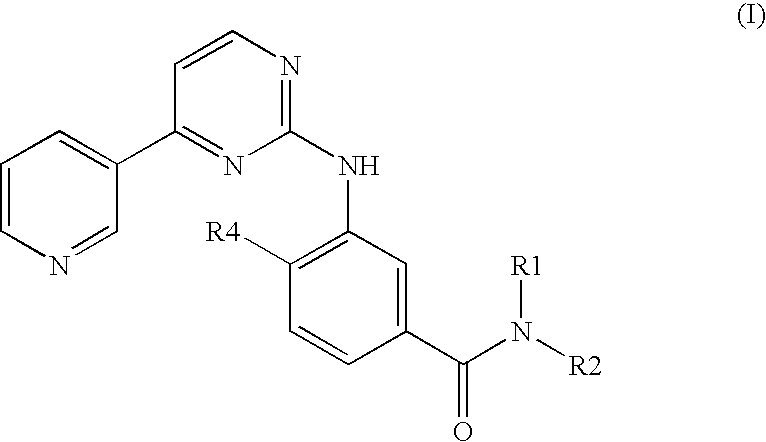
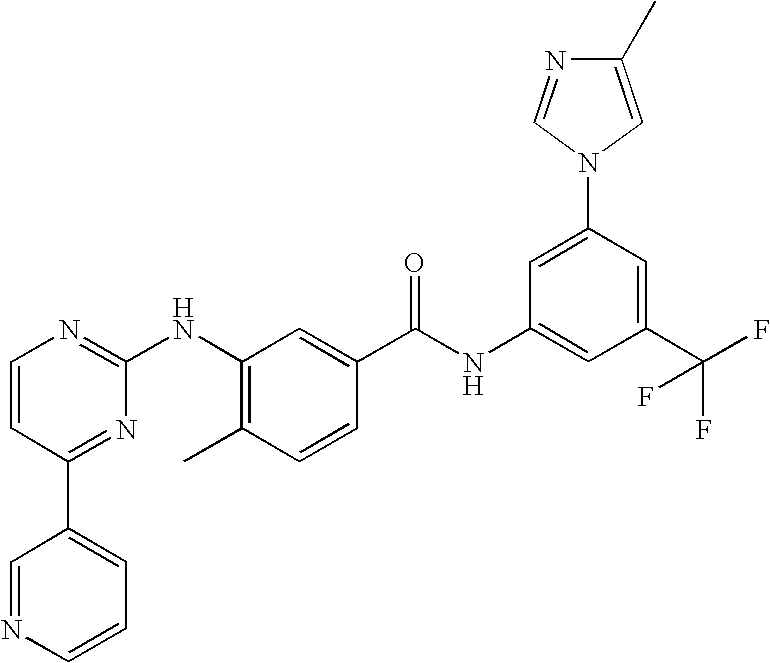
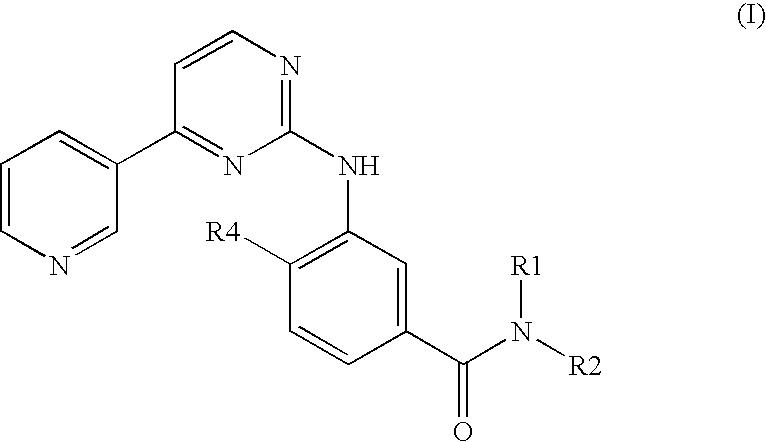
Pharmaceutical compositions comprising nilotinib or its salt
a technology which is applied in the direction of drug compositions, biocide, capsule delivery, etc., can solve the problems of difficult formulation and delivery of nilotinib and its salts, and poor water soluble compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0071]The therapeutic compound in this example is nilotinib hydrochloride monohydrate. This therapeutic compound has low solubility in aqueous media. Furthermore this therapeutic compound has a slight hygroscopic tendency.
[0072]Table 1 shows the formulation of Example 1
Amount perPercentageIngredientscapsule (mg)(w % / w %)GranuleNilotinib hydrochloride220.6055.2%monohydratePoloxamer 1883.180.8Lactose monohydrate78.4719.6%Polyvinyl pyrrolidone15.91 4%External PhaseLactose monohydrate77.6419.4%Colloidal silicon dioxide2.10 0.5%magnesium stearate2.10 0.5%Total400.0
[0073]The nilotinib hydrochloride monohydrate, lactose monohydrate and polyvinyl pyrrolidone are mixed together using a high shear mixer to form a powder blend. The poloxamer 188 is solubilized with purified water and then added to the powder blend in order to wet the powder blend. Then, the mixture is kneaded and dried in a fluid bed dryer to form granules. Lactose monohydrate and colloidal silicon dioxide (as part of the ext...
example 2
Dissolution Profile
[0076]Dissolution testing is performed using the basket method according to Ph. Eur. 2.9.3 ‘Dissolution test for solid dosage forms’ and USP ‘Dissolution’ at 100 rpm in 1000 ml 0.1 N HCL as dissolution material. The determination of the amount of drug substance dissolved (%) is performed with a UV detection method. The method has been validated for selectivity, accuracy, precision and linearity.
TABLE 2Dissolution Results of the Capsule of Example 1Time PointMean of Nilotinib hydrochloride(min)monohydrate dissolved in %529.81597.23098.56099.1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com