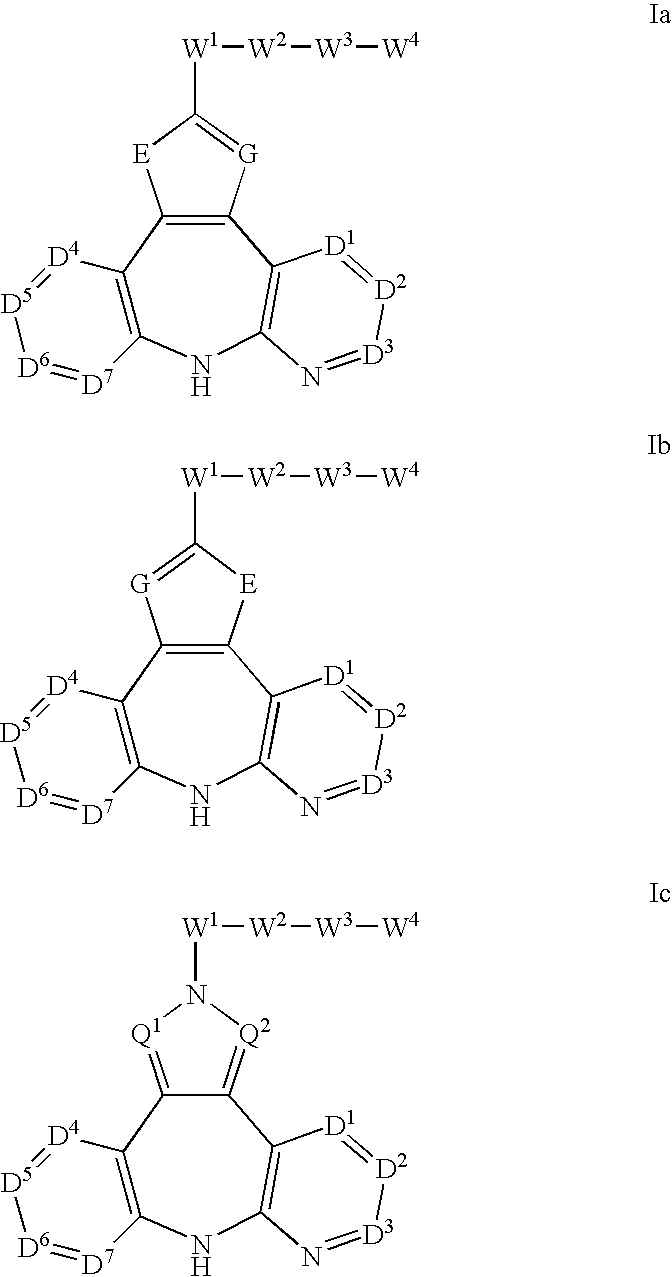
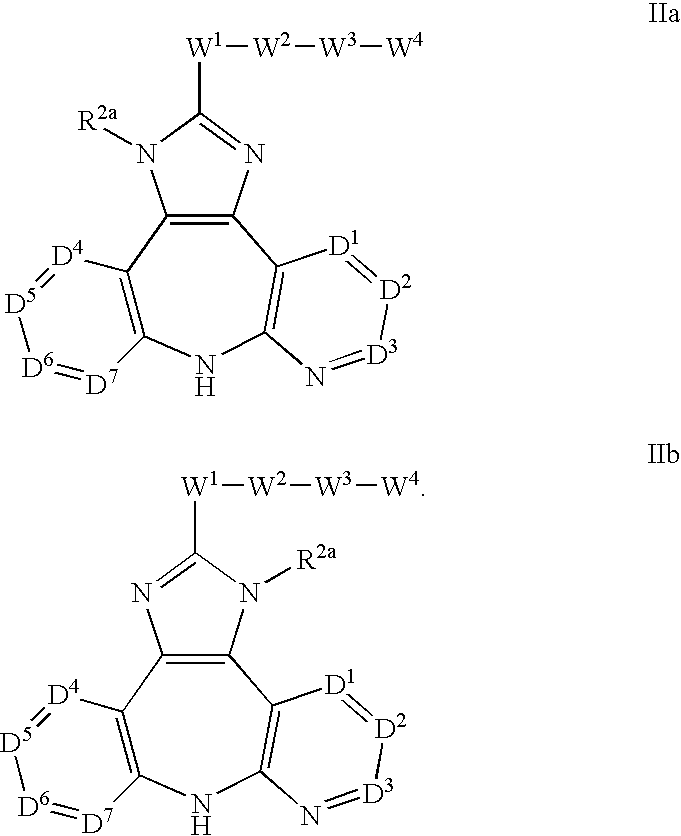
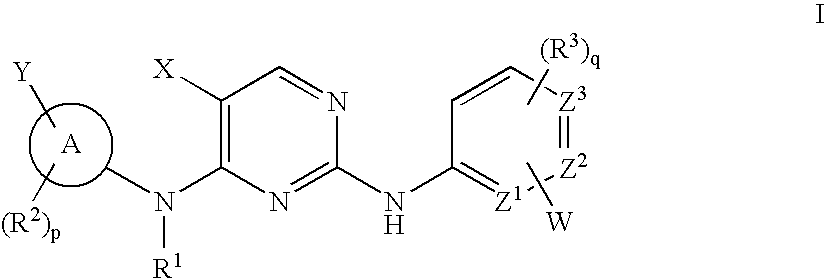
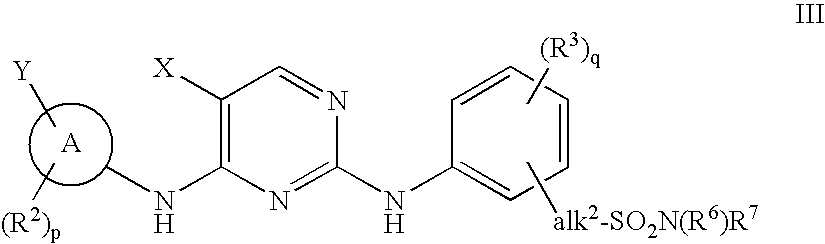
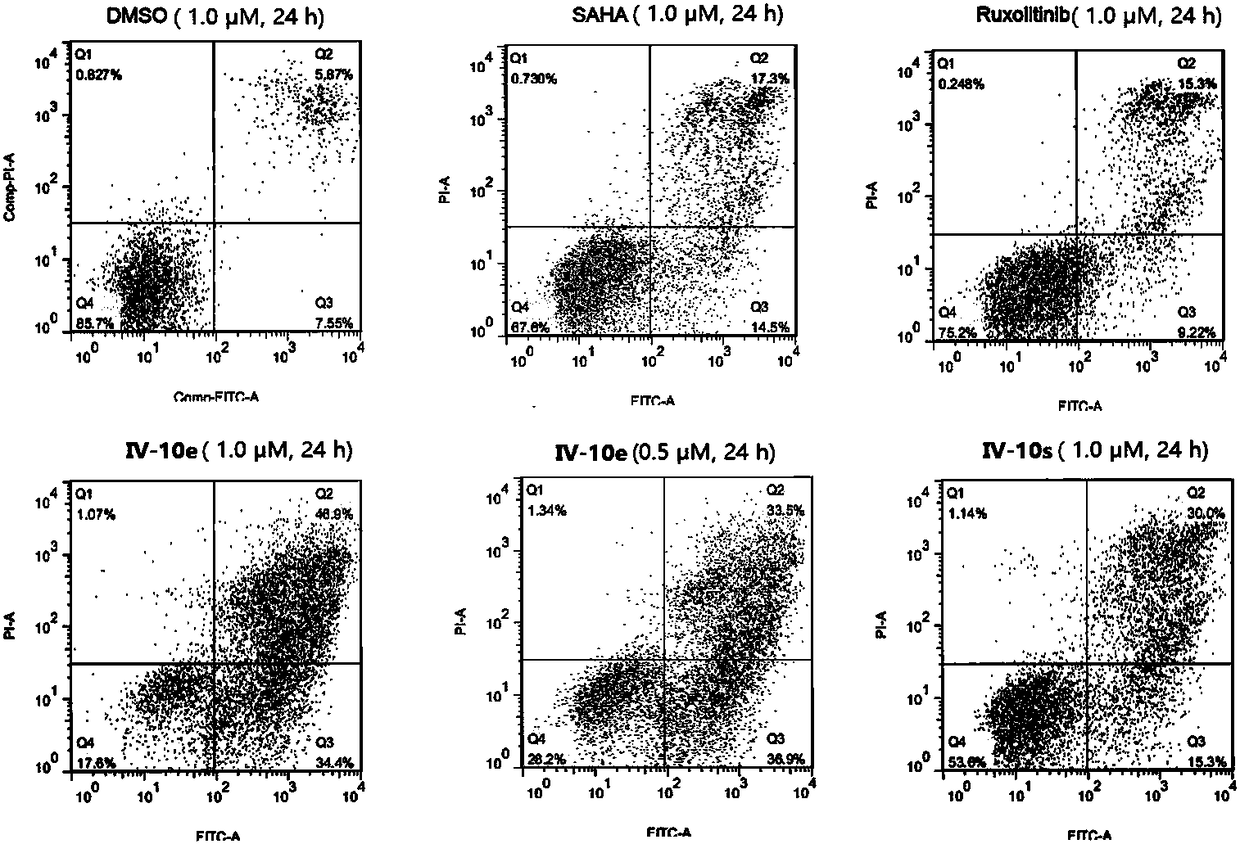
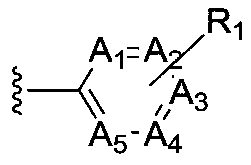
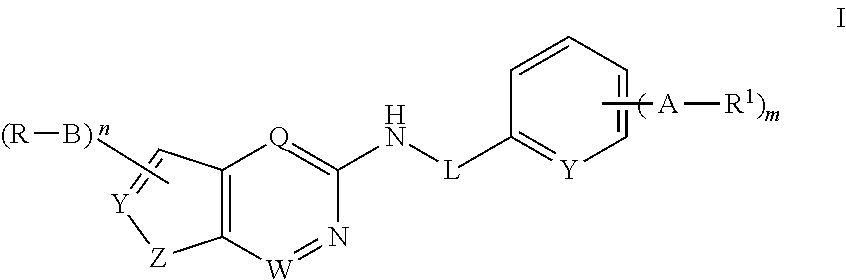
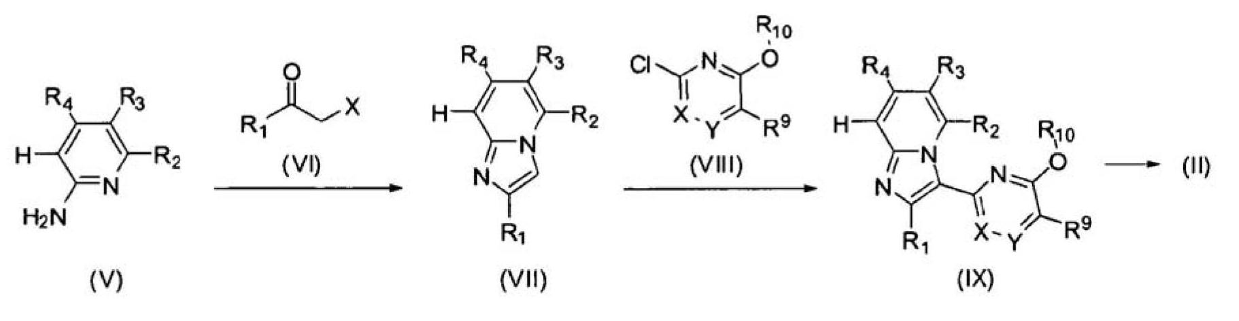
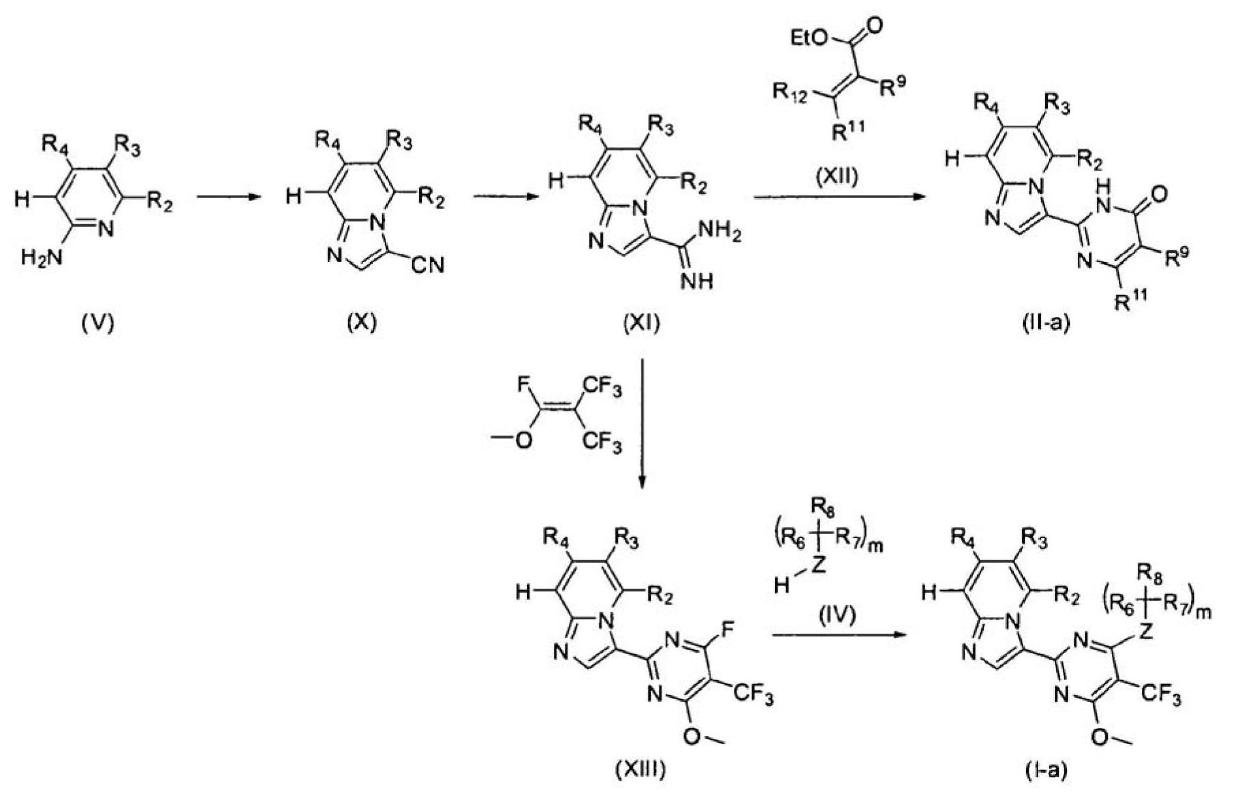
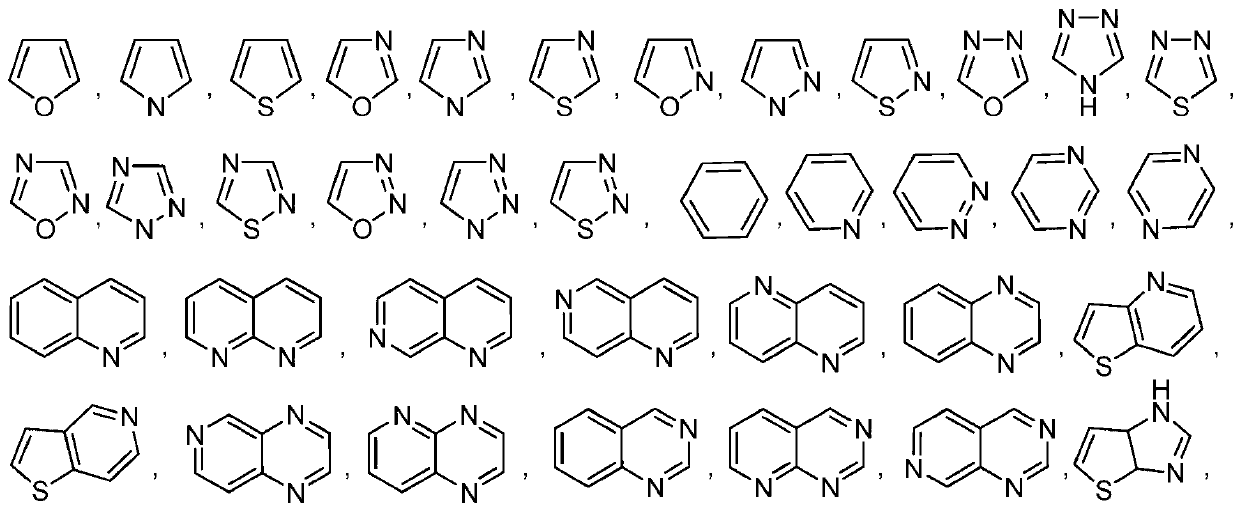
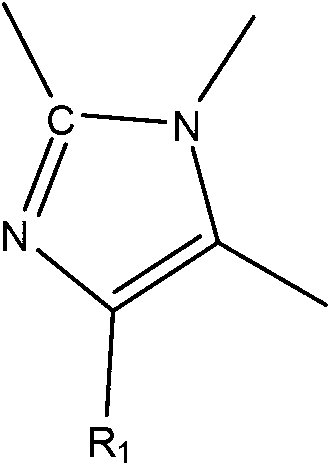
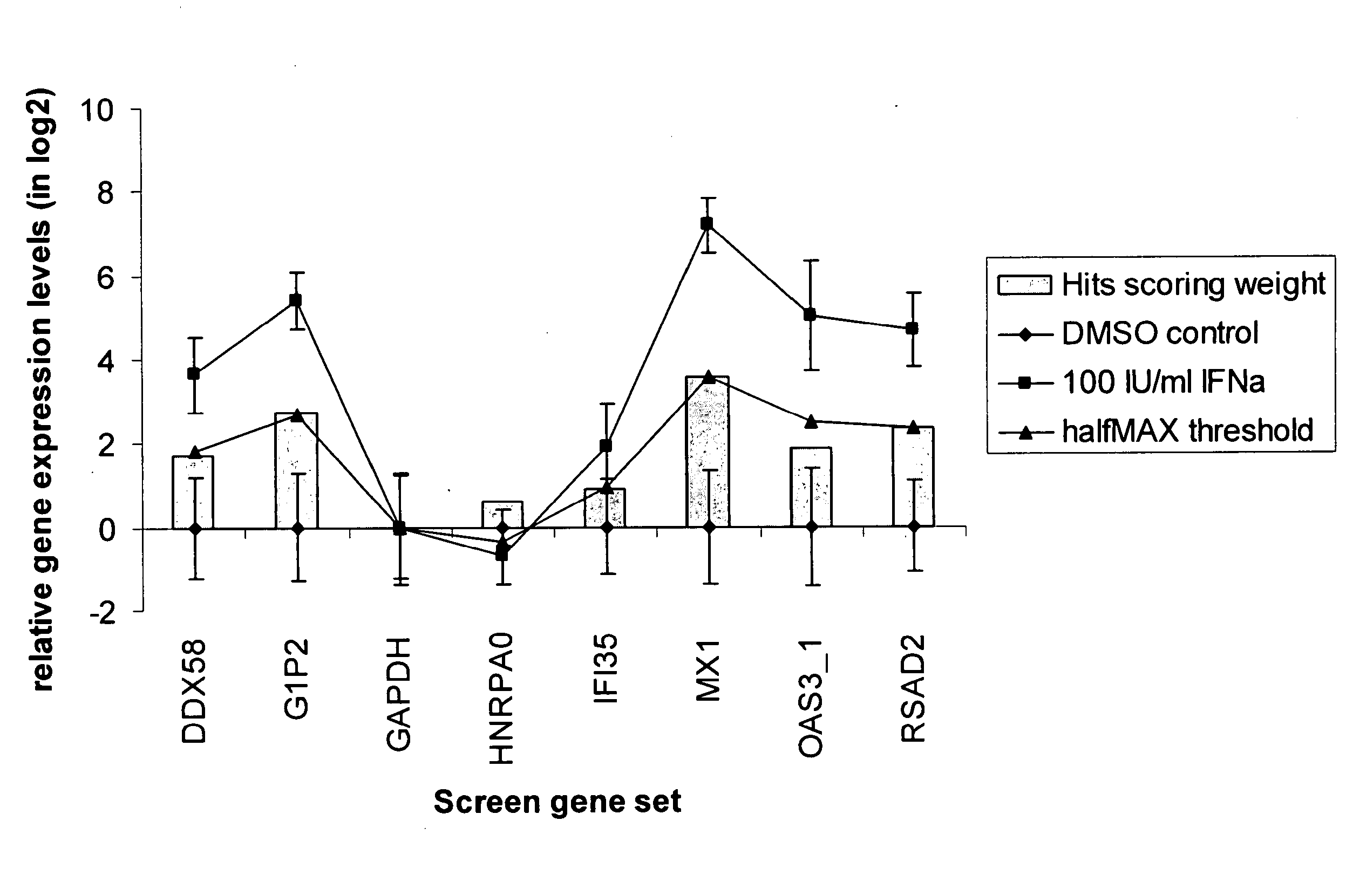
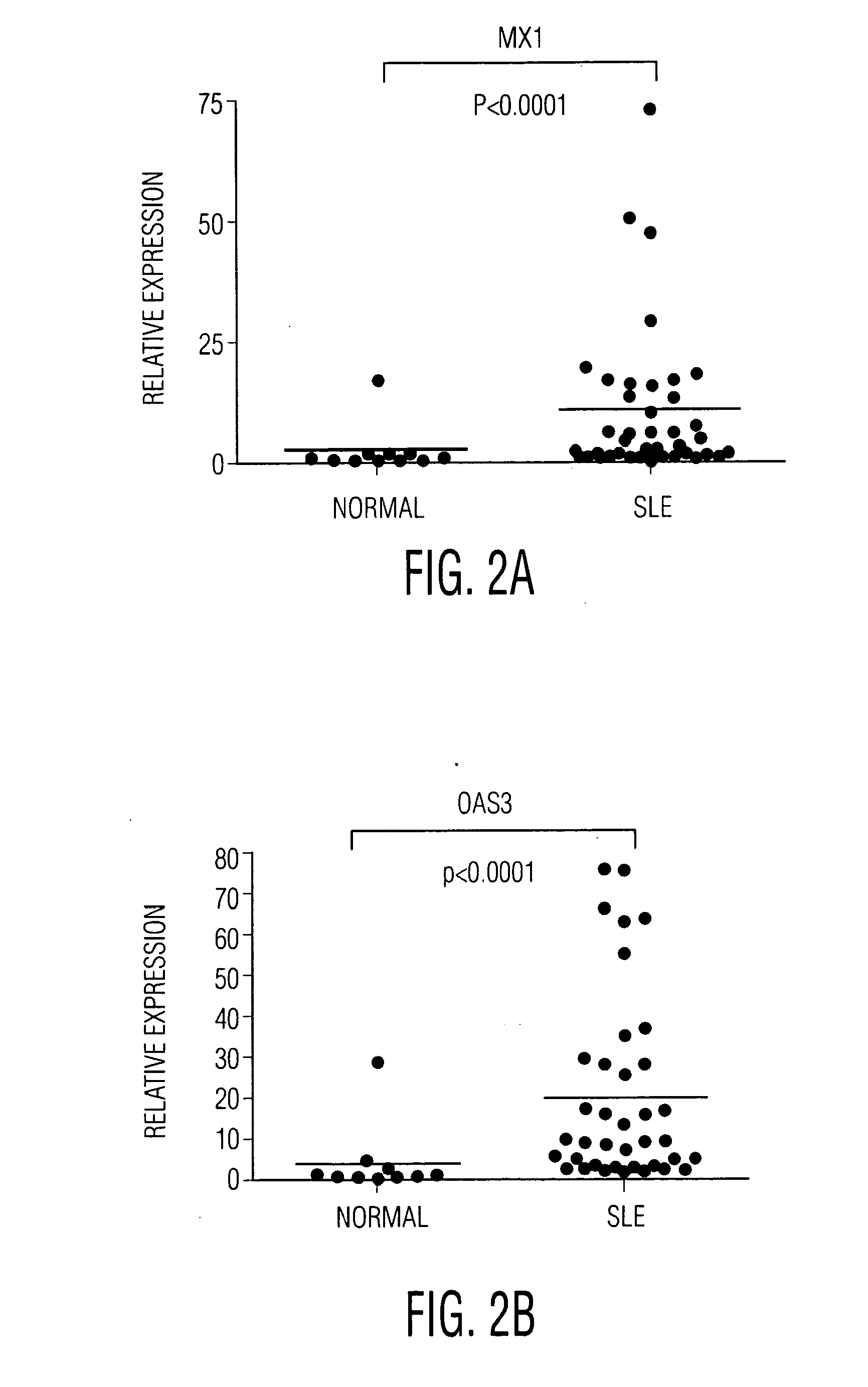
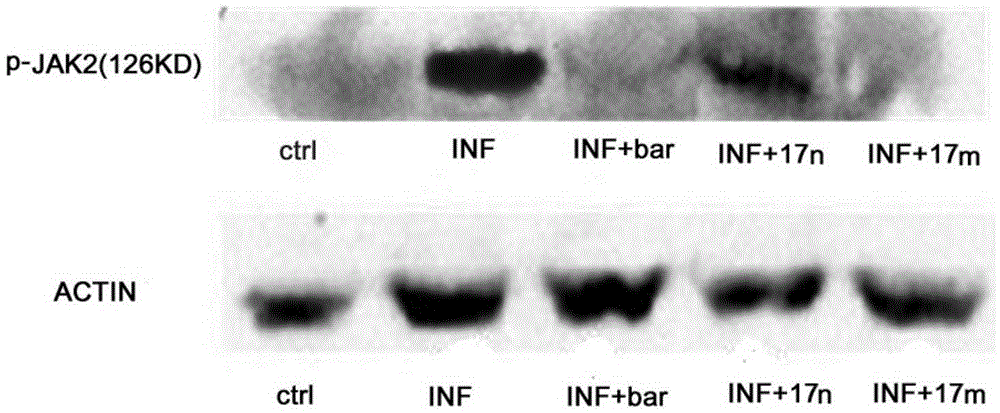
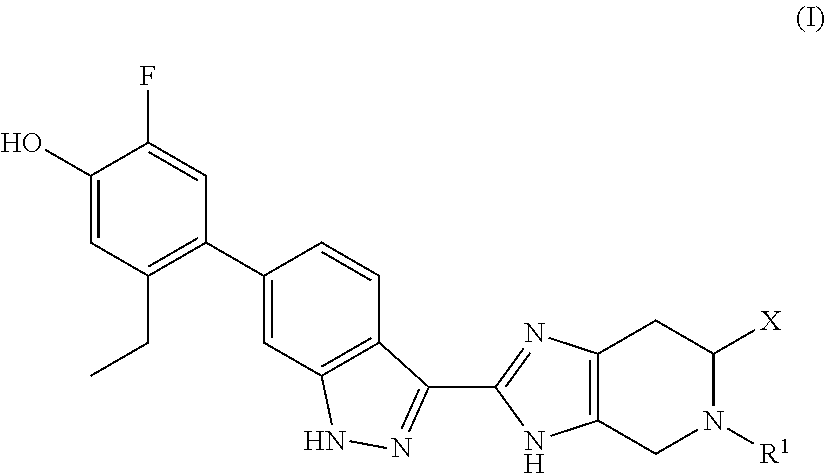
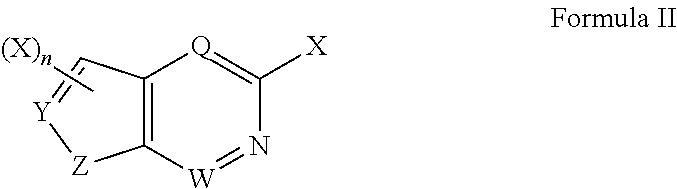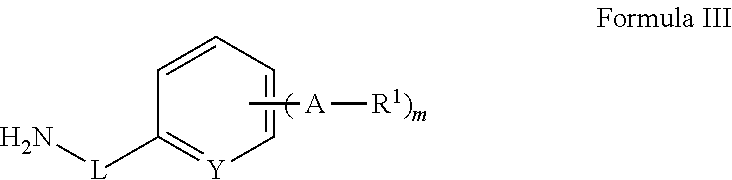Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
