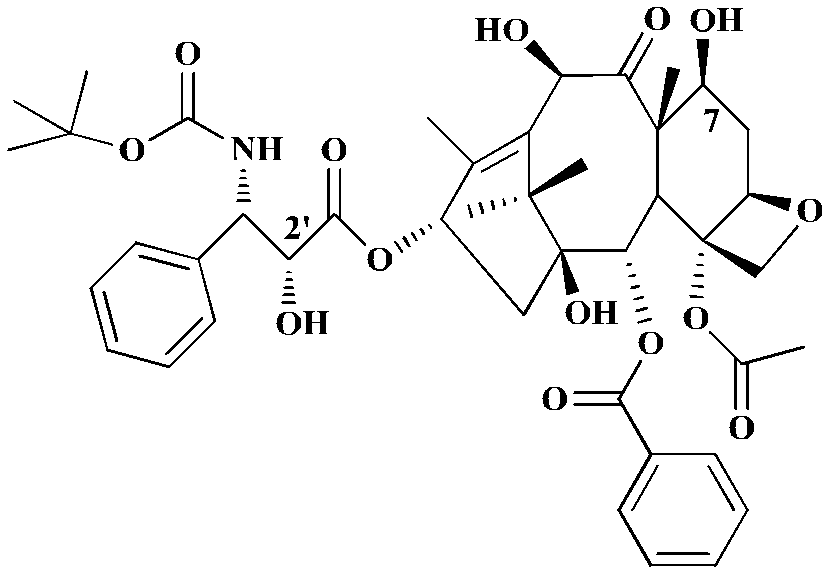
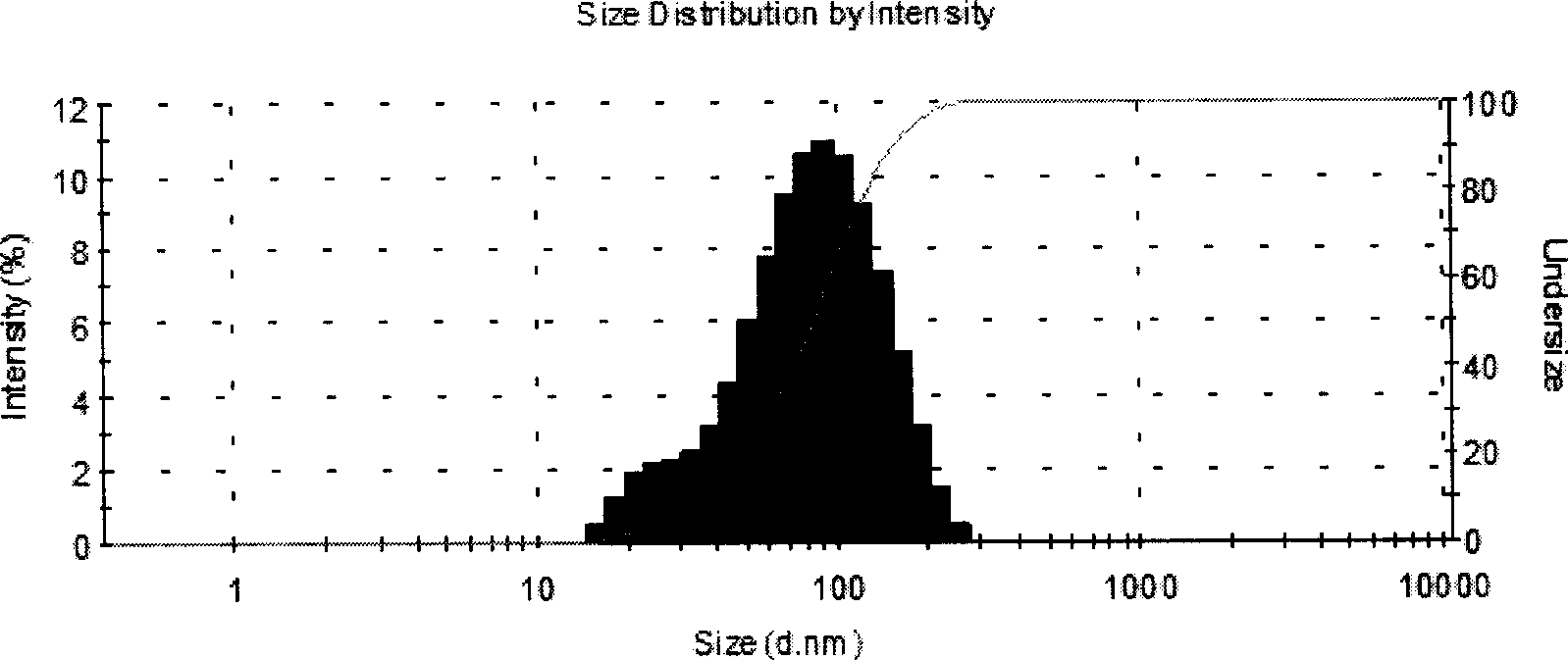
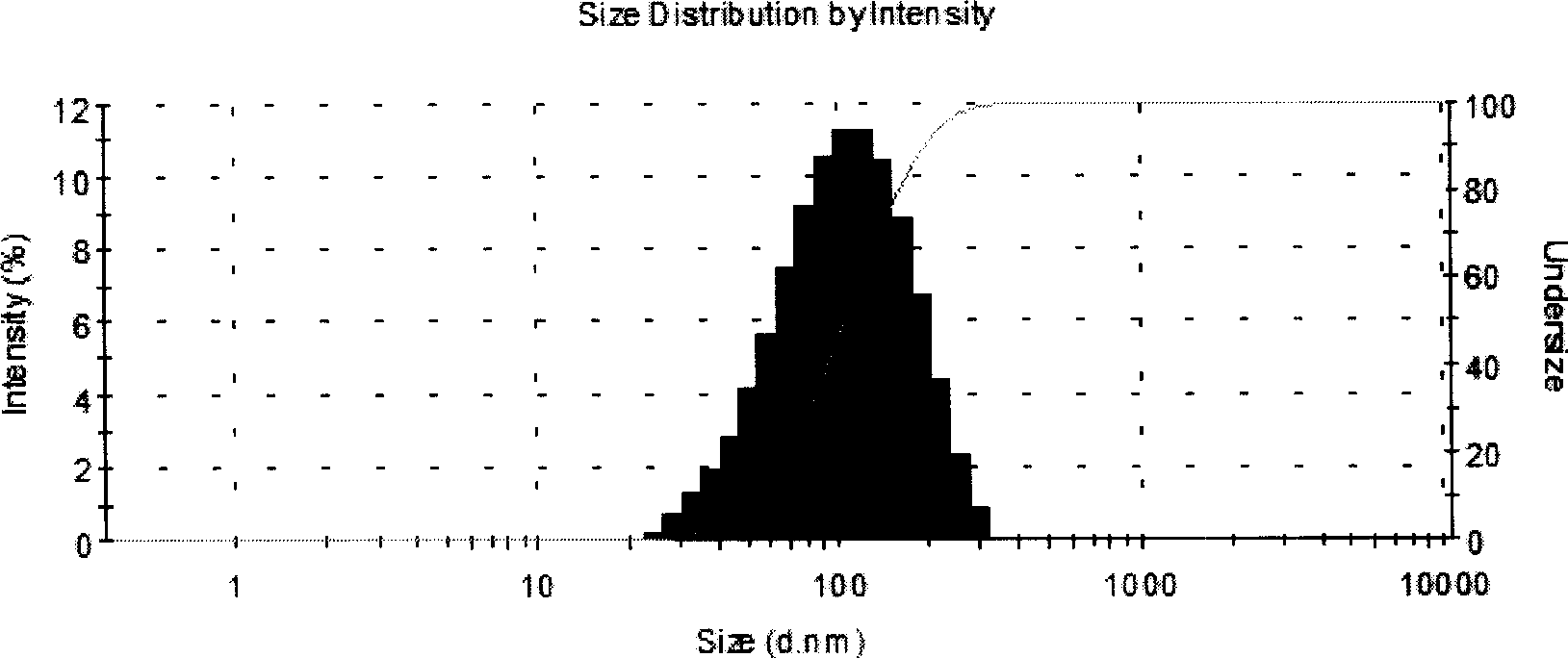
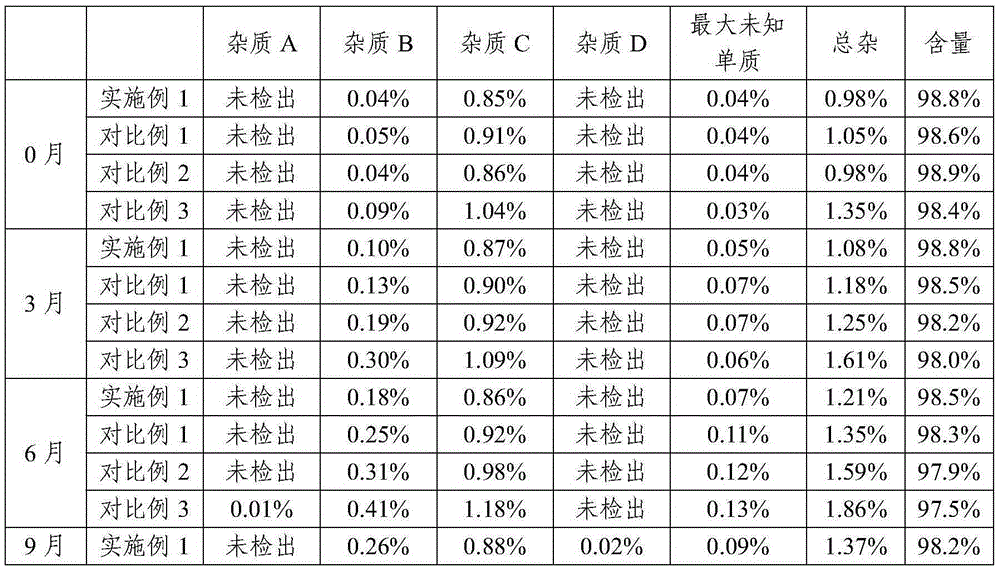
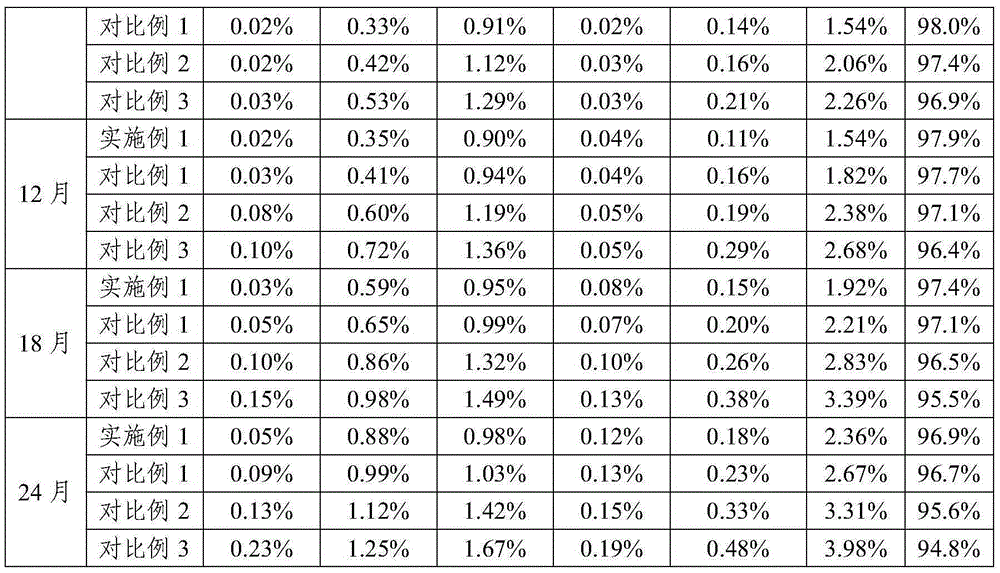
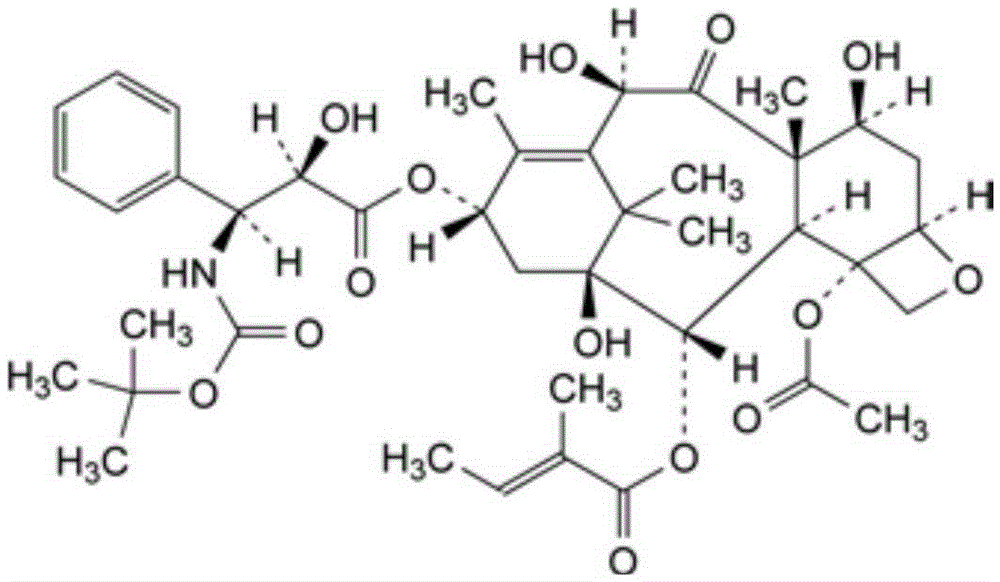
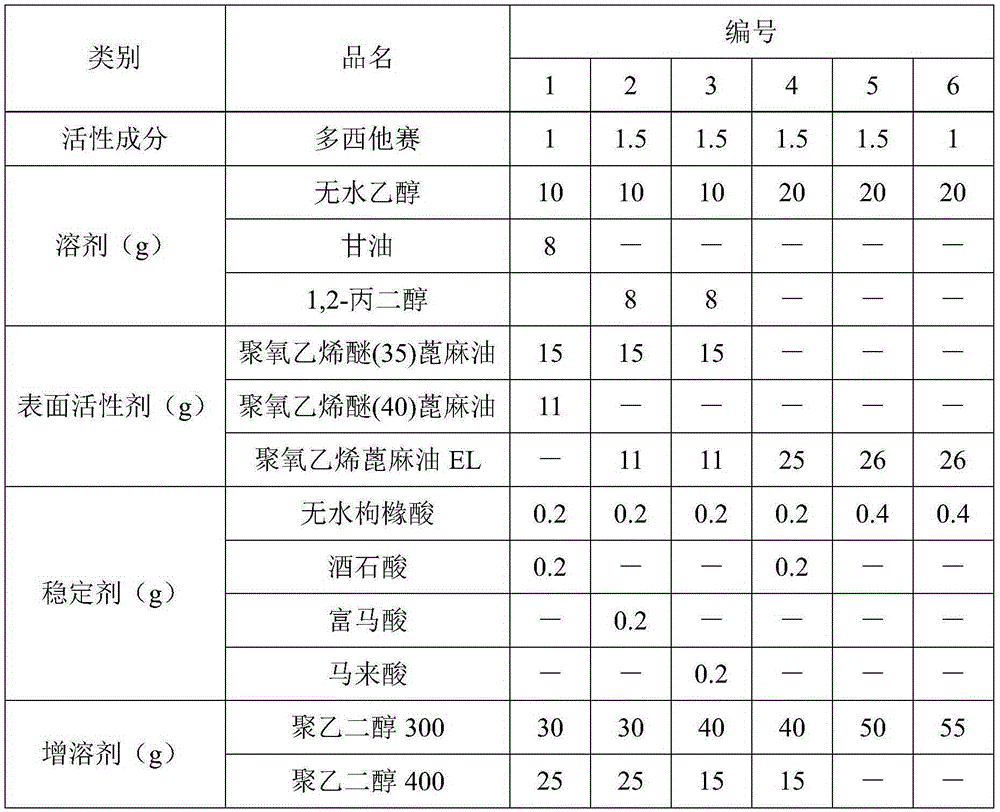
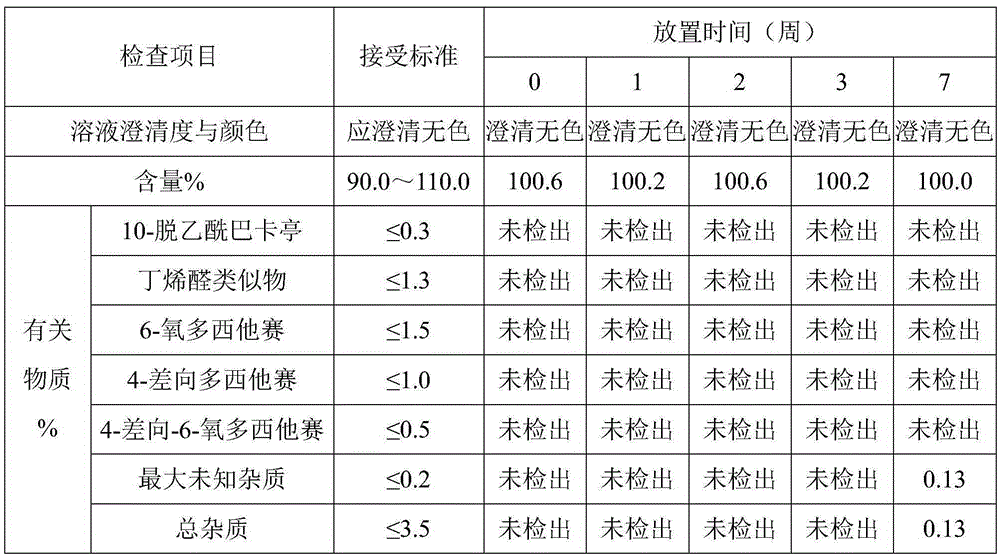
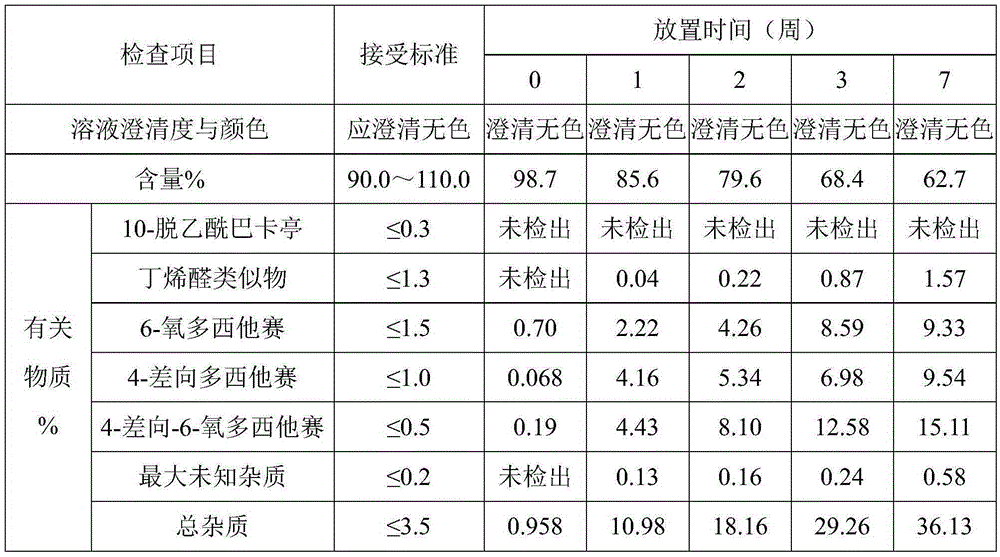
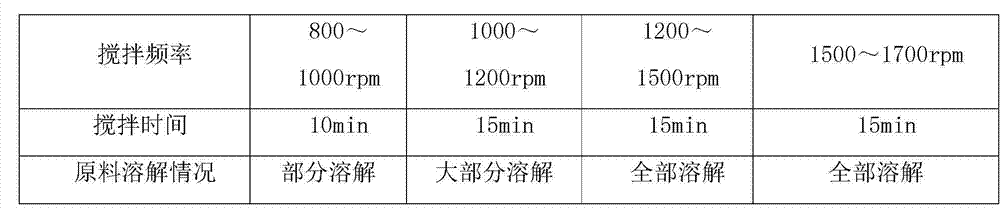
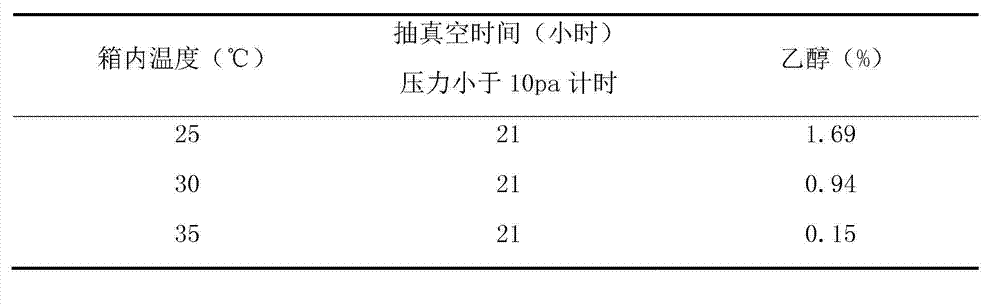
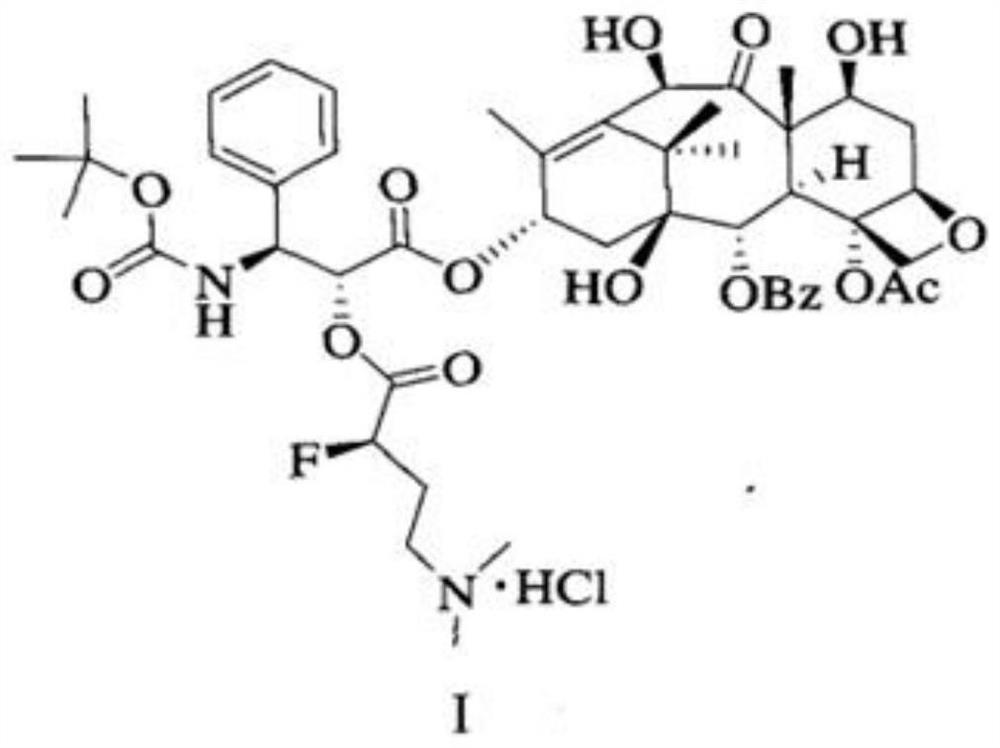
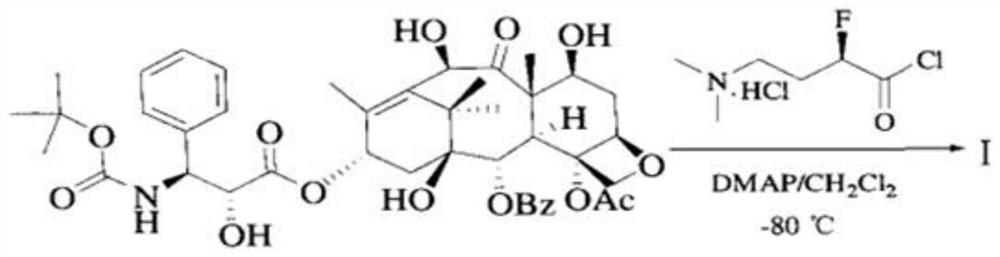
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Docetaxel Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
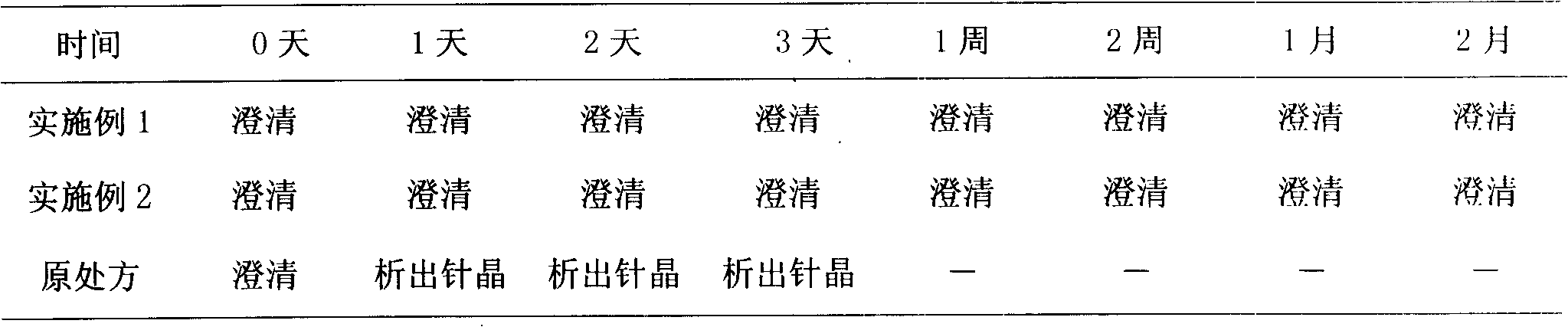
Use with docetaxel injection may either raise the chance of an infection or make the vaccine not work as well. Rarely, a bone marrow problem called myelodysplastic syndrome (MDS) has happened in patients treated with docetaxel injection.
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
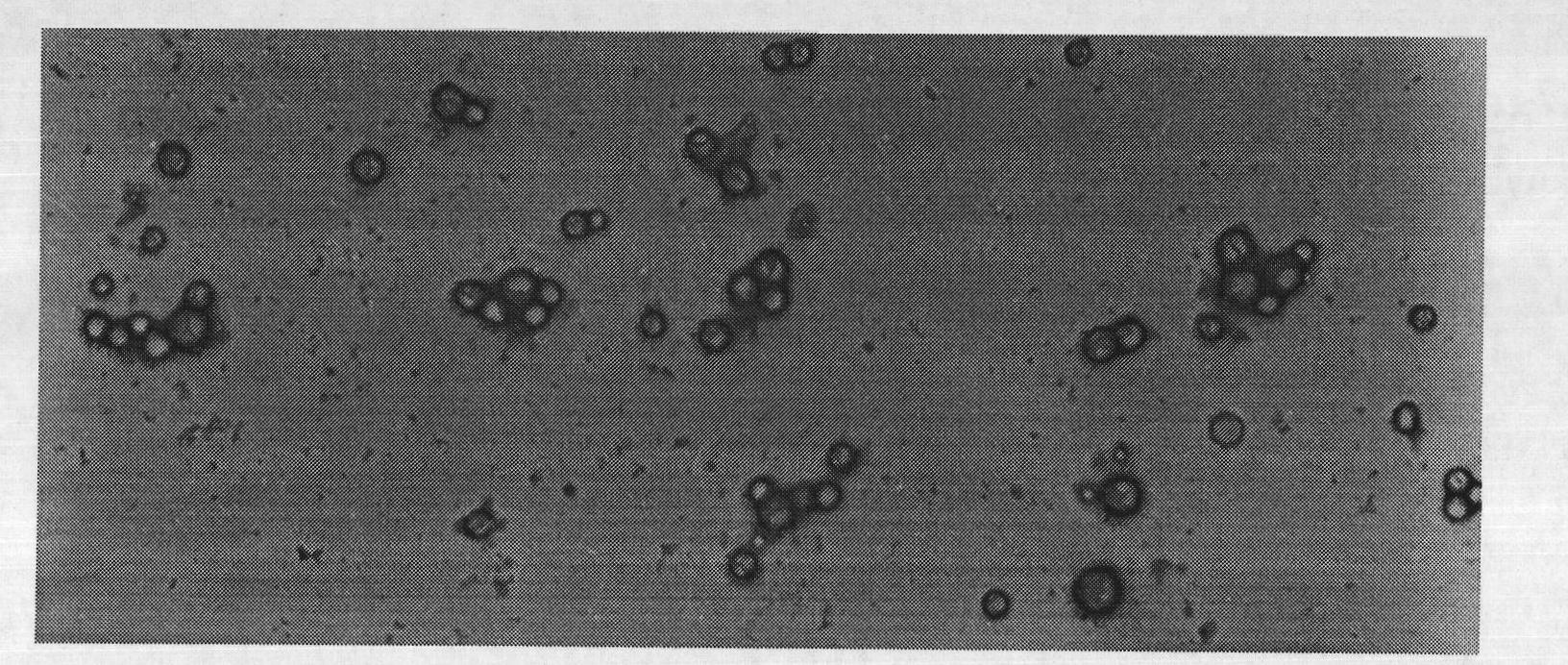
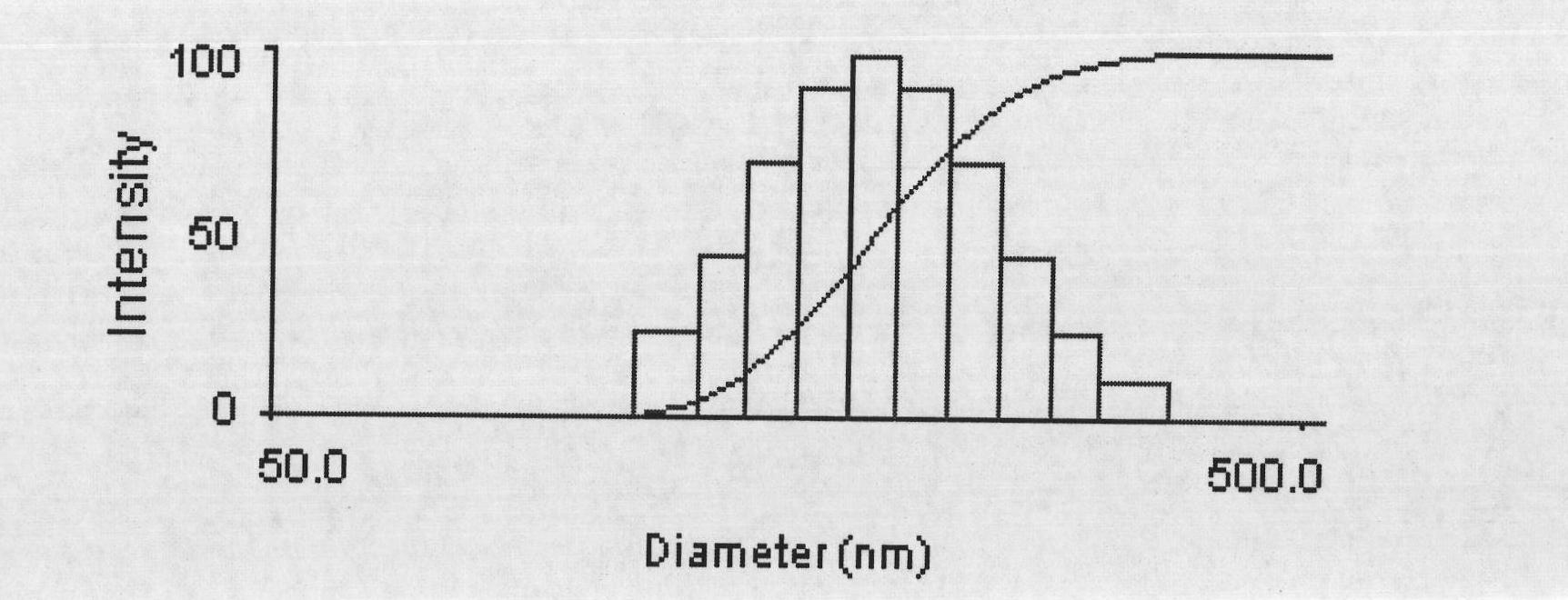
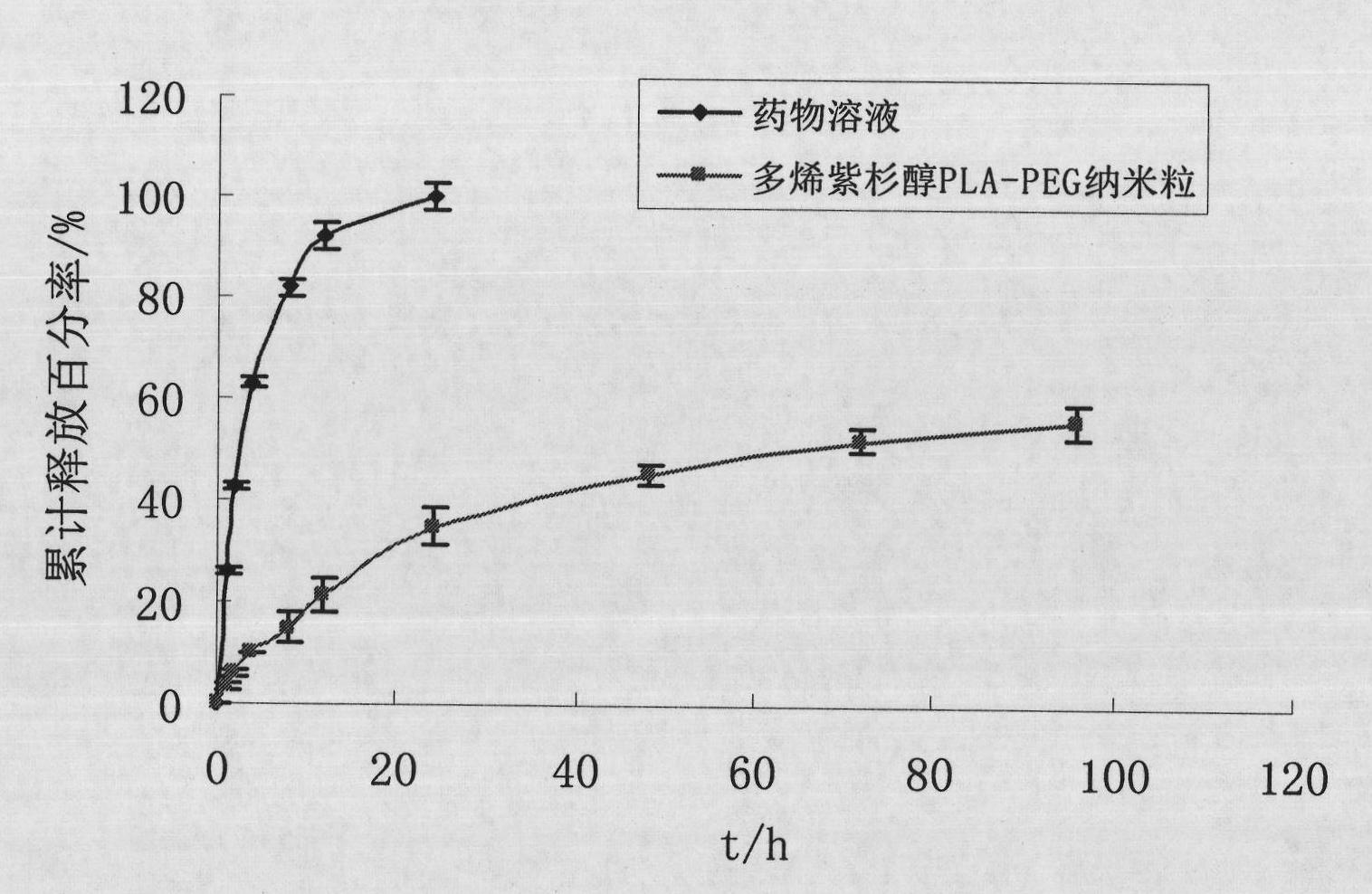
