Stabilized cell-penetrating peptide with hydrophobic side chain and preparation method and use of stabilized cell-penetrating peptide
A membrane-penetrating peptide and hydrophobic technology, applied in the field of biomedicine, can solve the problems of low cell penetrating ability and poor metabolic stability of cell-penetrating peptides, and achieve strong cell penetrating ability, improve metabolic stability, and fast penetration. The effect of penetration speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
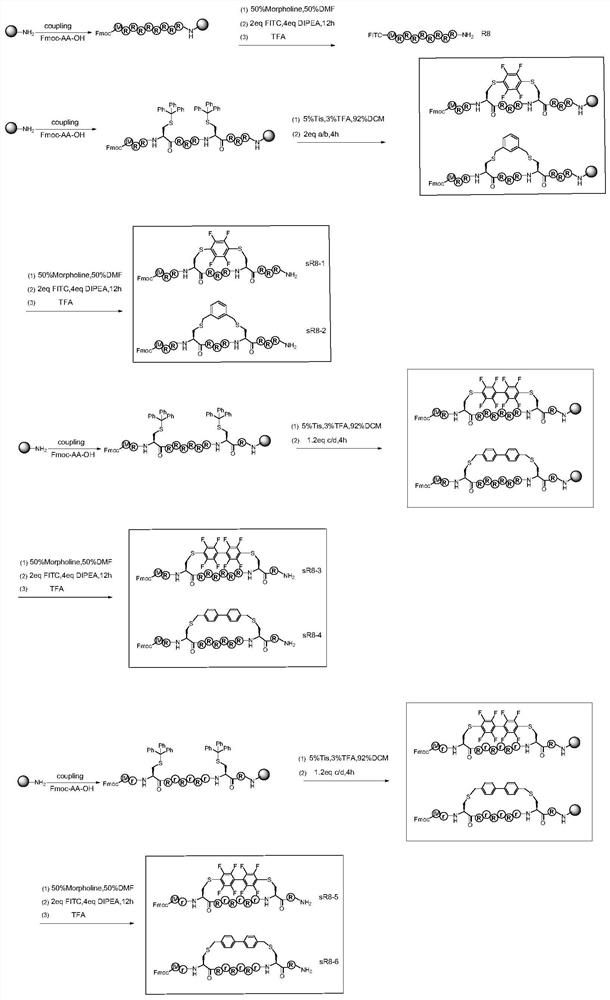
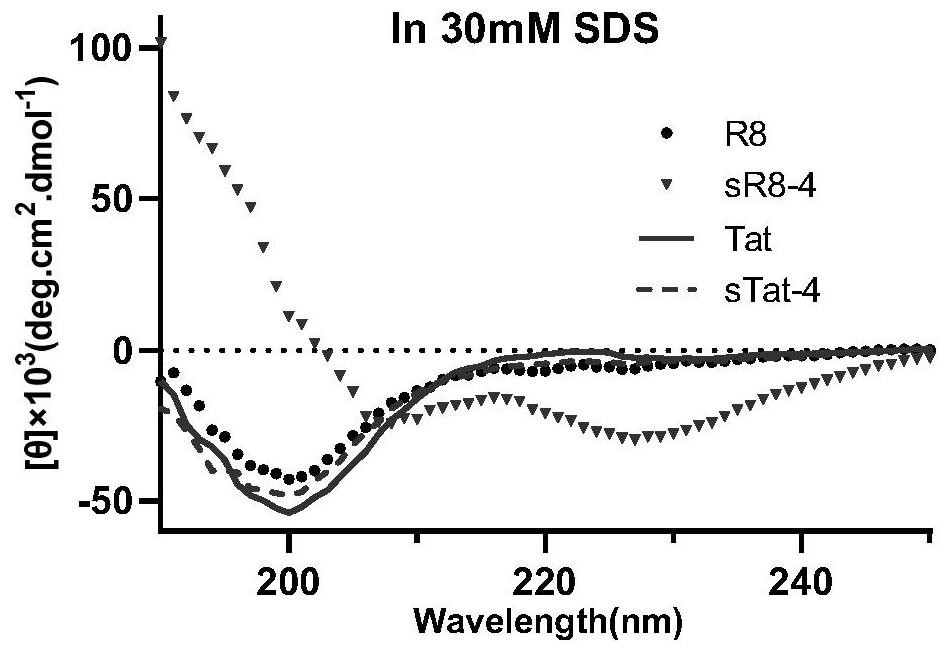
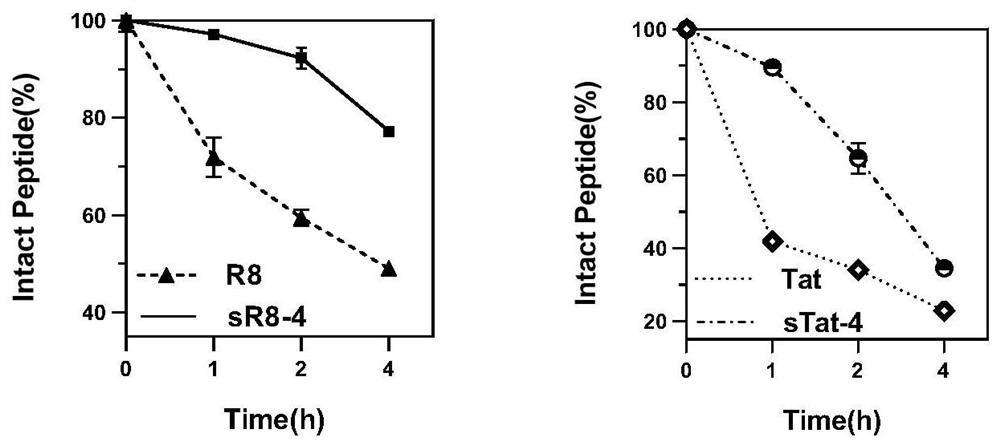
[0045] Since each ascending turn of the α-helix contains 3.6 amino acid residues, the corresponding cyclization positions need to be i+4, i+7. In order to explore the effect of hydrophobicity on the penetrating ability of CPPs, based on the sequences of R8 and Tat derivatives, small molecules with different hydrophilicity and hydrophobicity were used to cyclize the polypeptide, using weakly hydrophobic perfluorobenzene and 1,3 -Bis(bromomethyl)benzene is cyclized at the i+4 position, and decafluorobiphenyl and 4,4'-bis(bromomethyl)biphenyl with strong hydrophobicity are cyclized at the i+7 position. At the same time, in order to understand the effect of amino acid conformation on the penetration ability of CPPs, the same chirality (L) sequence and different chirality (L, D) alternating cyclic peptides were designed. Constructed 12 test examples of stabilized cell-penetrating cyclic peptides with hydrophobic side chains, named sR8-1, sR8-2, sR8-3, sR8-4, sR8-5, sR8-6 and sTat- ...
Embodiment 2
[0050] Take the synthetic route of R8, sR8-1, sR8-2, sR8-3, sR8-4, sR8-5, sR8-6 as an example, the synthetic route is as attached in the manual figure 1 As shown, the synthesis methods of Tat, sTat-1, sTat-2, sTat-3, sTat-4, sTat-5, and sTat-6 are the same.