125 results about "Janus kinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
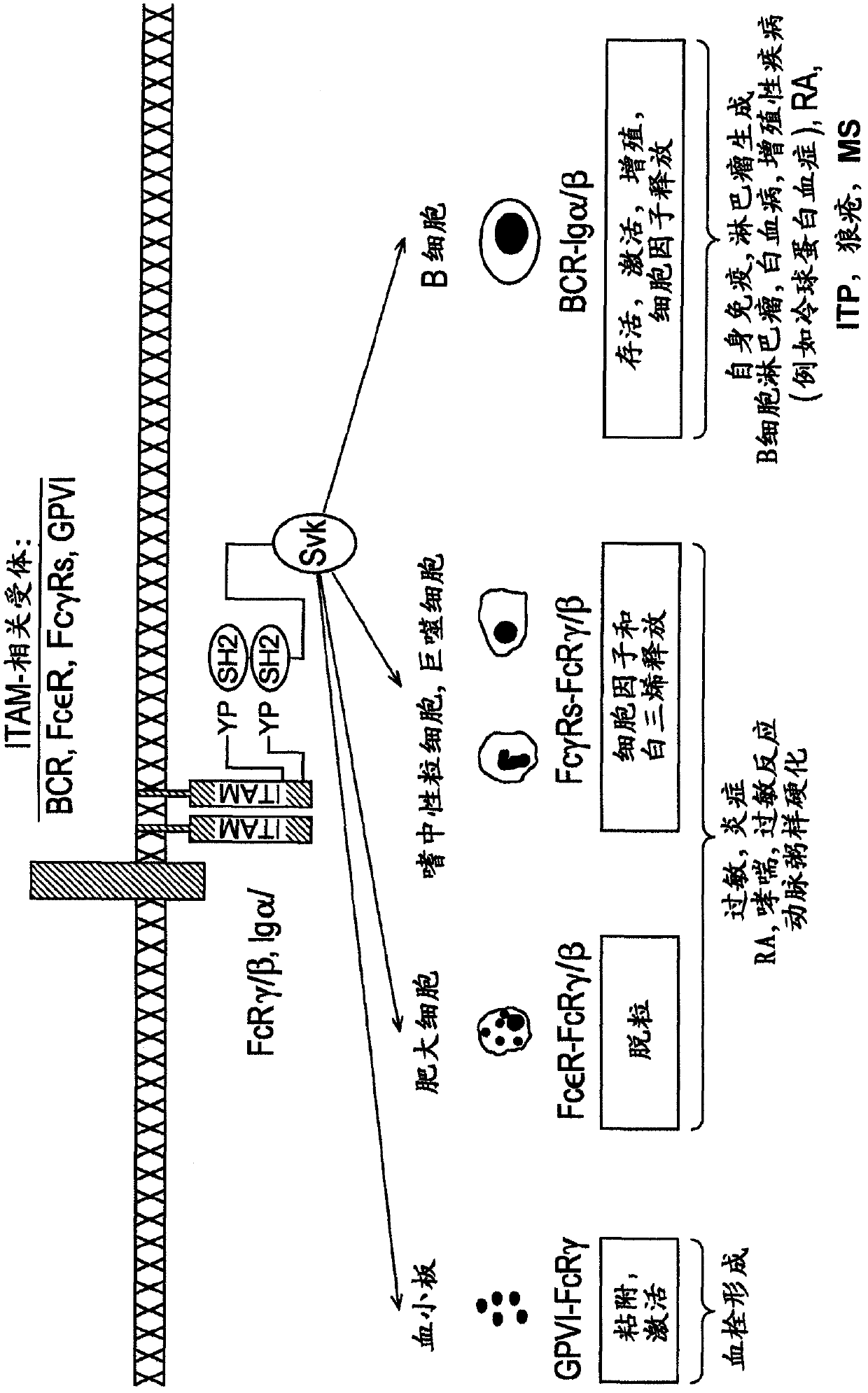
Janus kinase (JAK) is a family of intracellular, nonreceptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway. They were initially named "just another kinase" 1 and 2 (since they were just two of many discoveries in a PCR-based screen of kinases), but were ultimately published as "Janus kinase". The name is taken from the two-faced Roman god of beginnings, endings and duality, Janus, because the JAKs possess two near-identical phosphate-transferring domains. One domain exhibits the kinase activity, while the other negatively regulates the kinase activity of the first.