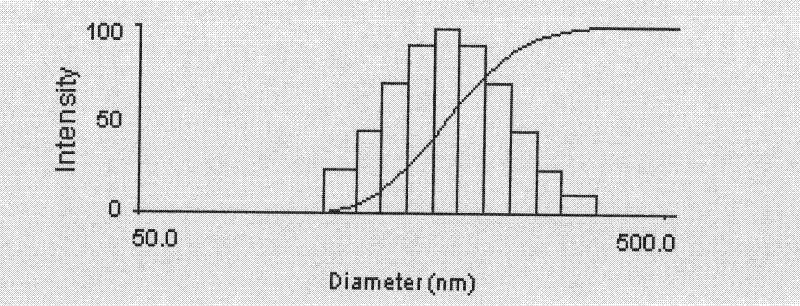
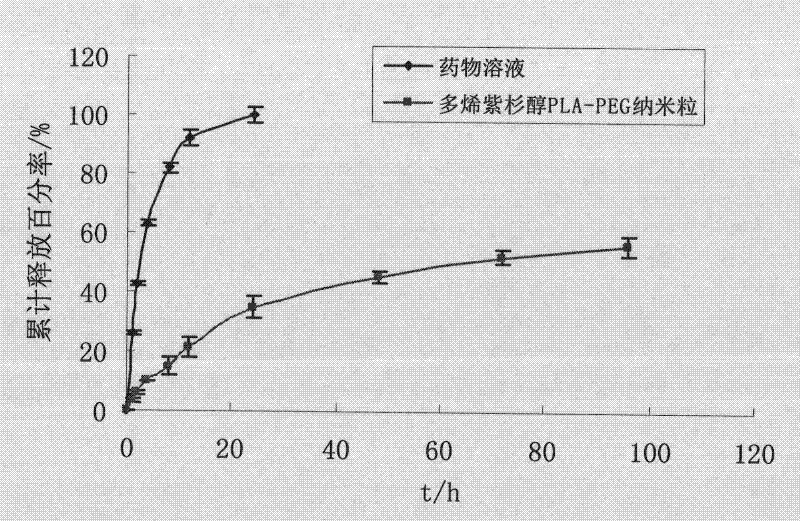
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
Prescription and preparation method of docetaxel injection
InactiveCN102451155AEasy to manufactureImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismMetastatic small cell carcinomaPharmaceutical formulation
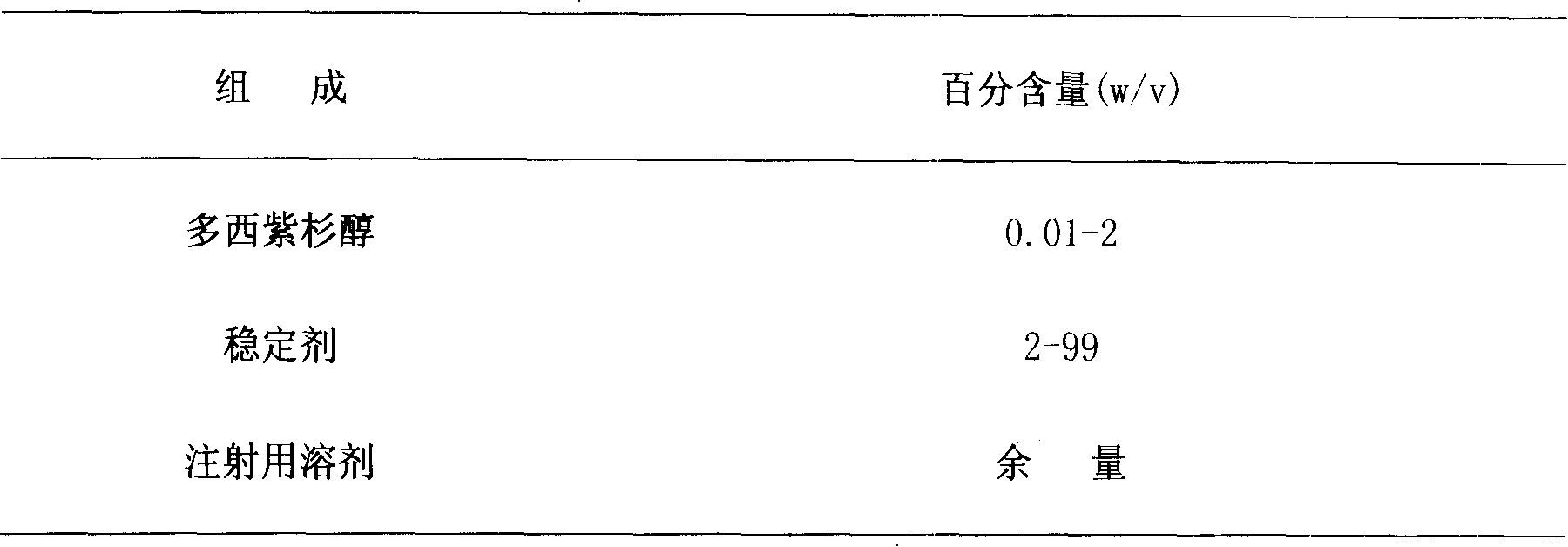
The invention provides a prescription and a preparation method of an anti-tumor medicament docetaxel injection which is used for early chemotherapy failure or metastatic breast cancer, late cisplatin chemotherapy failure or metastatic small cell carcinoma in the field of pharmacy. The preparation is an injection prepared by using docetaxel as an active component and adding injection auxiliary materials through heat source removing, degerming and alcohol volatilization. Aiming at the overcoming the defect of a medicinal preparation using docetaxel as the active component, the invention provides the prescription and preparation method of the docetaxel injection. The preparation prepared by virtue of the method is excellent in stability and convenient in preparation, so that the medicament safety and the patient adaptability are improved.
