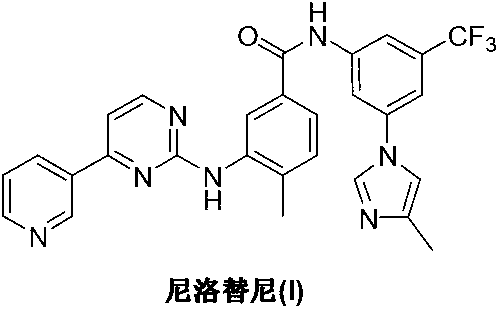
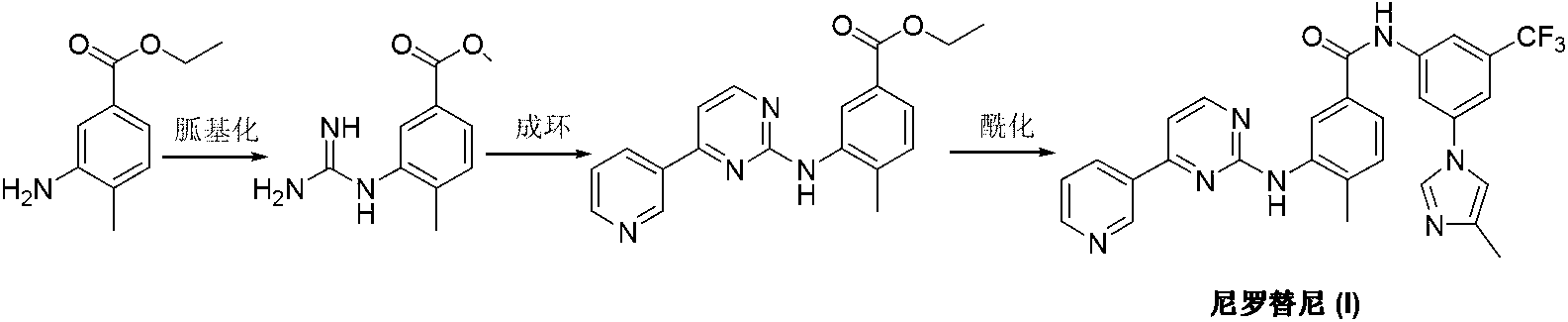
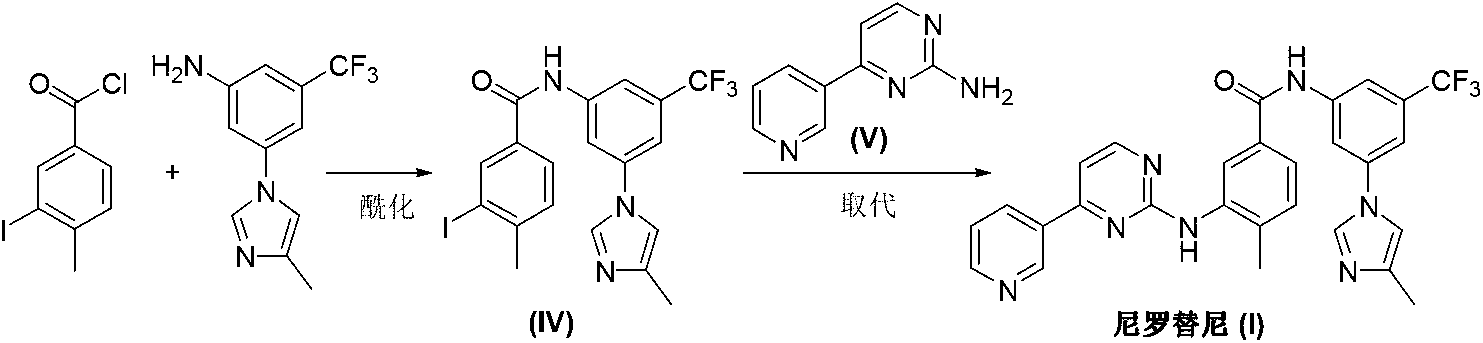
Preparation method of nilotinib
A technology of nilotinib and methyl, applied in the field of nilotinib preparation, can solve the problems of rare raw materials, high cost, long steps, etc., and achieve the effects of controllable production, improved product quality, and promotion of development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Under nitrogen protection, 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidinyl]amino]benzoic acid (II) (3.06g, 10mmol), benzotriazepam Azol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) (6.63 g, 15 mmol) and acetonitrile 50 mL. Under stirring, 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (2.28 g, 15 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 60° C., and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, dissolved in 100 mL of ethyl acetate, and washed with 20 mL of 2M sodium hydroxide. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in 100 mL of tetrahydrofuran, 5-(4-methyl-1H-imidazol-1-yl)-3-trifluoromethylaniline (3.13 g, 13 mmol) and sodium hydride (0.37 g, 15 mmol) were added, and the temperature was raised to 80° C., stirred for 5 hours, and TLC monitored the completion of the r...
Embodiment 2
[0026] Under nitrogen protection, 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidinyl]amino]benzoic acid (II) (3.06g, 10mmol), benzotriazepam Azol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) (6.63 g, 15 mmol) and acetonitrile 50 mL. Under stirring, 1,5-diazabicyclo[4.3.0]-non-5-ene (DBN) (1.86 g, 15 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 60° C., and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, dissolved in 100 mL of ethyl acetate, and washed with 20 mL of 2M sodium hydroxide. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in 100 mL of tetrahydrofuran, 5-(4-methyl-1H-imidazol-1-yl)-3-trifluoromethylaniline (3.13 g, 13 mmol) and sodium hydride (0.37 g, 15 mmol) were added, and the temperature was raised to 50° C., stirred for 5 hours, and TLC monitored the completion of the rea...
Embodiment 3
[0028] Under nitrogen protection, 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidinyl]amino]benzoic acid (II) (3.06g, 10mmol), benzotriazepam Azol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) (6.63g, 15mmol), 5-(4-methyl-1H-imidazol-1-yl)-3-tri Fluoromethylaniline (3.13 g, 13 mmol) and N,N-dimethylformamide 50 mL. Under stirring, 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (4.56 g, 30 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 60° C., and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, dissolved in 100 mL of ethyl acetate, and washed with 20 mL of 2M sodium hydroxide. The organic phase was separated, dried and concentrated under reduced pressure. The residue was recrystallized from tetrahydrofuran / ethyl acetate (4:1) to obtain 4.32 g of off-white solid nilotinib (I), with a yield of 81.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com