Compositions and methods for inhibition of the jak pathway
Owner:RIGEL PHARMA
Use of bi-aryl meta-pyrimidine inhibitors of kinases
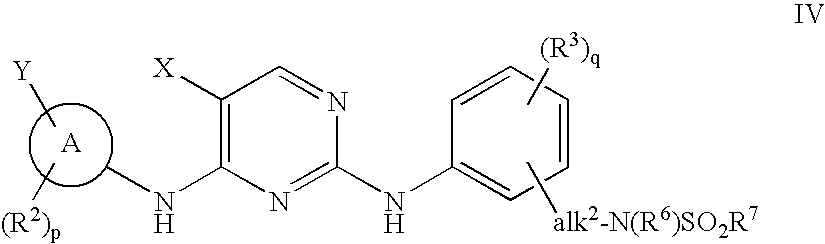
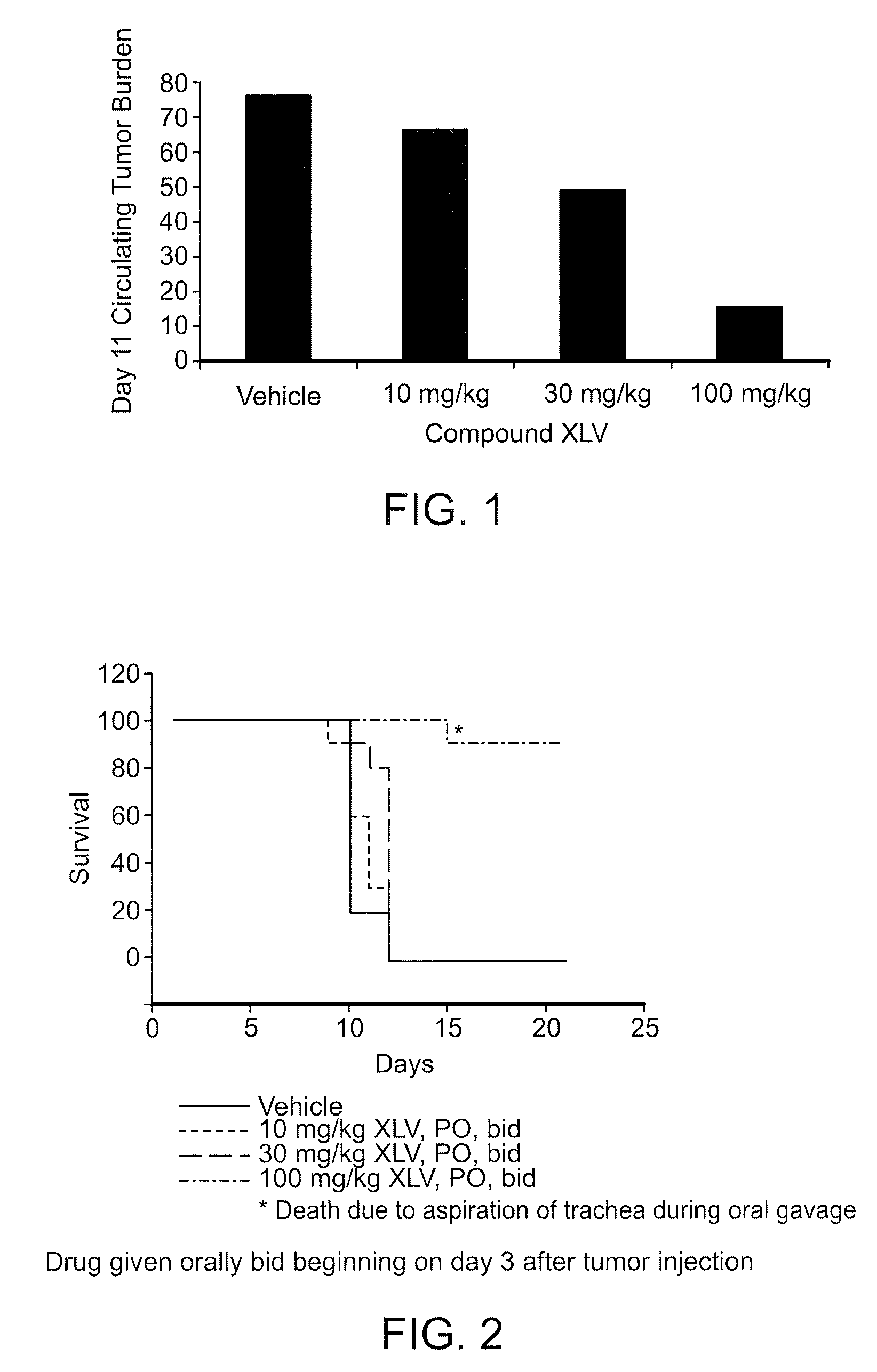
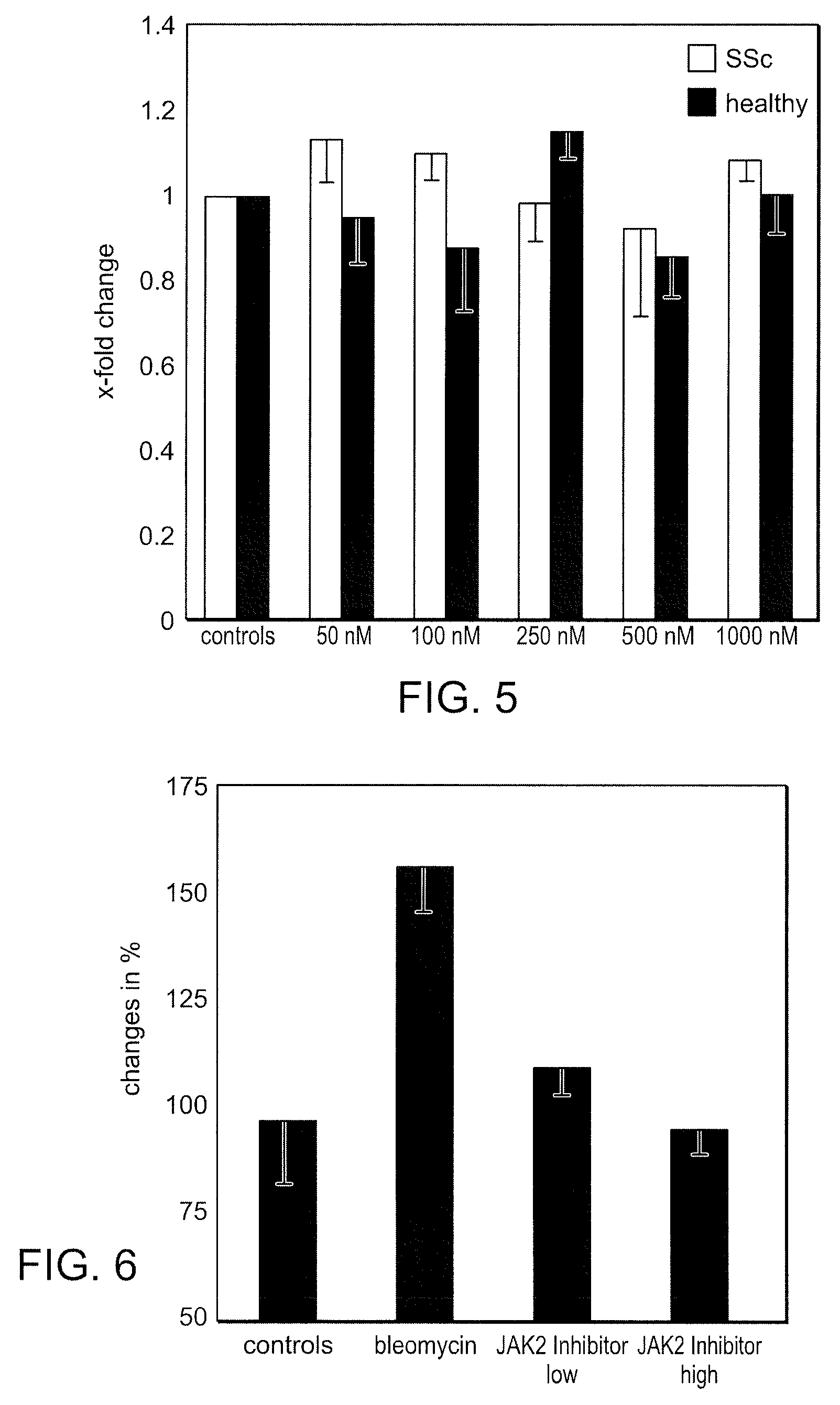
The invention provides methods of treating a disease selected from systemic sclerosis, rheumatoid arthritis, mastocytosis, and chronic eosinophilic leukemia comprising administering biaryl meta-pyrimidine compounds having the general structure (A) to a subject in need thereof. The pyrimidine compounds of the invention are capable of inhibiting kinases, such as members of the JAK kinase family, and various other specific receptor and non-receptor kinases.