Owner:双鹤药业(海南)有限责任公司
Docetaxel injection and preparation method thereof
InactiveCN101190213AImprove stabilityConvenient for clinical operationOrganic active ingredientsPharmaceutical delivery mechanismDocetaxel-PNPMedical prescription
The invention relates to the technical field of medicine, in particular to a novel preparation method of docetaxel injection. The prescription of the docetaxel injection of the invention consists of docetaxel, stabilizing agent and menstruum used for injection. The preparation method includes the following steps: (1) the stabilizing agent of a prescription amount is dissolved in the menstruum of the prescription amount used for injection; (2) the docetaxel of the prescription amount is dissolved in the solution obtained in the step (1); and then filtering, sub-package and sterilization are carried out to finish the preparation. The docetaxel injection of the invention has better stability and convenient use.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Docetaxel injection composition and preparation method thereof
ActiveCN102988285AImprove securityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPolyethylene glycol
The invention provides a docetaxel injection composition and preparation method thereof. The components of the docetaxel injection are as follows: the concentration of a main drug docetaxel ranges from 20 mg / mL to 60 mg / mL; the proportional range of the main drug to absolute ethyl alcohol is 1 : 1-1 : 50; the proportion of the main drug to poly(ethylene glycol stearate) 15 ranges from 1 : 1 to 1 : 50; anhydrous citric acid is used as a pH conditioning agent; and the pH range adjusted by anhydrous citric acid is from 3 to 4.5.
Owner:哈药集团股份有限公司 +1
Nanoscale docetaxel and preparation method thereof
ActiveCN102973490ABioavailability leapEasy to prepareOrganic active ingredientsPowder deliverySide effectOral medication
The invention discloses nanoscale docetaxel and a preparation method thereof, and relates to the docetaxel which is an antitumor drug. Aiming at the defects of low solubility and low oral bioavailability of