Hydrochloride of pyrrolo-pyrazine compound and application of hydrochloride
A technology of hydrochloride and pyrrole, which is applied in the application field of pyrrolopyrazine compounds and drug preparation, and can solve problems such as disease recurrence
- Summary
- Abstract
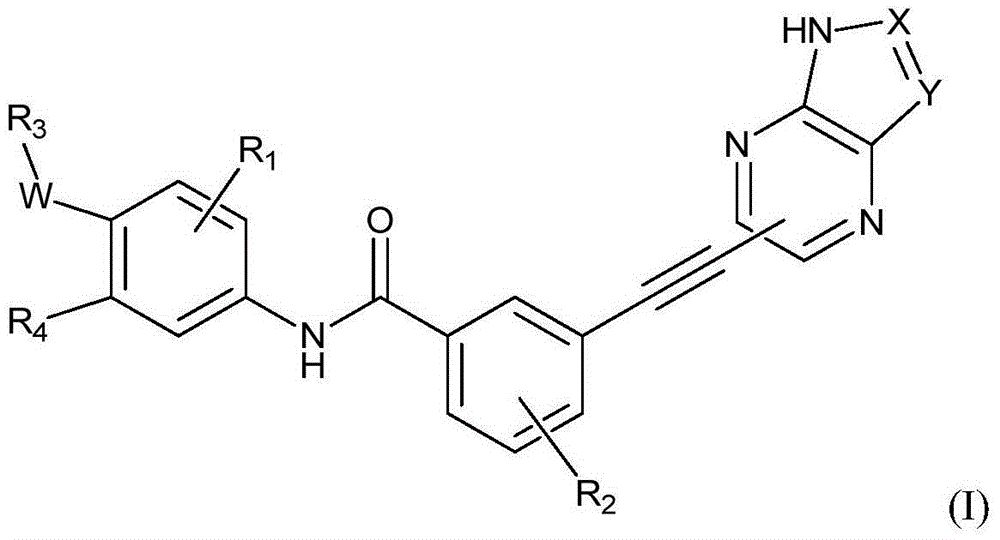
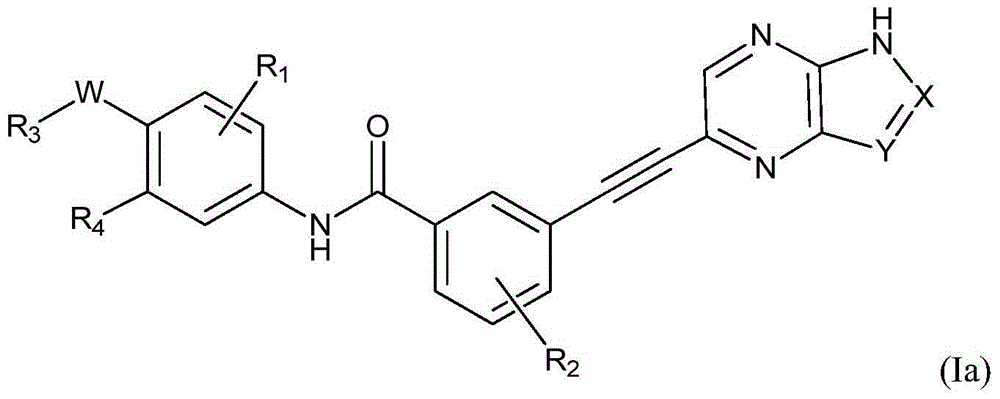
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1 Preparation of 3-ethynyl-4-methyl-N-[4-((4-methylpiperazin-1-yl)methyl)-3-trifluoromethylphenyl]benzamide
[0125]
[0126] Step 1 Preparation of 3-iodo-4-methyl-N-[4-(4-methylpiperazin-1-yl)methyl)-3-trifluoromethylphenyl]benzamide
[0127] 4-(4-Methylpiperazin-1-ylmethyl)-3-trifluoromethylaniline (2.27 g, 8.3 mmol), 3-iodo-4-methyl-benzoyl chloride ( 10mmol), 15ml tetrahydrofuran, 10ml triethylamine, stirred at room temperature for 4 hours. Add saturated NaHCO 3 Solution washing, ethyl acetate and water extraction, saturated NaCl solution washing, anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column to give the title compound as a yellow oil.