Owner:IMPACT BIOMEDICINES INC
Triazolopyridine JAK Inhibitor Compounds and Methods
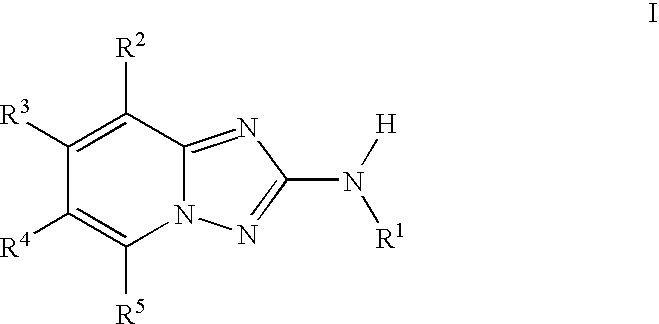
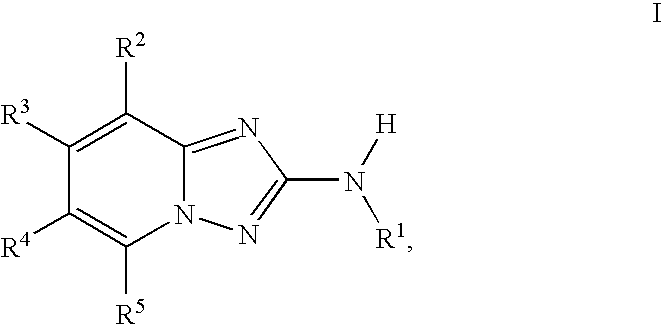
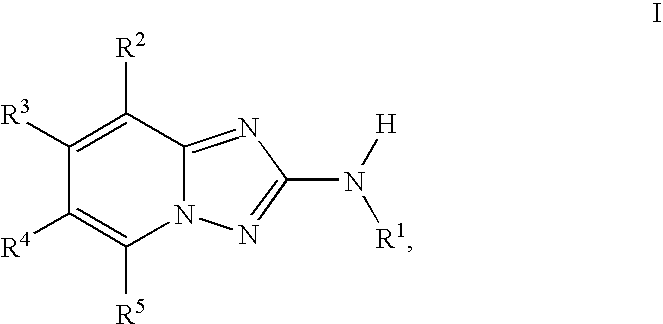
InactiveUS20100048557A1Reduce severityReduce the severity of the diseaseBiocideNervous disorderDiseaseKinase activity
A compound of Formula I, enantiomers, diasteriomers, tautomers or pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4 and R5 are defined herein, are useful as JAK kinase inhibitors. A pharmaceutical composition that includes a compound of Formula I and a pharmaceutically acceptable carrier, adjuvant or vehicle, and methods of treating or lessening the severity of a disease or condition responsive to the inhibition of JAK kinase activity in a patient are disclosed.