the docetaxel, and serious toxic and side effects of the conventional docetaxel injection, the invention aims at providing novel nanoscale docetaxel particles which are characterized by using silicon dioxide aerogel as a carrier of the docetaxel on the one hand; the invention aims at providing a preparation method of the novel nanoscale docetaxel particles on the other hand; and the preparation method is characterized by comprising the following steps: dissolving the docetaxel in anhydrous ethanol, adding the silicon dioxide aerogel according to proportion, drying after full absorption, adding purified water, feeding into an emulsifying machine for emulsification, homogenizing through a high pressure homogenizer, and drying the obtained homogenate to obtain the novel nanoscale docetaxel particles. The nanoscale docetaxel disclosed by the invention is especially suitable for oral administration.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Segmented copolymer - docetaxel combination, preparation thereof and preparation method thereof
InactiveCN102626521AAvoid burst phenomenonImprove bioavailabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the technical field of pharmaceutical chemicals and discloses segmented copolymer - docetaxel combination, a preparation thereof and a preparation method thereof. The combination is combined by bonding polyoxyethylene - polypropylene oxide - polyoxyethylene (PEO- b - PPO - b - PEO with a product name as Pluronics (BASF company)) triblock copolymer and docetaxel through ester bond, and the Pluronics - docetaxel combination is obtained. By utilizing amphipathy of the combination and adopting physical methods to prepare micelle aqueous solution, a freeze-dried preparation can also be prepared. Nanomicelle is capable of gathering at tumor parts through 'enhanced permeation and retention effects (EPR effects)', and tumor targeting of docetaxel is enhanced. Besides, an active targeting factor is connected on Pluronics polymer so as to have the function of active targeting. The segmented copolymer - docetaxel combination overcomes the defects that existing docetaxel injection is poor in water-solubility and severe in allergic reaction and the like.
