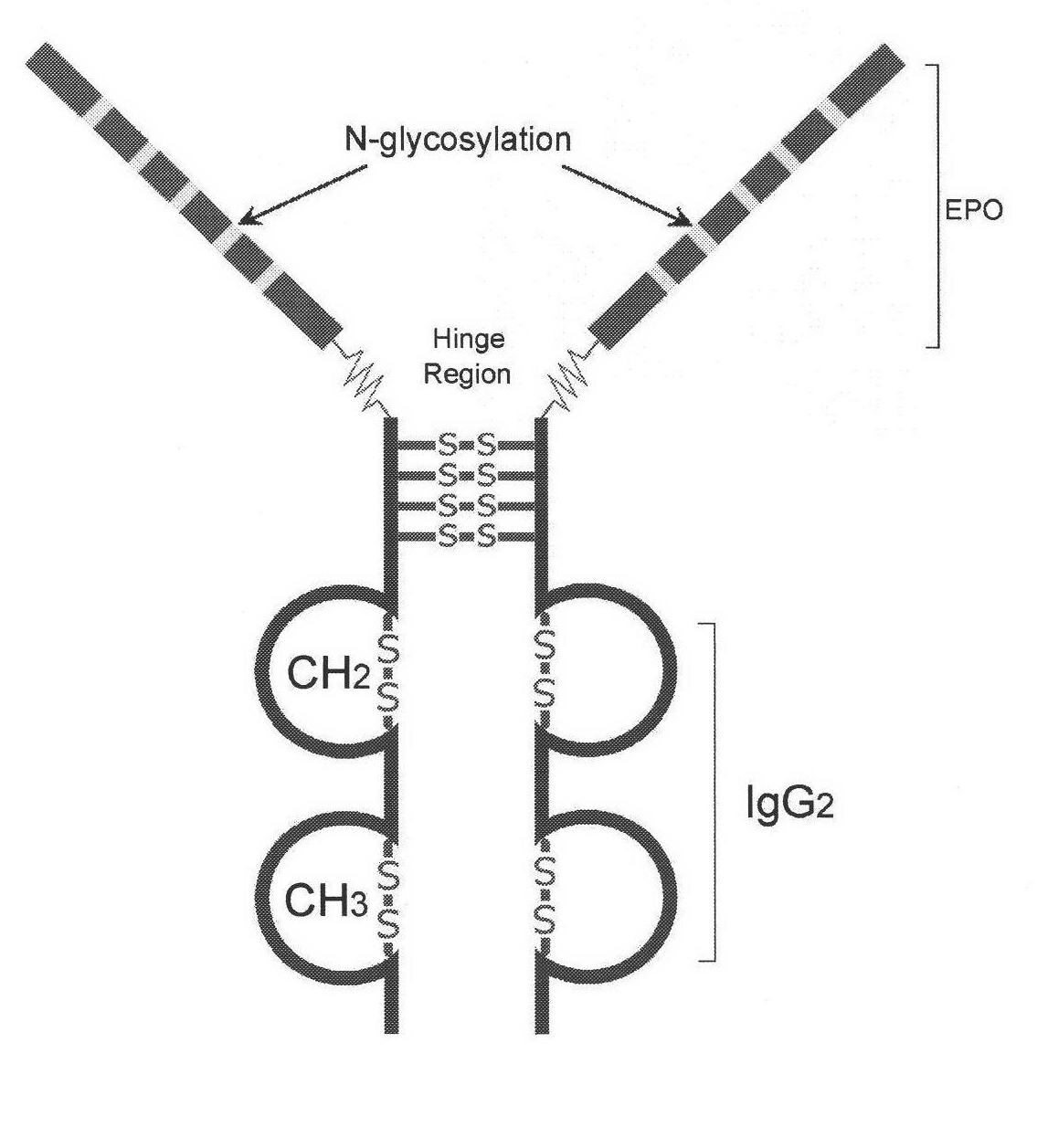
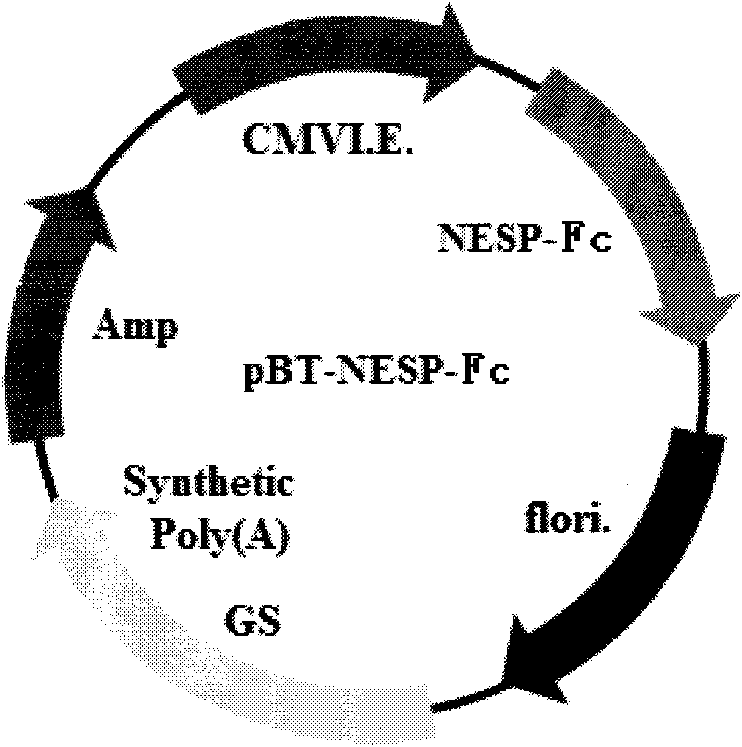
Novel high-glycosylation erythropoietin immune fusion protein
An erythropoietin and fusion protein technology, applied in the field of bioengineering, can solve the problems of inconvenience to patients, short efficacy of EPO monomer drugs, frequent drug use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of NESP-Fc immune fusion protein expression vector
[0052] 1. Optimization of NESP-Fc immune fusion protein expression gene
[0053] There are many factors that affect the expression yield of cell protein. When the gene contains a large number of rare codons, the expression yield of protein will be reduced. Because the fusion protein will be expressed in the cell, according to the biased codon table expressed by CHO (see Holm L. Nucleic Acids Res. 1986 Apr 11; 14 (7): 3075-87) for the new erythropoiesis-stimulating protein (NESP) and The DNA sequence encoded by IgG2 was optimized, and the amino acid residues of the encoded protein were kept unchanged. For the protein amino acid residue sequence of the new erythropoiesis-stimulating protein (NESP), see patent US20030104996, and for the IgG2 protein amino acid residue sequence, see >gi|243169|gb|AAB21082.1|. In order to ensure the high-efficiency expression of the protein, the signal peptide seq...
Embodiment 2
[0089] Example 2: Expression and purification of NESP-Fc immune fusion protein