Owner:GENENTECH INC
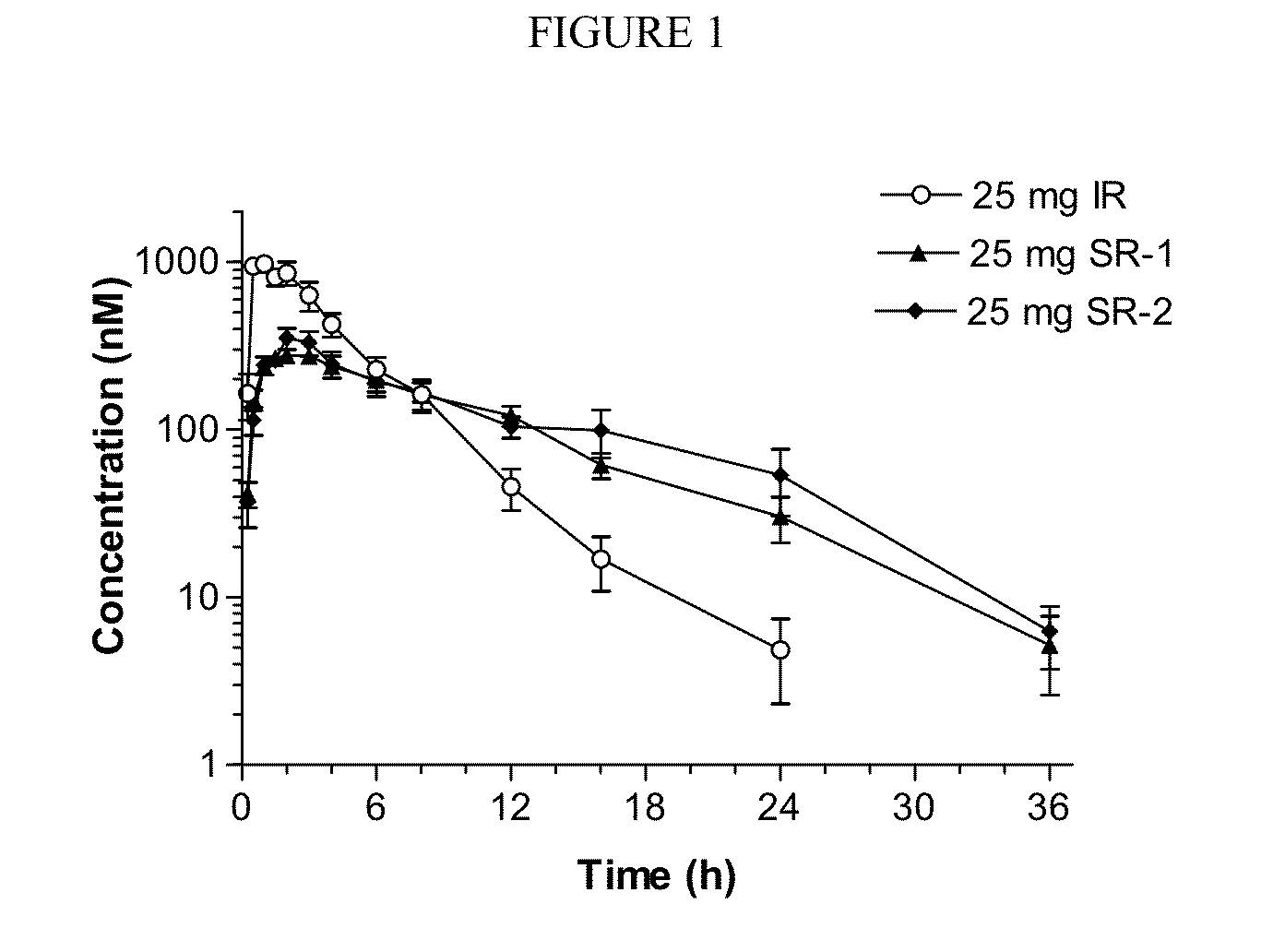
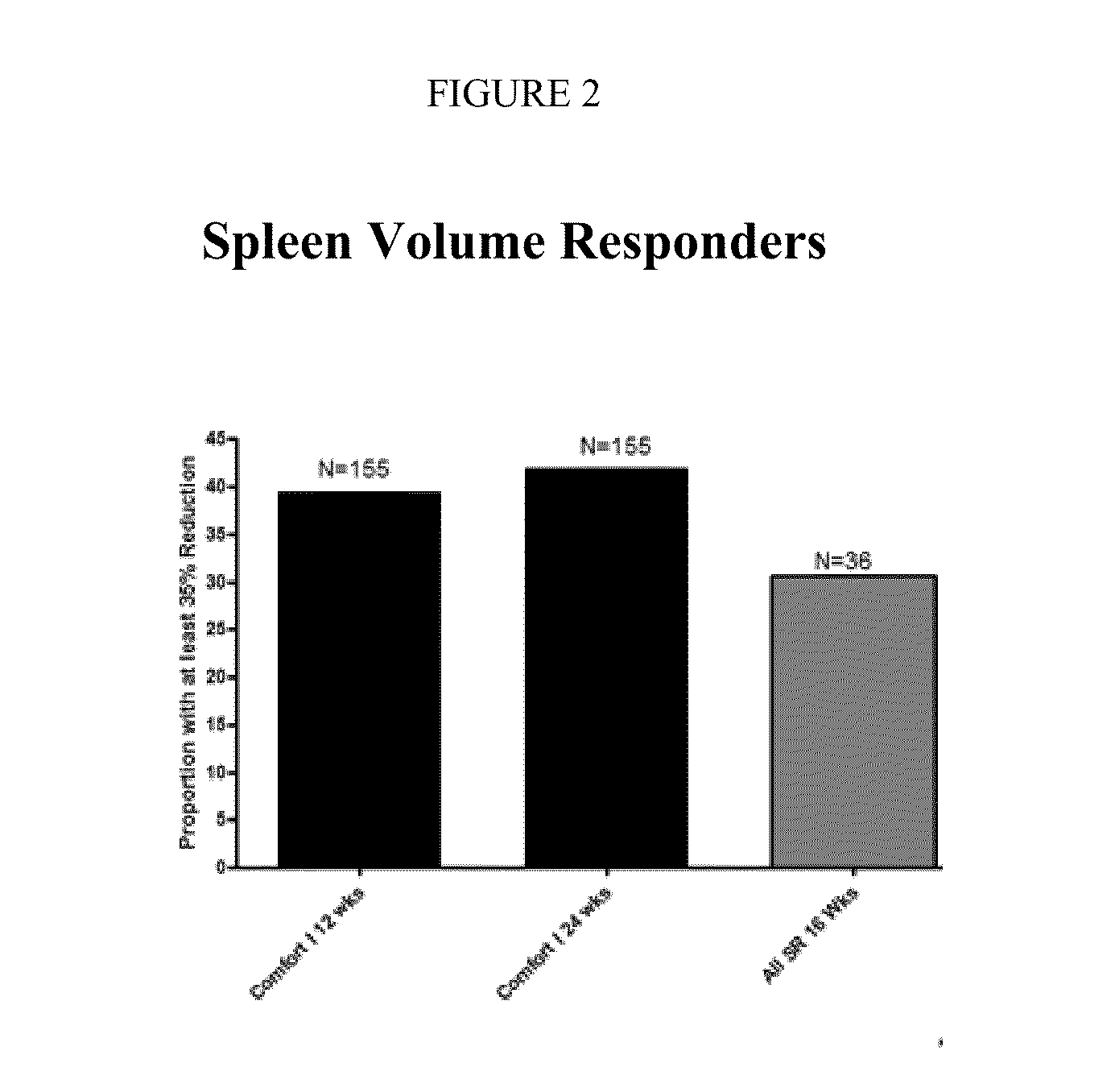
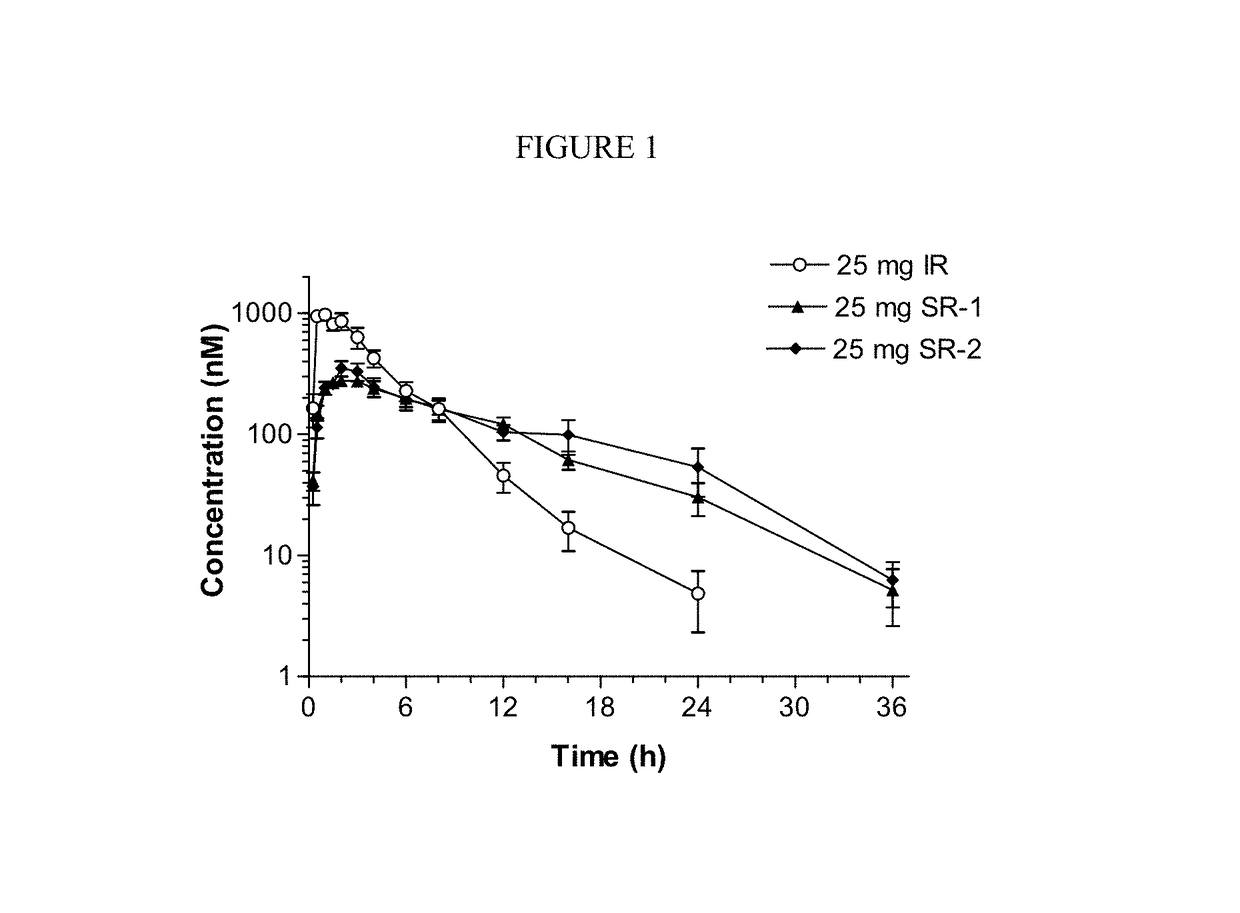
Sustained-release dosage forms of ruxolitinib
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
Bicyclic compounds and their uses as dual c-SRC / JAK inhibitors
Owner:AURIGENE DISCOVERY TECH +1
Inhibitors of protein kinases
InactiveCN102066338AInhibits syk kinase activityNervous disorderOrganic chemistryKinase activityJanus kinase
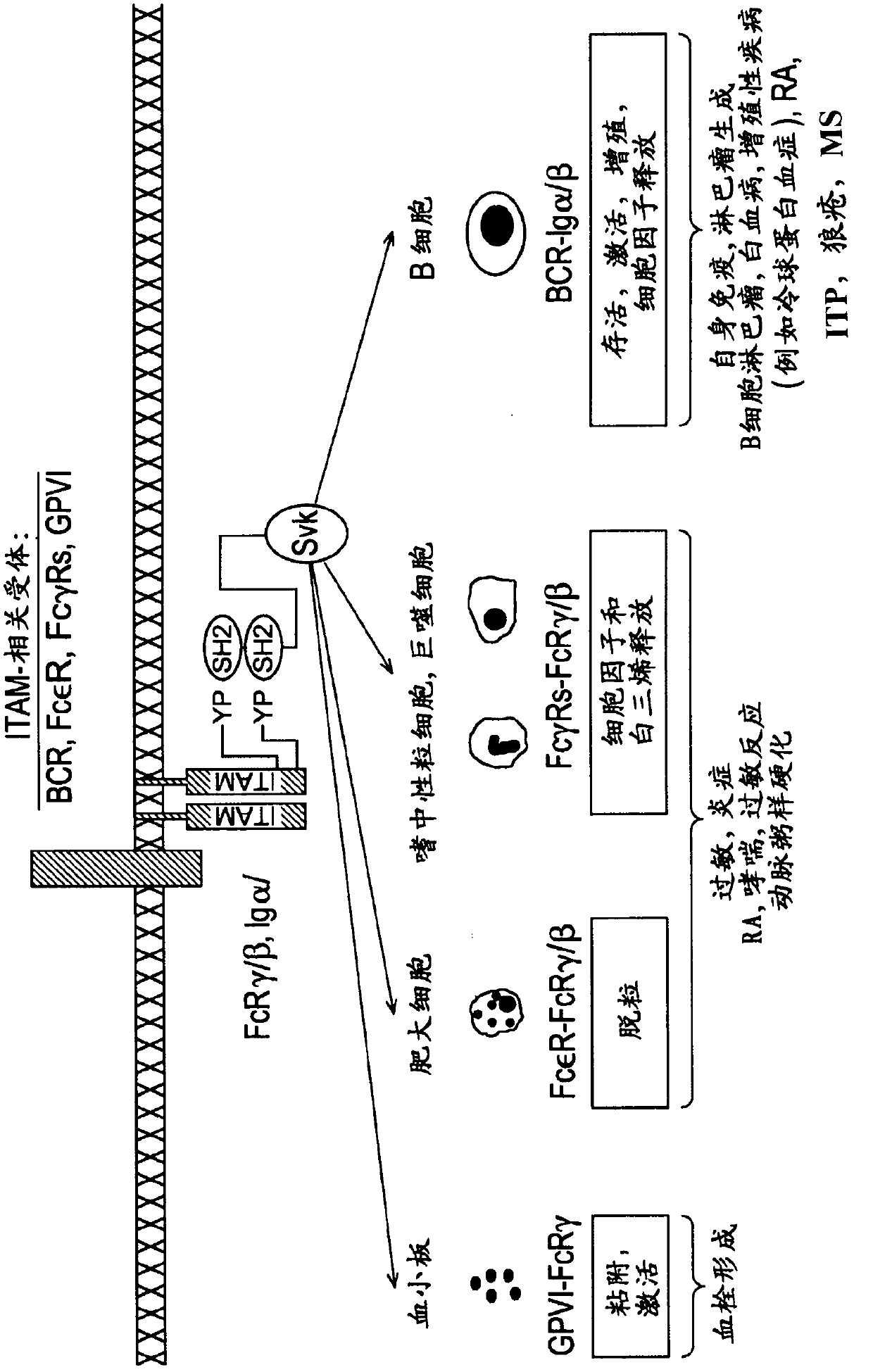
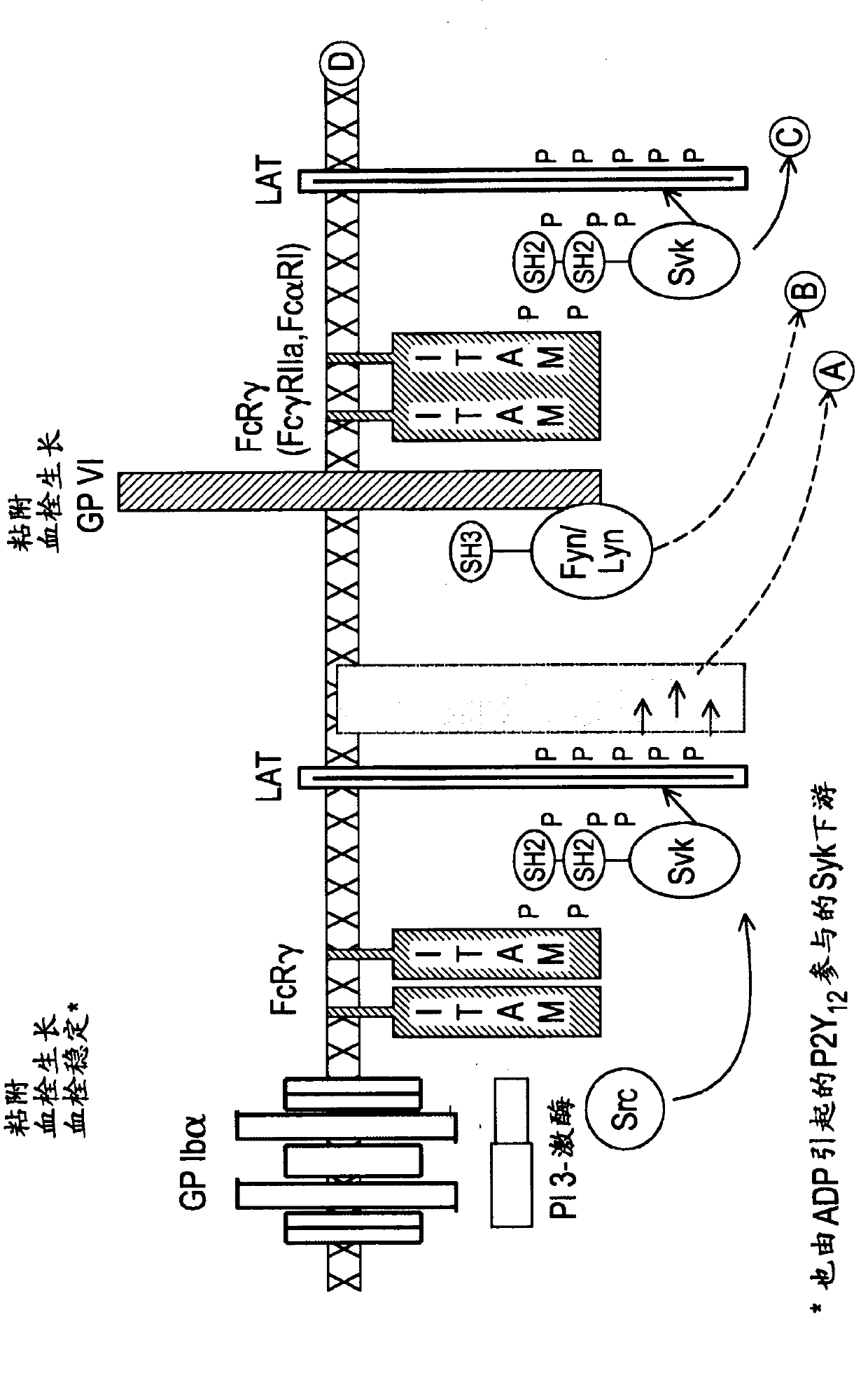
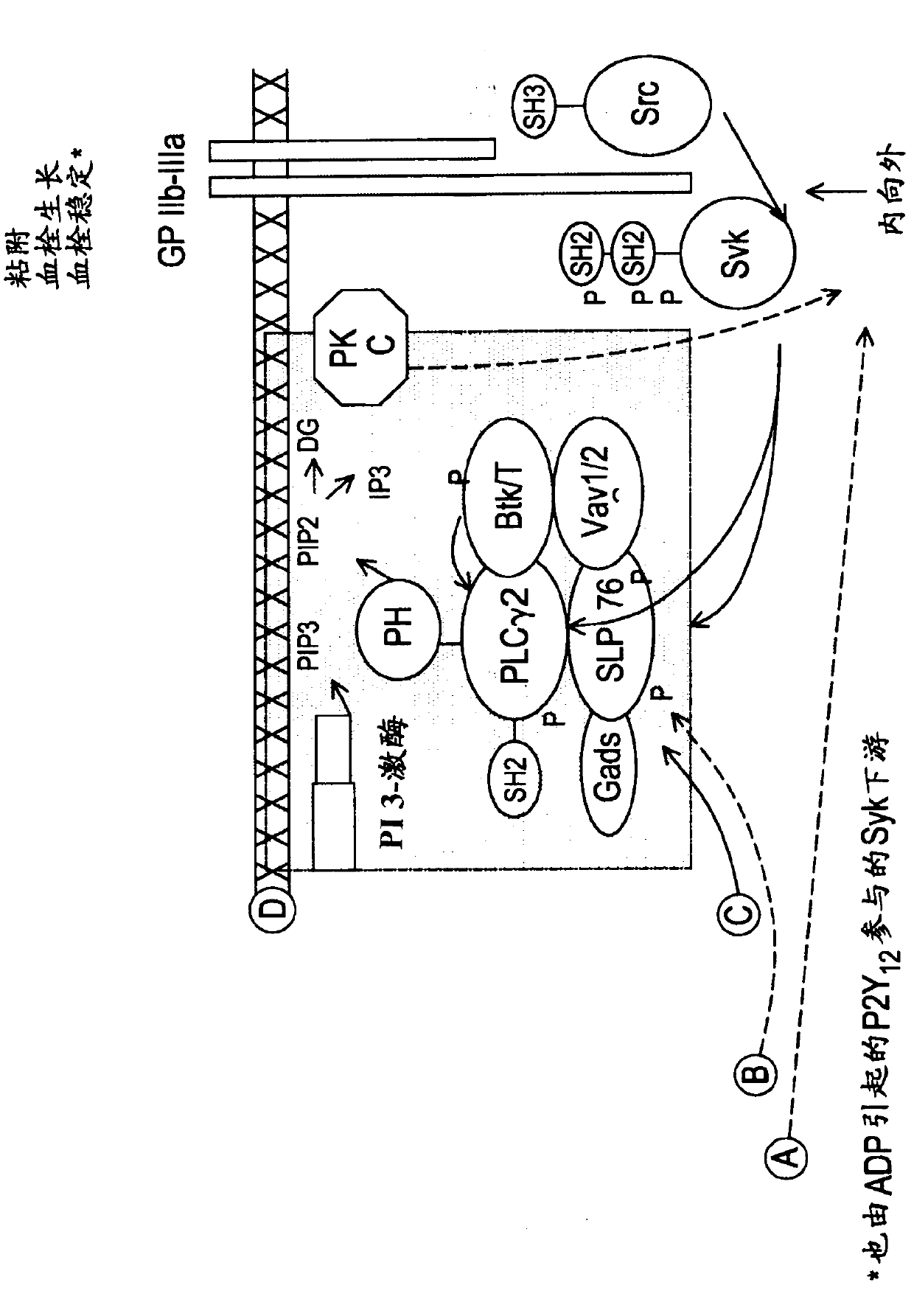
