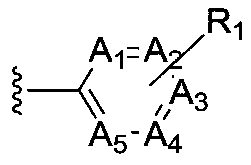
Small molecule compound
A technology of small molecule compounds and compounds, applied in the fields of drug combination, organic chemistry, digestive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1, the general method of synthetic compound TDM-180712
[0082]
[0083] Step 1: Example 112b
[0084] The original compound 112a, methyl 1H-indole-3-carboxylate (554mg, 3.16mmol) and sodium hydride (183mg, 4.59mmol, 60%wt.) were added to the three-necked flask, pumped under the condition of the system water pump Gas was replaced with nitrogen three times. Under ice-bath conditions, anhydrous DMF (50 mL) was added via syringe with stirring, and then the mixture was stirred for 30 min. A solution of compound B0, 4-chloro-N-(1-methyl-1H-pyrazol-4-yl)pyrimidin-2-amine (600 mg, 2.87 mmol) in DMF (10 mL) was slowly injected into the reaction solution. The resulting reaction solution was refluxed at 100° C. for 4 hours. After the reaction was completed, it was poured into water (30 mL), extracted with ethyl acetate (60 mL x 3). The organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pr...
Embodiment 2
[0093] Embodiment 2, the general method of synthetic compound 125 (TDM-180725)
[0094]
[0095] Step 1: Example 125b
[0096] (1-(2-((1-Methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)-1H-indol-3-yl)carbamate tert-butyl ester
[0097] Add compound 125a (0.25g, 0.75mmol), THF (10mL) mixture of DIPEA (193mg, 1.5mmol) to inject t-BuOH (15mL) and DPPA (309mg , 1.13 mmol). The mixture was heated to 105°C and refluxed for 18 hours. Starting material remained by LCMS, then another batch of DIPEA (193 mg) and DPPA (309 mg) was added and the resulting mixture was stirred at 105 °C under Ar for 20 h. The reaction mixture was concentrated and purified by column chromatography (petroleum ether / ethyl acetate=0 / 100) to obtain compound 125b as a yellow solid (80 mg, yield <12.5%).
[0098] [Note: Response reproducibility is not good. ]
[0099] LCMS[M+1] + =406.2
[0100] Step 2: Example 125c
[0101] 1-(2-((1-Methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)-1H-indol-3-amine
[0102] T...
example 3
[0109] Example 3: General method for the synthesis of compound 122 (TDM-180722)
[0110]
[0111] Step 1: Example 122c
[0112] At room temperature, to compound 122a (200mg, 0.96mmol) ie 4-chloro-N-(1-methyl-1H-pyrazol-4-yl)pyrimidin-2-amine, compound 122b (287mg, 0.96mmol) ie 4,4,5,5-Tetramethyl-2-(4-nitrophenyl)-1,3,2-dioxaborinane, [1,1'-bis(diphenylphosphino)bis To a mixture of ferrocene]palladium dichloride (70.2 mg, 0.096 mmol) and sodium carbonate (210 mg, 1.92 mmol) were added 1,4-dioxane (20 mL) and water (3 mL). The mixture was then degassed under vacuum, and the mixture was heated to 105° C. and stirred for 3 hours under nitrogen protection. After the reaction was complete, the mixture was concentrated under reduced pressure. It was purified by silica gel column (petroleum ether:ethyl acetate=25:75) to obtain red solid compound 122c (242 mg, yield 56.8%), namely N-(1-methyl-1H-pyrazol-4-yl )-4-(4-nitrophenyl)pyrimidin-2-amine. LCMS[M+1] + =297.
[0113] St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com