Ratio fluorescence probe for identification of nilotinib, preparation and identification method thereof
A ratiometric fluorescent probe, nilotinib technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of excessive use of organic solvents, expensive detection instruments, high analysis costs, etc., to achieve the preparation process Simple, direct recognition, and visible recognition results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
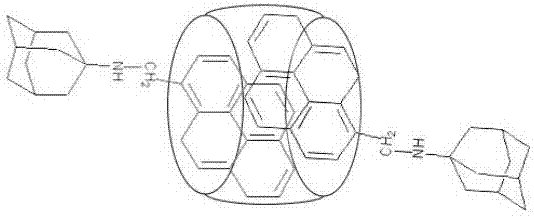
[0043] Embodiment 1. A ratio fluorescent probe for identifying nilotinib, the molecular formula is: C 114 H 118 O 20 N 42 , Structural formula as attached figure 1 Shown.
[0044] The preparation method of the ratio fluorescent probe is as follows:
[0045] 1) Mix amantadine hydrochloride and sodium hydroxide, add anhydrous methanol, stir until a suspension is obtained, react for 1.5 hours, filter, and take the filtrate to obtain product A;
[0046] 2) Take pyrene formaldehyde and dissolve it in anhydrous methanol to obtain product B;
[0047] 3) Add product B to product A, reflux at 65°C for 18 hours, then cool to room temperature, add sodium borohydride, reflux at 65°C for 18 hours, then cool to room temperature, and finally distill under reduced pressure. Light yellow solid, product C is obtained;
[0048] 4) Dissolve product C in dichloromethane, add water for extraction, and take the organic phase to obtain product D;
[0049] 5) Distill product D under reduced pressure, filter, an...
Embodiment 2
[0051] Embodiment 2. A ratio fluorescent probe for identifying nilotinib, the molecular formula is: C 114 H 118 O 20 N 42 , Structural formula as attached figure 1 Shown.
[0052] The preparation method of the ratio fluorescent probe is as follows:
[0053] 1) Mix amantadine hydrochloride and sodium hydroxide, add anhydrous methanol, stir until a suspension is obtained, react for 1 hour, filter, and take the filtrate to obtain product A;
[0054] 2) Take pyrene formaldehyde and dissolve it in anhydrous methanol to obtain product B;
[0055] 3) Add product B to product A, reflux at 60°C for 12 hours, then cool to room temperature, add sodium borohydride, reflux at 60°C for 12 hours, then cool to room temperature, and finally distill under reduced pressure. Light yellow solid, product C is obtained;
[0056] 4) Dissolve product C in dichloromethane, add water for extraction, and take the organic phase to obtain product D;
[0057] 5) Distill product D under reduced pressure, filter, and d...
Embodiment 3
[0059] Example 3. A ratio fluorescent probe for recognizing nilotinib, the molecular formula is: C 114 H 118 O 20 N 42 , Structural formula as attached figure 1 Shown.
[0060] The preparation method of the ratio fluorescent probe is as follows:
[0061] 1) Mix amantadine hydrochloride and sodium hydroxide, add anhydrous methanol, stir until a suspension is obtained, react for 2 hours, filter, and take the filtrate to obtain product A;
[0062] 2) Take pyrene formaldehyde and dissolve it in anhydrous methanol to obtain product B;
[0063] 3) Add product B to product A, reflux at 70°C for 24 hours, then cool to room temperature, add sodium borohydride, reflux at 70°C for 24 hours, then cool to room temperature, and finally distill under reduced pressure. Light yellow solid, product C is obtained;
[0064] 4) Dissolve product C in dichloromethane, add water for extraction, and take the organic phase to obtain product D;
[0065] 5) Distill product D under reduced pressure, filter, and dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
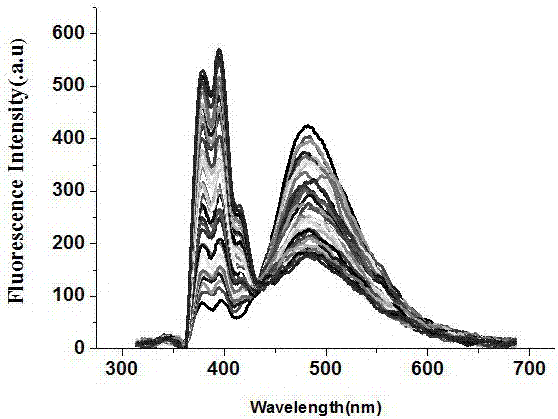
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com