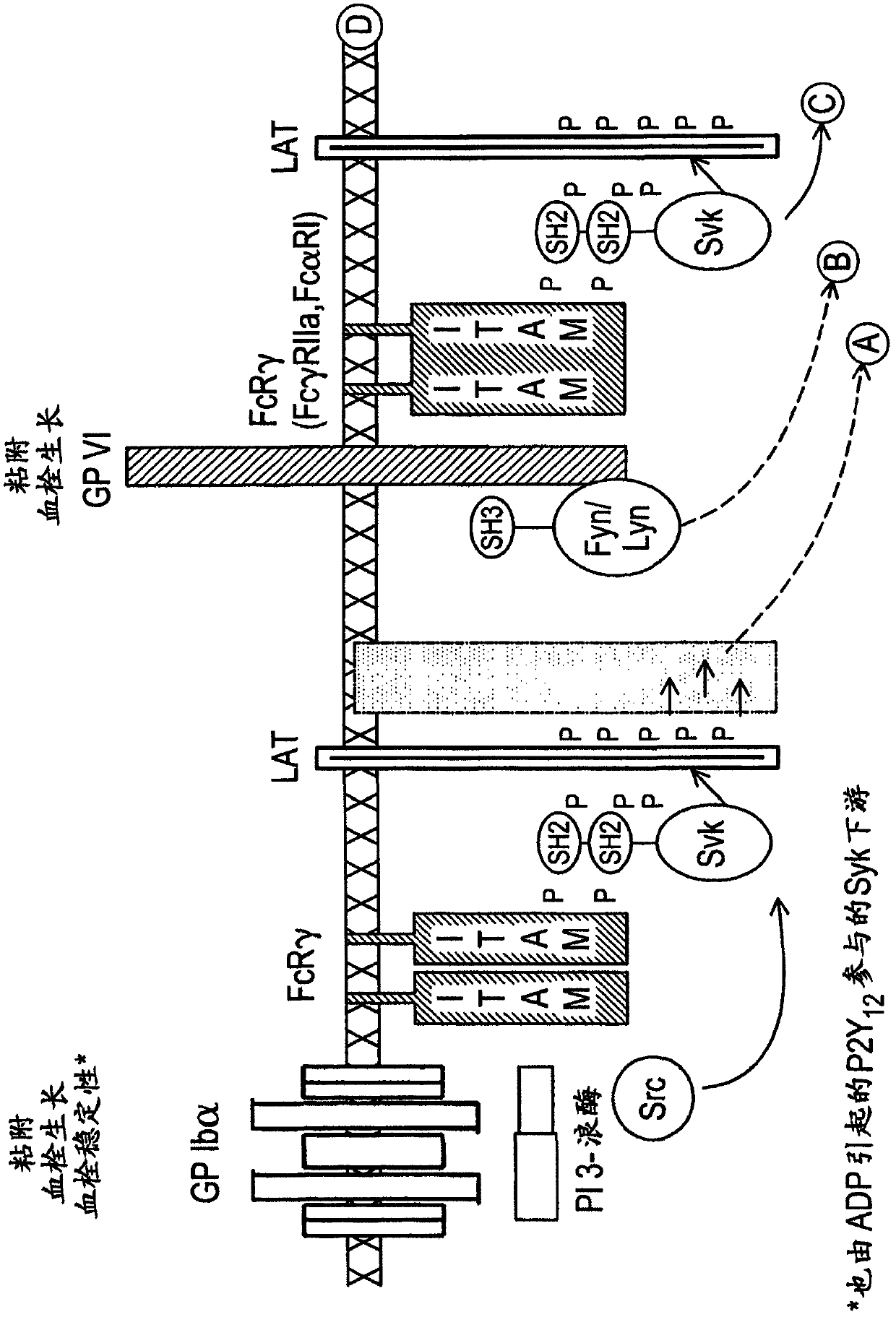
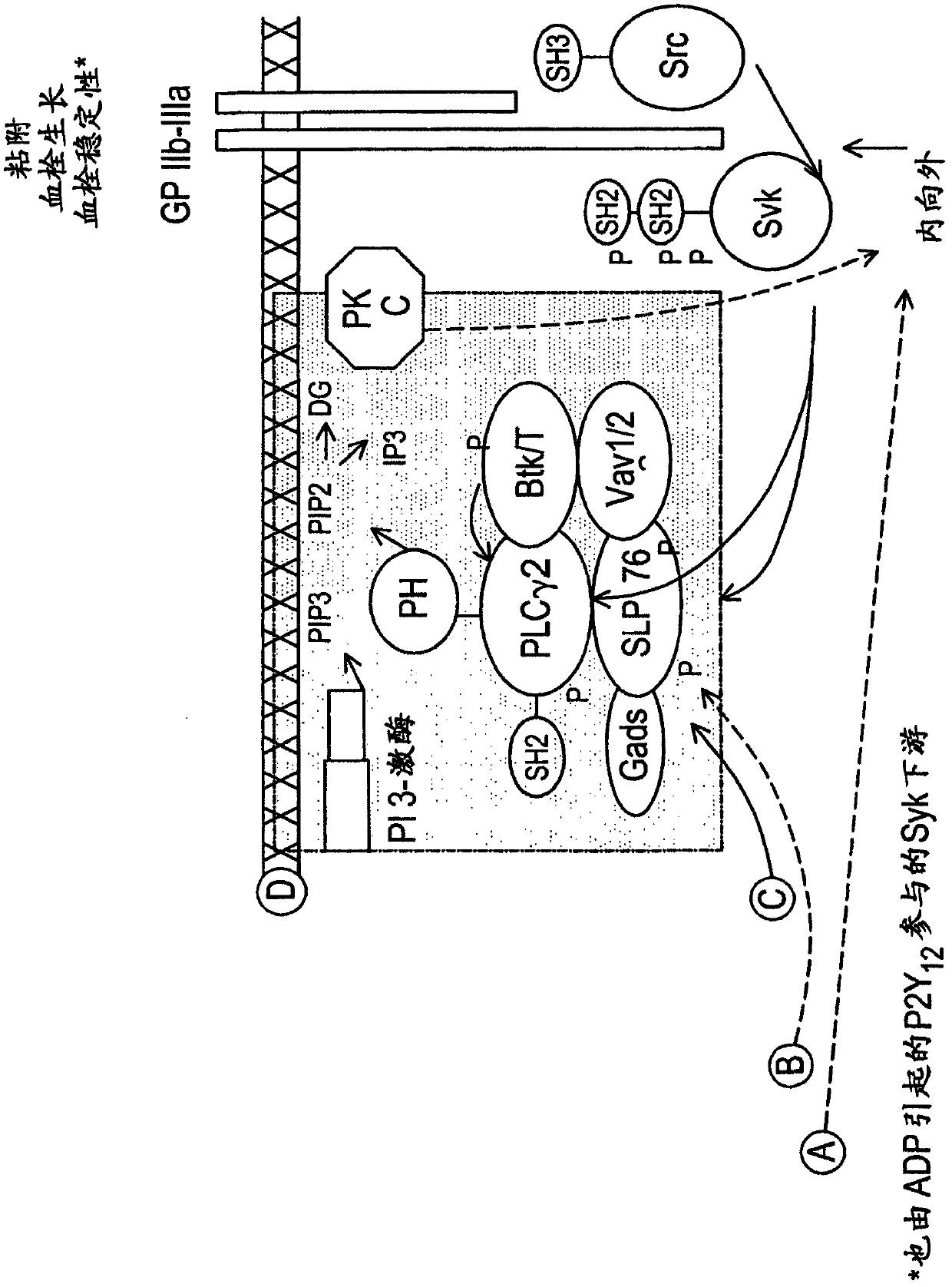
The present invention is directed to compounds of formula (I)-(II) and pharmaceutically acceptable salts, esters, and prodrugs thereof which are inhibitors of syk and / or JAK kinase. The present invention is also directed to intermediates used in making such compounds, the preparation of such a compound, pharmaceutical compositions containing such a compound, methods of inhibition syk and / or JAK kinase activity, methods of inhibition the platelet aggregation, and methods to prevent or treat a number of conditions mediated at least in part by syk and / or JAK kinase activity, such as undesired thrombosis and Non Hodgkin's Lymphoma.