Owner:SHANDONG UNIV
Construction of intravenous-injection self-assembled nano-particles of triglyceride prodrug
ActiveCN112494458AMeeting urgent needsThe synthesis method is simpleOrganic active ingredientsPharmaceutical non-active ingredientsDisulfide bondingAdjuvant
The invention belongs to the fields of novel adjuvants and novel dosage forms of pharmaceutical preparations and relates to lipase-reducing-environment-programmed-triggered synthesis of a triglycerideprodrug, construction of intravenous-injection self-assembled nano-particles of the prodrug and application of the prodrug in drug delivery. According to the disulfide-bond-bridged triglyceride prodrug, Docetaxel is selected as a model drug and is connected to a structural framework of triglyceride through dithiodiglycolic acid, and fatty acids of 1 and 3 positions are (a) stearic acid, (b) oleicacid and (c) linoleic acid. The disulfide-bond-bridged triglyceride prodrug is designed and synthesized, and the synthesis method is easy and feasible; meanwhile, uniform micromolecular prodrug self-assembly nano-particles are prepared, the preparation method is easy and feasible, the stability is good, and the efficient entrapment of the Docetaxel is achieved; and the micromolecular prodrug self-assembly nano-particles of the triglyceride prodrug can be applied to injection administration, problems of Docetaxel injections in the prior art are overcome, and thus, the treatment effect is improved.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation of antineoplastic drug docetaxel injection
InactiveCN101342143AIdea novel scienceEasy to acceptOrganic active ingredientsPharmaceutical delivery mechanismAlcoholMedicine
The invention relates to a preparation process of anticancer drug docetaxel injecta, which is applied to the field of chemical medicine. The product preparation process comprises that 20g to 25g of docetaxel is weighed precisely according to the formula dosage and is added into 2000ml to 3000ml of absolute ethyl alcohol to be stirred till being dissolved completely, and then 500ml to 800ml sterilized polysorbate 80 is added to be stirred for mixing till clarification; the mixture is filtered two times with a microporous filtering film and a degermation and heat-extraction filter under the aseptic condition, and then is subpackaged in vials after is in sure of asepsis and no heat source, and the half cap-pressing is carried out on line; the mixture is dried in vacuum under the prescriptive temperature, vacuum degree and time and then is sealed after the ethanol is removed, and finically the cap is pressed. The process has complete sterilization, simple heat-extraction preparation flow and low cost, is easy to be accepted by the patients, alleviates the pain of the patients, and has obvious healing efficacy and safe and reliable medication.
Owner:辽宁嘉事堂药业有限公司
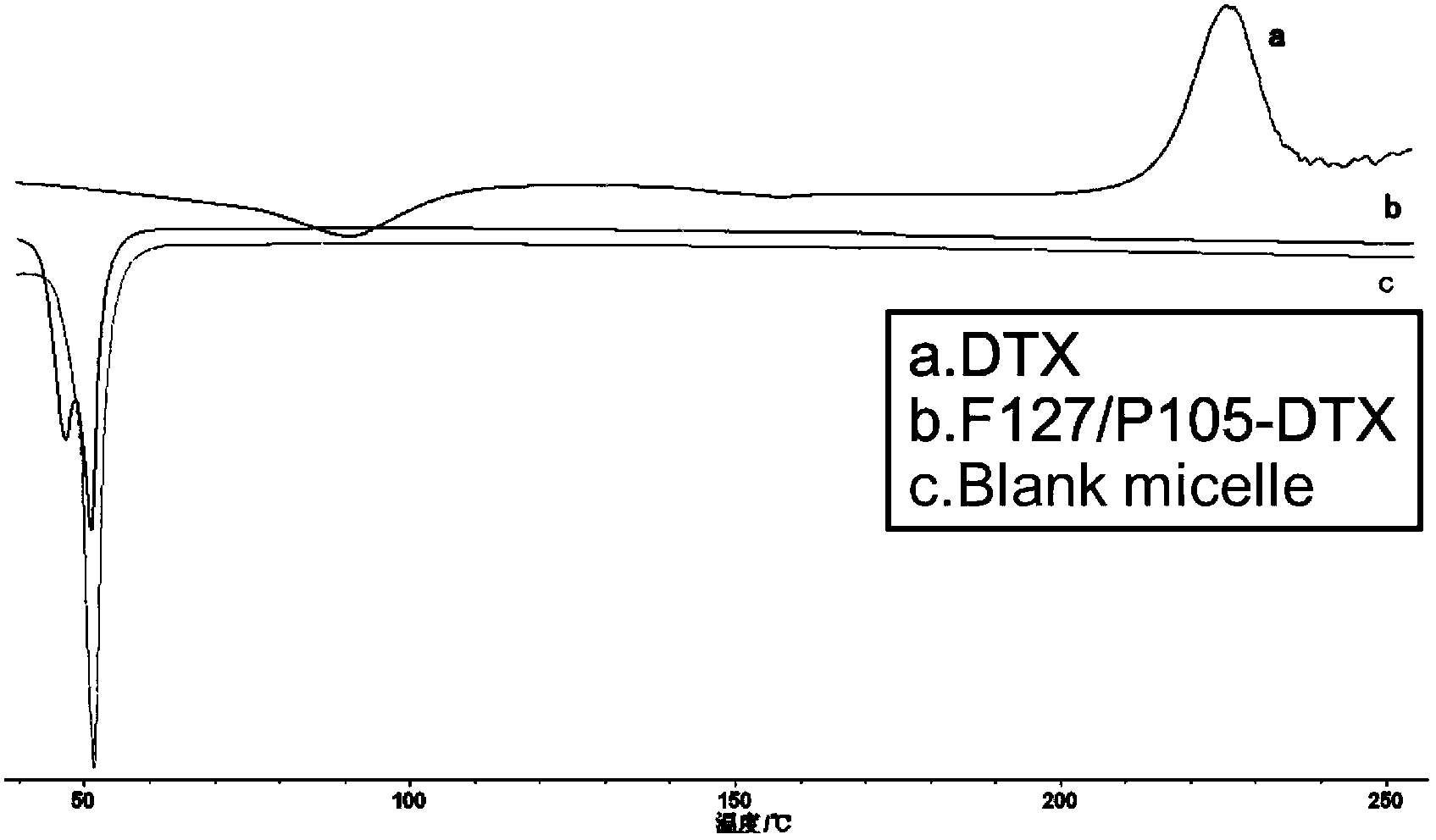
Docetaxel-coated polymeric micelle and preparation method thereof
InactiveCN103446040AImprove solubilityLow toxicityOrganic active ingredientsPharmaceutical delivery mechanismSolubilitySide effect
The invention belongs to the technical field of biological medicines, and relates to a docetaxel-coated polymeric micelle and a preparation method thereof. The preparation method is characterized in that a thin-film dispersion method is used for preparing a docetaxel Pluronic F127 / P105 mixed micelle, Pluronic is used as a carrier, and docetaxel is coated in a hydrophobic inner core PPO (poly-p-phenylene oxide) of Pluronic. The mixed micelle prepared by the method can be used for effectively improving the solubility of the docetaxel; the PEO (polyoxyethylene) long chain of F127 can play a role in long circulation and is prevented from being phagocytized by a reticulo-endothelial system, thus the half-life period of the mixed micelle in blood is prolonged; the P105 in the mixed micelle can be used for inhibiting the excretion of P-gp to the maximum degree and can efficiently reverse the multidrug resistance of tumors so as to improve the treatment effect on the tumors. The mixed micelle prepared by the method does not contain Tween-80 or alcohol. Compared with commercially available docetaxel injection, the mixed micelle can be used for obviously improving the toxic and side effects of the medicines and the safety of clinical application.
Owner:FUDAN UNIV
Novel docetaxel injection pharmaceutical composition and application thereof
InactiveCN102309444AImprove stabilityEasy to storeOrganic active ingredientsSolution deliveryMicro nanoMedicine
The invention discloses a novel docetaxel injection pharmaceutical composition, which comprises an A agent (micro-nano-docetaxel) and a B agent (a drug solvent). The docetaxel injection pharmaceutical composition disclosed by the invention is simple in production process and convenient to use. The docetaxel injection pharmaceutical composition can be stored at room temperature. The drug stability is prior to homemade and importation like products.
