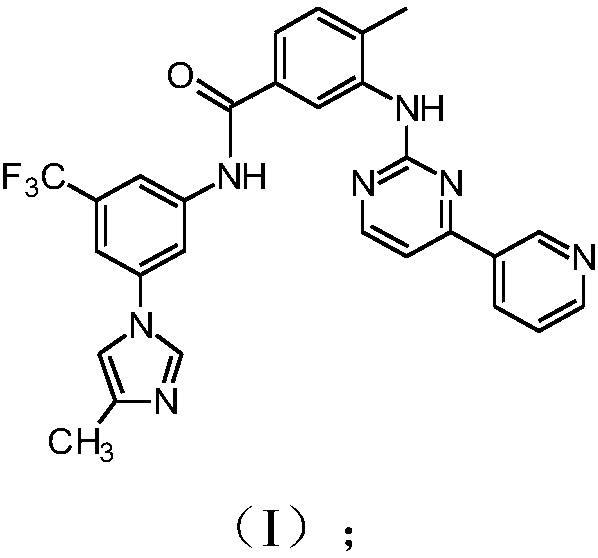
[0144] Example 3: Synthesis of compounds of formula (I)
[0145]
[0146] To a mixture of compound (I) (10 g, 18.88 mmol), acid 5 (1 g, 3.26 mmol) and aniline 6 (1 g, 4.156 mmol) in methanol (150 mL) was added 32% aqueous hydrochloric acid until the pH was 2.5-3.5 ( Indicative volume is 1.8 mL). The resulting mixture was then heated at 60-65°C and stirred at the same temperature for 1 hour until completely dissolved. To the resulting solution was then added 30% aqueous sodium hydroxide until pH 9 (indicative amount 2.1 mL). The resulting mixture was then cooled to T=40°C within 1 hour and then filtered at the same temperature. The obtained filtrate containing Compound (I) was washed with methanol (10 mL). The resulting solid was dried in vacuo to give Compound (I) as a white solid (8.89 g, 89.9% yield, HPLC purity 99.90%, acid 5 impurity 0.02%, aniline 6 impurity 0.00%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com