Owner:PORTOLA PHARMA INC
Thiopyrimidine-based compounds and uses thereof
ActiveUS20100239631A1Suppressing immune systemBiocideOrganic active ingredientsJanus kinaseKinase inhibition
The present invention relates to thiopyrimidine-based compounds that are inhibitors of protein kinases including JAK kinases. In particular, the compounds are selective for JAK1, JAK2 or JAK3 kinases and combinations thereof such as JAK1 and JAK2. The kinase inhibitors can be used in the treatment of kinase associated diseases such as immunological and inflammatory diseases including organ transplants; hyperproliferative diseases including cancer and myeloproliferative diseases; viral diseases; metabolic diseases and vascular diseases.
Owner:YM BIOSCI AUSTRALIA
2, 6-diamino-pyrimidin- 5-yl-carboxamides as SRK or JAK kinases inhibitors
The present invention is directed to compounds of formula I-II and pharmaceutically acceptable tautomers, salts, or stereoisomers thereof which are inhibitors of syk and / or JAK kinase. The present invention is also directed to intermediates used in making such compounds, the preparation of such a compound, pharmaceutical compositions containing such a compound, methods of inhibition syk and / or JAK kinase activity, methods of inhibition the platelet aggregation, and methods to prevent or treat a number of conditions mediated at least in part by syk and / or JAK kinase activity, such as cardiovascular disease, inflammatory disease, autoimmune disease and cell proliferative disorder, thrombosis, allergy, asthma, rheumatoid arthritis, leukemia, or non-Hodgkin's lymphoma. The formula I-II is shown in the description.
Owner:PORTOLA PHARMA INC
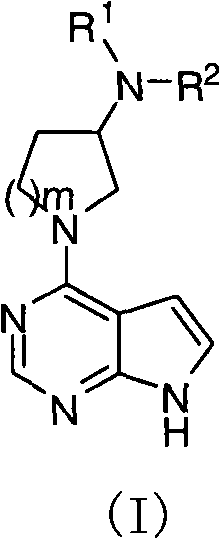
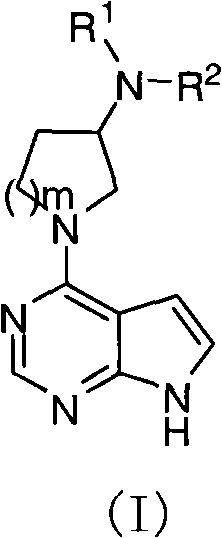
Pyrrolopyrimidine compound and use thereof
The present invention provides a pyrrolopyrimidine compound and a use thereof. Specifically that the present invention provides a class of pyrrolopyrimidine compounds with the inhibitory activity of JAK kinase. The present invention further provides a drug composition containing the pyrrolopyrimidine compound, and an application of the drug composition in treatments of inflammatory diseases, cancer and other diseases.
Owner:HUTCHISON MEDIPHARMA LTD
Sustained-release dosage forms of ruxolitinib
ActiveUS10166191B2Lower the volumeOrganic active ingredientsSenses disorderDiseaseSustained release drug
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
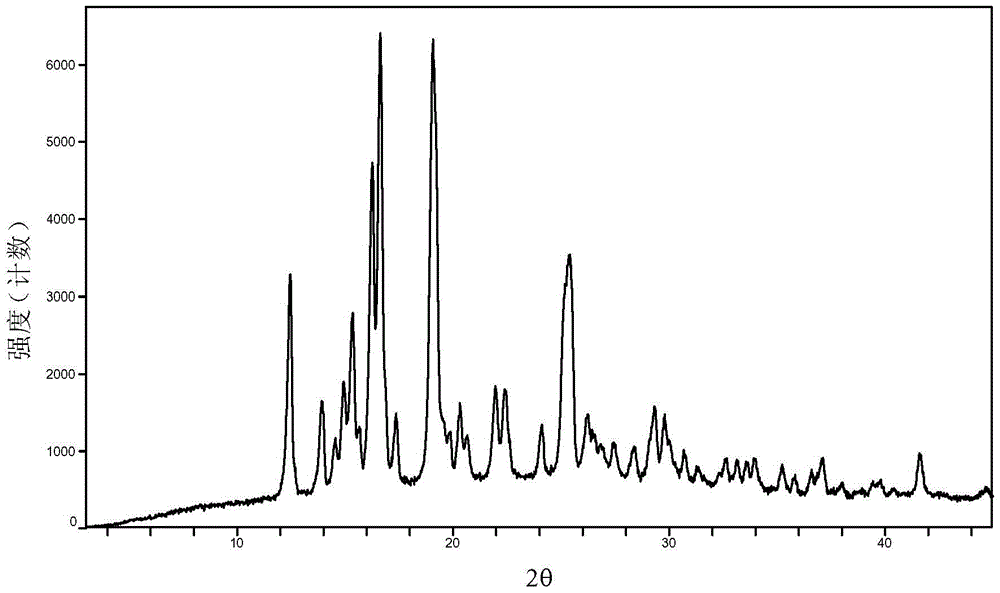
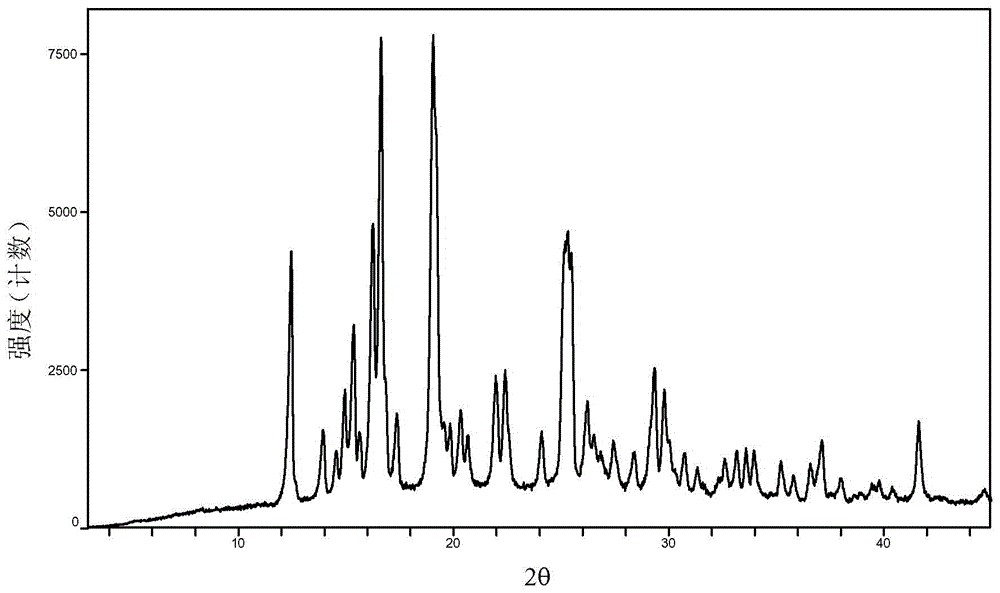
Baricitinib polymorph A and preparation method thereof
InactiveCN105693731AImprove high temperature stabilityImprove stabilityOrganic active ingredientsAntipyreticDiseaseHigh humidity
The invention provides a baricitinib polymorph A. The baricitinib polymorph A is characterized in that diffraction peaks are arranged on an XRPD (X Ray Powder Diffraction) map when values of 2 theta are equal to 12.46, 13.921, 14.94, 15.359, 16.26, 16.639, 17.36, 19.08, 20.321, 21.961, 22.381, 24.118, 25.42, 27.441, 28.381, 29.321, 29.799, 32.675, 33.14, 33.563, 33.923 and 41.6, wherein an error range of the values of the 2 theta is + / - 0.2. The baricitinib polymorph A provided by the invention has good high-temperature stability, good high-humidity stability and good illumination stability, can be applied to medicine for treating or preventing diseases related to JAK (Janus Kinase) and has better biological availability; meanwhile, provided qualitative and quantitative information has important significance in further studying the therapeutic effect of such solid medicine.
Owner:SHANGHAI SUNTRONG BIOTECH
Compositions and methods for inhibition of the JAK pathway
Owner:RIGEL PHARMA
JAK (Janus kinase) and HDAC (histone deacetylase) double-target-spot inhibitor with 4-aminopyrazole structure, and preparation method and application thereof
ActiveCN108864057AHigh yieldInhibit side effectsOrganic active ingredientsOrganic chemistryDiseaseHistone deacetylase
The invention relates to a JAK (Janus kinase) and HDAC (histone deacetylase) double-target-spot inhibitor with a 4-aminopyrazole structure, a preparation method thereof and application of the compoundto preparation of drugs for preventing or treating blood-related diseases, solid tumors, inflammations and autoimmune diseases. The compound comprises structures shown as general formulas (I), (II),(III) and (IV). (The formulas are shown in the description.).
Owner:SHANDONG UNIV
Small molecule compound
The invention provides a small molecular compound, The small molecular compound is characterized by having a structure as shown in the following molecular general formula, wherein X1 and X2 are selected from carbon or nitrogen, G1 is a carbon ring or a heterocyclic ring with aromaticity, any one or more hydrogen atoms on the G1 ring are substituted by R1, wherein R1 is selected from nitrogen-containing groups. The small molecule compound can be used as an efficient and specific JAK kinase inhibitor, especially a Tyk2 inhibitor, and / or a JAK1 inhibitor, and / or a JAK1 / Tyk2 dual inhibitor, or a Tyk2 / JAK1 dual inhibitor or a Tyk2 / Jak2 dual inhibitor.
Owner:TECHNODERMA MEDICINES
N-containing heterocyclic compounds
The present invention relates to N-containing heterocyclic compounds that are inhibitors of protein kinases including JAK kinases. In particular, the compounds are selective for JAK1, JAK2, JAK3 or TYK2 kinases and combinations thereof such as JAK1 and JAK2. The kinase inhibitors can be used in the treatment of kinase associated diseases such as immunological and inflammatory diseases including organ transplants; hyperproliferative diseases including cancer and myeloproliferative diseases; viral diseases; metabolic diseases; and vascular diseases.