[0051] Concrete preparation process is as follows:
[0052] 1. Cyclization method
[0053] Decafluorobiphenyl and 4,4'-bis(bromomethyl)biphenyl cyclization method: Swell 200mg (RCRRRRRRCR, rCRrRrRrCR, GRKCRRQRRRC and GrKCRrQrRrC) resin with DCM for 10 minutes, remove DCM, add the mixed solution to the reaction tube (2%TFA / 3%TIS / 95%DCM, v / v / v) selectively remove the Cys(Trt) protecting group, each time for 2 minutes, until the yellow color in the solution disappears, wash with DCM and DMF alternately for several times. Dissolve 1.2eq. of decafluorobiphenyl or 4,4'-bis(bromomethyl)biphenyl and 2.4eq. of DIPEA in DMF, mix well and add to the reaction tube to react for 4 hours.
[0054] Perfluorobenzene and 1,3-bis(bromometh...
Embodiment 3
[0062] The hydrophilicity and hydrophobicity of the synthesized CPPs were compared using the retention time of reversed-phase HPLC. The lyophilized peptide was dissolved in aqueous solution and detected by reversed-phase HPLC (Agilent Poroshell 120 EC-C18: 4.6×150mm, 4μm, flow rate 1.0mL / min) The peak time of each polypeptide can be used to judge the hydrophobicity of the polypeptide. The later the peak time, the stronger the hydrophobicity of the polypeptide. Experimental conditions: a gradient system in which solvent B (solvent A: 1‰ (v / v) TFA in water, solvent B: ACN) rises from 10% to 80% at a flow rate of 1.0ml / min in 30 minutes, detects 220nm and UV signal at 254nm.
[0063] The retention times of 14 CPPs including linear peptides detected on reversed-phase HPLC are shown in Table 2 and Table 3. The results show that the retention times of cyclized CPPs are increased compared with the corresponding linear CPPs, proving that cyclized CPPs The hydrophobicity has been impr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com