[0128] 1 H NMR (500MHz, CDCl 3 )δ:8.39(s,1H,N-H),8.29(s,1H,Ar-H),7.88(d,1H,Ar-H),7.86(s,1H,Ar-H),7.75(d,1H ,Ar-H),7.73(d,1H,Ar-H),7.28(d,1H,Ar-H),3.62(s,2H,PhCH 2 ),2.60(b,8H,4×-CH 2 ),2.47(s,3H,-CH 3 ),2.31(s,3H,-CH 3 )....
Embodiment 2
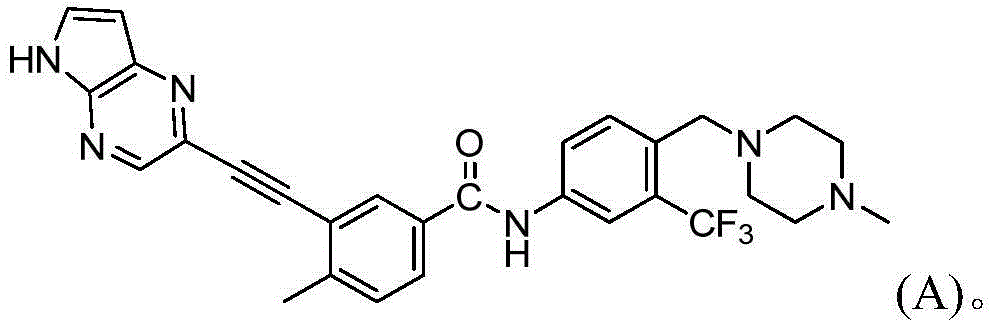
[0135] Example 2 3-((1H-pyrrolo[2,3-b]pyrazin-5-yl)ethynyl)-4-methyl-N-[4-((4-methylpiperazine-1- Base) methyl) -3-trifluoromethylphenyl] benzamide preparation
[0136]
[0137] Add the compound prepared in Example 1 (126mg, 0.3mmol), 5-bromo-1H-pyrrolo[2,3-b]pyrazine (59mg, 0.3mmol), Pd(PPh 3 ) 2 Cl 2 (63mg, 0.006mmol), CuI (18mg, 0.09mmol), 1mL Et 3N and 5mL DMF, under the protection of inert gas, stirred at 80°C for 8 hours. After the reaction was completed, extract with ethyl acetate and water, combine the organic layers, wash with saturated NaCl solution, anhydrous NaCl 2 SO 4 dry. Concentration under reduced pressure and the residue was purified by silica gel column to obtain the compound as an off-white solid.
[0138] 1 H NMR (500MHz, CDCl 3 )δ:8.91(br,1H,-NH),8.46(s,1H,Ar-H),8.02(d,1H,Ar-H),7.98(s,1H,Ar-H),7.87(s, 1H,Ar-H),7.85(s,-NH,1H),7.78-7.80(m,1H,Ar-H),7.69-7.70(d,1H,Ar-H),7.60-7.62(m,1H ,Ar-H),7.35(d,1H,Ar-H),6.72-6.73(m,1H,Ar-H),3.61(s,2H,-CH 2 ...
Embodiment 3
[0140] Example 3 3-((1H-pyrrolo[2,3-b]pyrazin-5-yl)ethynyl)-4-methyl-N-[4-((4-methylpiperazine-1- Base) methyl) -3-trifluoromethylphenyl] benzamide hydrochloride
[0141] Weigh 3-((1H-pyrrolo[2,3-b]pyrazin-5-yl)ethynyl)-4-methyl-N-[4-((4-methylpiperazin-1-yl )methyl)-3-trifluoromethylphenyl]benzamide (30mg) was dissolved in 5mL of methanol, and ethyl hydrogen chloride solution was added dropwise to a pH value of about 3, stirred at room temperature for 3h, and evaporated under reduced pressure to remove the volatile The material was dried in vacuo at 50 °C for 5 h to afford the title compound.
[0142] 1 H NMR (300MHz, DMSO-d 6 )δ:12.34(s,1H),10.61(s,1H),10.25(b,1H),8.56(s,1H),8.26(s,2H),8.14(d,1H),7.96-8.01(m ,2H),7.73(d,1H),7.56(d,1H),6.67-6.69(m,1H),3.70(s,2H),3.37(m,4H),2.89-3.06(m,4H), 2.77(s,3H), 2.61(s,3H).
[0143] 1 H NMR (300MHz, DMSO-d 6 +D 2 O)δ:10.62(s,1H),8.57(s,1H),8.22(s,2H),8.07(d,1H),7.93-7.99(m,2H),7.74(d,1H),7.56( d,1H),6.71(d,1H),3.70(s,2H),3.38-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com