[0090] 1. Transfection of NESP-Fc immune fusion protein expression vector into 293F cells
[0091] 293F (purchased from Invitrogen, Cat No. 11625-019) cells were suspended and cultured in serum-free CD293 medium (purchased from Invitrogen, Cat No. 11913-019), centrifuged before transfection to replace fresh medium, and the cell concentration was adjusted 1×10 6 cells / ml. Taking 100ml cells as an example, add DNA (250ug) and PEI (500ug, Sigma, Cat. No: 408727) into 1ml 293 culture medium, mix well, and let stand for 5min. Place at room temperature for 8 minutes. Add the PEI / DNA suspension dropwise into the shaker flask, mix gently, and place in 5% CO 2 1. Collect the culture supernatant after 5 days of shaking culture (115 rpm) at 37°C.
[0092] 2. Detection of the concentration of immune fusion protein
[0093] The transient expression of the immune fusion protein in the collected culture su...
Embodiment 3
[0098] Example 3: Confirmation of the structure of NESP-Fc immune fusion protein:
[0099] The purified fusion protein was subjected to protein electrophoresis to analyze the purified product.
[0100] There is no change in the molecular weight of the protein before and after dialysis, and the molecular weights of the fusion protein are 72kDa and ~200kDa under reducing and non-reducing conditions, respectively. See attached Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com