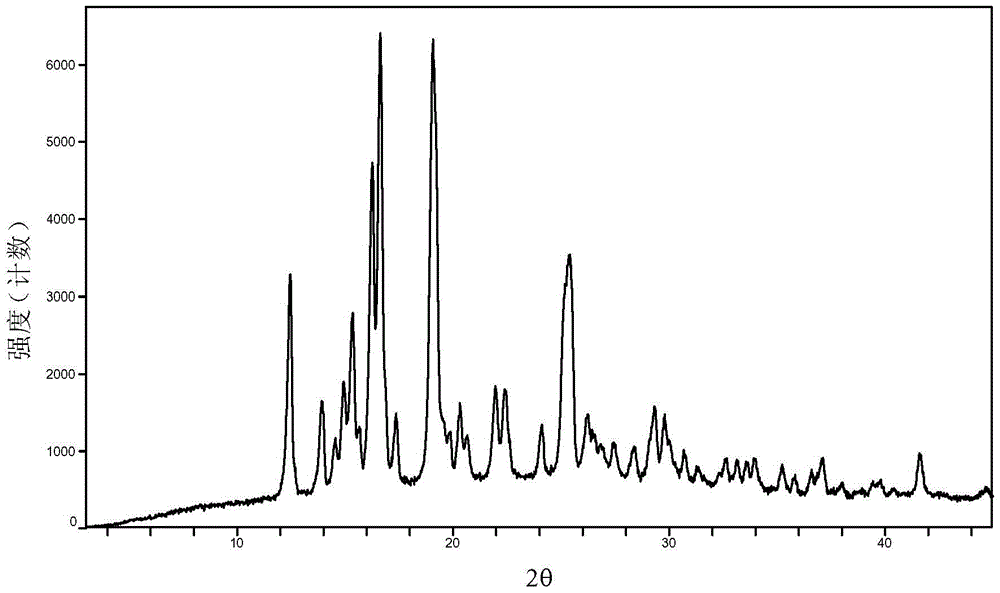
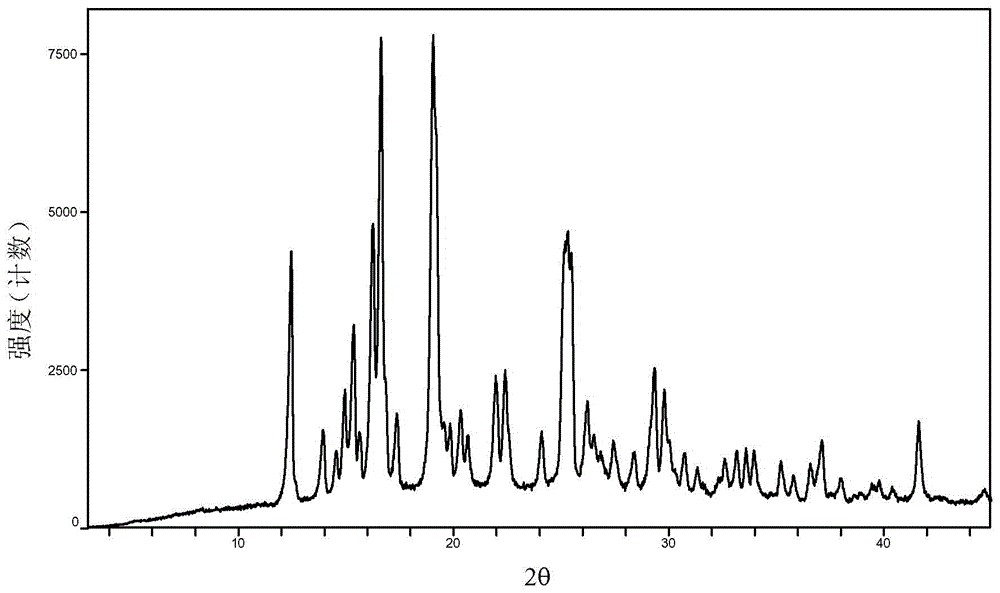
Baricitinib polymorph A and preparation method thereof
A baricitinib and crystal form technology, applied in the field of baricitinib crystal form A and its preparation, can solve the instability of baricitinib, unfavorable crystal form transformation, strong hygroscopicity, etc. problem, to achieve the effect of good bioavailability and excellent high temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 6
[0035] Embodiments 1 to 6 Preparation of Baricitinib A Crystal Form
[0036] Weigh 800mg of baricitinib raw material into a container, add 1.5mL N,N dimethylformamide to completely dissolve it. Slowly add 50 mL of the solvent in Table 1 (analytical grade, water is deionized water) to the obtained solution respectively, let stand overnight, filter and vacuum dry to obtain an off-white solid. Weigh and calculate its yield, and the results are shown in Table 1.
[0037] Table 1 Preparation of baricitinib A crystal form
[0038] Example
Embodiment 7 to 12
[0039] Examples 7 to 12 Preparation of crystal form A of baricitinib
[0040] Weigh 1.0 g of baricitinib raw material into a container, add 1.0 mL of N,N dimethylformamide to completely dissolve it. Slowly add 60 mL of the solvent in Table 1 (analytical grade, water is deionized water) to the obtained solution respectively, let it stand overnight, filter, and vacuum-dry to obtain an off-white solid.
Embodiment 13 to 18
[0041] Examples 13 to 18 Preparation of crystal form A of baricitinib
[0042] Weigh 100mg of baricitinib raw material into a container, add 0.5mL N,N dimethylformamide to completely dissolve it. Slowly add 10 mL of the solvent in Table 1 (analytical grade, water is deionized water) to the obtained solution respectively, let stand overnight, filter and vacuum dry to obtain an off-white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com