Owner:YM BIOSCI AUSTRALIA
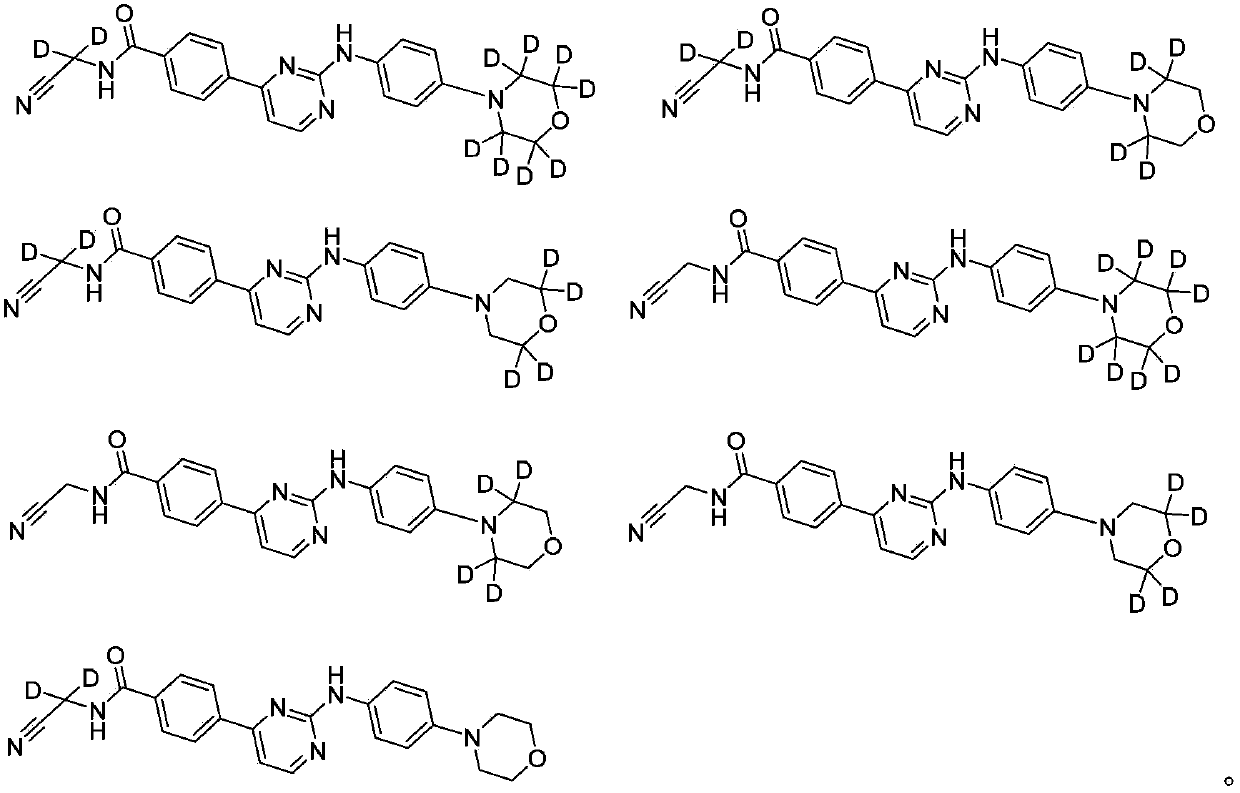
Deuterated phenylamino pyrimidine compound and drug composition containing the same
The present invention relates to a deuterated phenylamino pyrimidine compound and a drug composition containing the deuterated phenylamino pyrimidine compound. Particularly the present invention discloses a deuterated phenylamino pyrimidine compound represented by a formula (I), and a drug composition containing the deuterated phenylamino pyrimidine compound, or a crystal form, a pharmaceutically acceptable salt, a hydrate or a solvate thereof. The compound can be used for treating and / or preventing JAK kinase-associated diseases such as myeloproliferative diseases, cancers, immunologic diseases and the like.
Owner:SUZHOU ZELGEN BIOPHARML
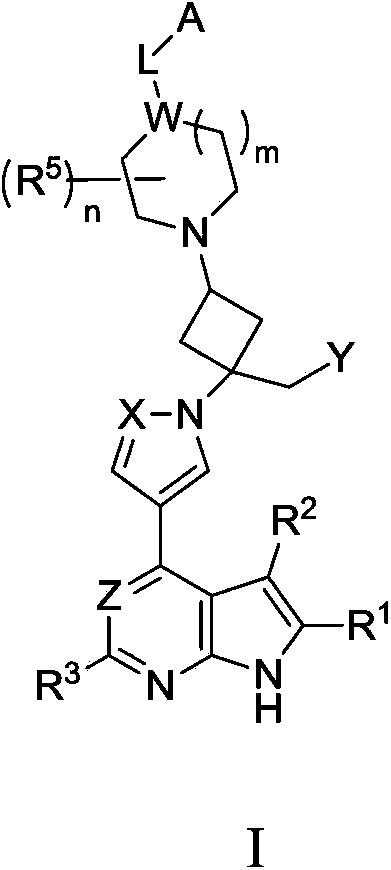
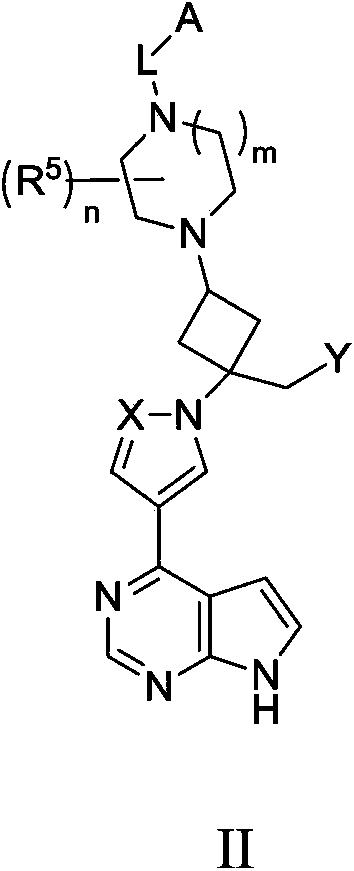
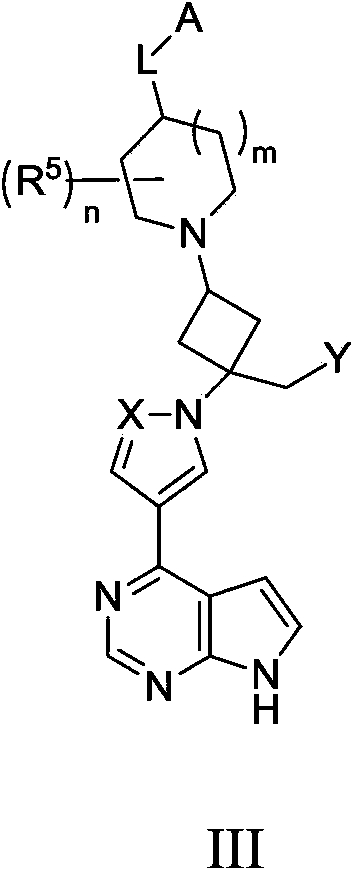
Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors
InactiveCN103415515AAvoid disintegrationLasting effectOrganic active ingredientsBiocideJanus kinasePyrrole
The present invention provides cyclobutyl substituted pyrrolopyrimidines and pyrrolopyridines of Formula I: wherein X, Y, Z, L, A, R5, n and m are defined above, as well as their compositions and methods of use, that modulate the activity of Janus kinases (JAKs) and are useful in the treatment of diseases related to the activity of JAKs including, for example, inflammatory disorders, autoimmune disorders, cancer, and other diseases.
Owner:INCYTE HLDG & INCYTE
Phenyl amino pyrimidine compounds and uses thereof
The present invention relates to phenyl amino pyrimidine compounds which are inhibitors of protein kinases including JAK kinases. In particular the compounds are selective for JAK2 kinases. The kinase inhibitors can be used in the treatment of kinase associated diseases such as immunological and inflammatory diseases including organ transplants; hyperproliferative diseases including cancer and myeloproliferative diseases; viral diseases; metabolic diseases; and vascular diseases.
Owner:GLAXO SMITHKLINE LLC
Methods for treating myeloproliferative disorders
ActiveUS20170037100A1Effective therapyPreventing and reducing rateOrganic active ingredientsSenses disorderMyelofibrosisMyeloproliferative Disorders
In part, the present disclosure relates methods for treating, preventing, or reducing the severity of a myeloproliferative disorder (e.g., polycythemia vera, essential thrombocythemia, and myelofibrosis) or one or more complications of a myeloproliferative disorder. The present disclosure further relates methods for treating, preventing, or reducing the severity of a Janus kinase-associated disorder or one or more complications of a Janus kinase-associated disorder. In certain aspects the disclosure provides TβRII antagonists for treating, preventing, or reducing the severity of a myeloproliferative disorder (e.g., polycythemia vera, essential thrombocythemia, and myelofibrosis) or a Janus kinase-associated disorder or one or more complications of a myeloproliferative disorder or a Janus kinase-associated disorder.
Owner:ACCELERON PHARMA INC
Phenyl amino pyrimidine compounds and uses thereof
The present invention relates to phenyl amino pyrimidine compounds which are inhibitors of protein kinases including JAK kinases. In particular the compounds are selective for JAK2 kinases. The kinase inhibitors can be used in the treatment of kinase associated diseases such as immunological and inflammatory diseases including organ transplants; hyperproliferative diseases including cancer and myeloproliferative diseases; viral diseases; metabolic diseases; and vascular diseases.
Owner:GLAXO SMITHKLINE LLC
5-member and 6-member rings heterocyclic compound, its preparation method, pharmaceutical composition and its application
The invention discloses a 5-member and 6-member rings heterocyclic compound shown as a formula I, its pharmaceutically acceptable salt, a metabolite, a metabolism precursor or its medicine precursor, a preparation method, a pharmaceutical composition and an application. The 5-member and 6-member rings heterocyclic compound has activity by being as a Janus kinases (JAK) inhibitor, and be used for treating diseases due to abnormal activity of kinases, such as cell proliferation diseases such as cancer, and can be used for preparing the medicines for treating the disease.
Owner:GUANGZHOU MAXINOVEL PHARMA CO LTD
Substituted heteroaryl compound and composition and application thereof
The invention provides a substituted heteroaryl compound and a pharmaceutical composition and application thereof. The compound is a compound as shown in formula (I) or the stereisomer, tautomer, nitric oxide, solvate, metabolite, pharmaceutically acceptable salt or prodrug of the compound as shown in formula (I). The pharmaceutical composition containing the compound can regulate the activity ofprotein kinase and especially the activity of Aurora kinase and JAK kinase and is used for preventing, treating and relieving diseases or disorder mediated by protein kinase and especially JAK kinase.
Owner:SUNSHINE LAKE PHARM CO LTD
Tricyclic fused thiophene derivatives as JAK inhibitors
The present invention provides tricyclic fused thiophene derivatives, as well as their compositions and methods of use, that modulate the activity of Janus kinase (JAK) and are useful in the treatment of diseases related to the activity of JAK including, for example, inflammatory disorders, autoimmune disorders, cancer, and other diseases.
Owner:INCYTE
Imidazopyridine derivatives as JAK inhibitors
New imidazopyridine derivatives having the chemical structure of formula (I) are disclosed; as well as process for their preparation, pharmaceutical compositions comprising them and their use in therapy as inhibitors of Janus Kinases (JAK).
Owner:ALMIRALL
Pyrrolopyrimidine compounds and uses thereof
Disclosed are pyrrolopyrimidine compounds of formula (I) capable of inhibiting JAK kinase, wherein R1, R2 and m are defined as in the description. The pharmaceutical compositions containing said pyrrolopyrimidine compounds and uses of said pyrrolopyrimidine compounds in the treatment of disorders or diseases such as inflammatory diseases and cancers are also disclosed.
Owner:HUTCHISON MEDIPHARMA LTD
Heteroaryl imidazolone derivatives as JAK inhibitors
New heteroaryl imidazolone derivatives having the chemical structure of formula (I) disclosed; as well as process for their preparation, pharmaceutical compositions comprising them and their use in therapy as inhibitors of Janus Kinases (JAK).
Owner:ALMIRALL
Compounds and methods for treating or preventing autoimmune diseases
Methods of treating, ameliorating, preventing, or reducing the risk of developing an auto-immune disease and / or an inflammatory condition, such as systemic lupus erythematosus, in a patient, such as a human being, using a therapeutically effective amount of an agent(s) that inhibits the activity of one or more of histone deacetylase (HDAC), IKB kinase (IKK-2), nuclear factor kB (NF-kB), ubiquitin / proteasome and Janus kinase (JAK) are disclosed. Compounds useful in such methods are also presented.
Owner:HORRIGAN STEPHEN K +8
JAK kinase inhibitor containing 4-aminopyrazole structure, preparation method and application thereof
The invention relates to a JAK kinase inhibitor containing a 4-aminopyrazole structure, a preparation method and an application thereof. Compounds of the series have structures shown in a general formula (I) or a general formula (II). The invention also provides the preparation method of the kinase inhibitor, and the application of the kinase inhibitor in preparation of medicines for preventing or treating inflammation, tumor and blood-related diseases.
Owner:SHANDONG UNIV
Jak inhibitors containing a 4-membered heterocyclic amide
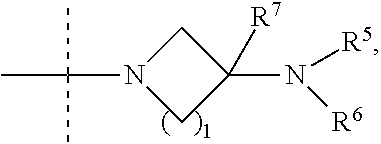
ActiveUS20180258087A1Useful in preparationOrganic active ingredientsOrganic chemistryPulmonary circulation diseasesJanus kinase
The invention provides compounds of formula (I):which contain a 4-membered heterocyclic amide, where the variables are defined in the specification, or a pharmaceutically-acceptable salt thereof, that are useful as JAK kinase inhibitors. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat respiratory diseases, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com