Owner:张伟 +1
Method for purifying docetaxel
ActiveCN109836401AReduce contentReduce the introductionOrganic chemistryBulk chemical productionTolueneImpurity
The invention discloses a method for purifying docetaxel. A docetaxel solid precipitates from a dichloromethane and toluene mixed solution. The purifying method has the advantages of great reduction of the single content of every impurity in docetaxel, introduction of few impurities, improvement of the purity of the product, and high yield, and is very suitable for industrial large-scale production, and the obtained product meets preparation demands, and can be directly used to prepare a docetaxel injection.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation of docetaxel long-circulating liposome and freeze-dried powder injection thereof
ActiveCN101322689BHigh drug loadingImprove stabilityOrganic active ingredientsSolution deliveryCholesterolAntioxidant
The invention discloses a preparation technique of non-pegylation docetaxel long-circulating liposome which meets the requirements for clinical and large-scale production and a freeze-dried powder-injection; the liposome consists of docetaxel, neutral phospholipid, charged phospholipid, cholesterin, an antioxidant, an excipient, a buffering agent and water used for injection; the preparation technique includes the following steps: preparing multilamelar liposome, homogenizing the liposome, fixing volume, sterilizing, split charging, freeze drying, etc. The liposome increases the solubility ofdrugs, overcomes poor stability of docetaxel injection and toxicity caused by compound solvent and prolongs the in vivo circulating time of drugs; compared with pegylation long-circulating liposome, the toxicity is reduced and the inhibiting effect on tumor cells is enhanced.
Owner:HAINAN SIMCERE PHARMA CO LTD
Docetaxel injection and preparation method thereof
InactiveCN105395477AAntioxidantImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPolyethylene glycol
The invention relates to a docetaxel injection. The docetaxel injection is prepared from docetaxel, lipoic acid, medicinal absolute ethyl alcohol and polyethylene glycol stearate. The lipoic acid is added into the docetaxel injection, and stability of double bonds in the docetaxel is facilitated. In addition, the lipoic acid has the anti-oxidation effect and can slow down product degradation. In the injection preparation process, compared with an existing technology, the medicinal absolute ethyl alcohol is not removed. During clinical use, the step of adding ethyl alcohol for dilution is omitted, and use is safer, more convenient and faster. The four components in the injection are compounded for use, the docetaxel injection meets the USP35 docetaxel injection standard within the two-year life time, and the clinical application is safer.
Owner:李宏 +1
Productive technology of docetaxel injection
InactiveCN102078295AGuaranteed contentReduce generationOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonFiltration
The invention relates to a productive technology of a docetaxel injection, and in particular relates to a common productive process of a thick injection in the market, wherein polysorbate 80 is taken as a primary solvent in the productive process. The main processes of the productive technology are as follows: a docetaxel bulk pharmaceutical chemical (BPC) is dissolved into the polysorbate 80 by ultrasound after homogenate; an ethanol solution of citric acid is utilized to regulate the pH value of the polysorbate 80 solution to 4.3-5.5; a proper amount of activated carbon is added to eliminate pyrogen; the activated carbon is eliminated by multistage millipore filtration; and filling is performed under the condition of sterile operation after millipore filtration sterilization is carried out. The injection prepared by the productive technology meets the requirements in tentative standard YBH10802005 from State Food and Drug Administration (SFDA).
Owner:北京东方协和医药生物技术有限公司
Preparation method of docetaxel injection
ActiveCN107157926AImprove solubilitySimple manufacturing methodOrganic active ingredientsPharmaceutical delivery mechanismDrugs preparationsGlucose polymers
The invention relates to the field of drug preparations, in particular to a docetaxel injection and a preparation method thereof. The docetaxel injection diluted to the clinical application concentration with a 5% glucose solution or a 0.9% sodium chloride solution can be stored for a long time without separating out precipitations. With the adoption of the preparation method, the defects that docetaxel diluted to the clinical application concentration with the 5% glucose solution or the 0.9% sodium chloride solution is poor in stability and likely to separate out precipitations and cannot be stored for a long time are overcome, and the medicinal safety is improved.
Owner:SICHUAN HUIYU PHARMA
Method for preparing tween-free docetaxel injection
InactiveCN102793678AImprove solubilityExtended stayPowder deliveryOrganic active ingredientsSolubilityChemical industry
The invention relates to a method for preparing a changed formulation of an antitumor medicine sold on the market, in particular to a method for preparing a tween-free docetaxel injection, and belongs to the technical field of medicines and chemical industry. According to the method, poloxamer 407 is used as a carrier of docetaxel, precursor polymer micelles are prepared by a melting method, a powder injection or an injection is prepared, and a stable and clarified docetaxel polymer micelle solution can be obtained by melting the powder injection in water or diluting the injection before the powder injection and the injection are used, so that the solubility of docetaxel is improved, the in-vivo detention time is prolonged, and the adverse reaction caused by using tween-80 is avoided.
Owner:邓世明
Docetaxel injection and preparation method thereof
InactiveCN105395540ANo hemolysisImprove securityOrganic active ingredientsPharmaceutical delivery mechanismHemolysisGlycerol
The invention discloses docetaxel injection. The docetaxel injection is prepared from the following components in parts by weight: 1-2 parts of docetaxel, 18-25 parts of a solvent, 20-30 parts of a surfactant, 0.3-0.6 part of a stabilizer, and 40-60 parts of a solubilizer, wherein the solvent is one or more of absolute ethyl alcohol, glycerol and propylene glycol, the surfactant is polyoxyethylenated castor oil, the stabilizer is one or more of citric acid, tartaric acid, fumaric acid and maleic acid, and the solubilizer is polyethylene glycol 300 and / or polyethylene glycol 400. The docetaxel injection provided by the invention is free of hemolysis, good in safety, good in stability, stable in content of active ingredients, and low in contents of related substances and impurities, and can be stored under the condition with the temperature being 20-40 DEG C.
Owner:HAINAN GENERAL & KANGLI PHARMA
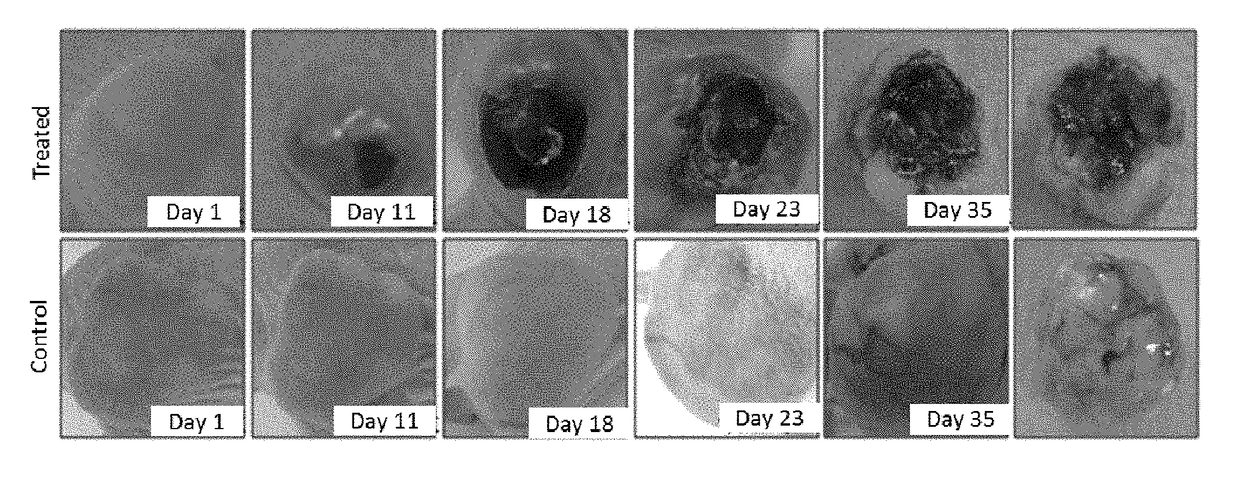
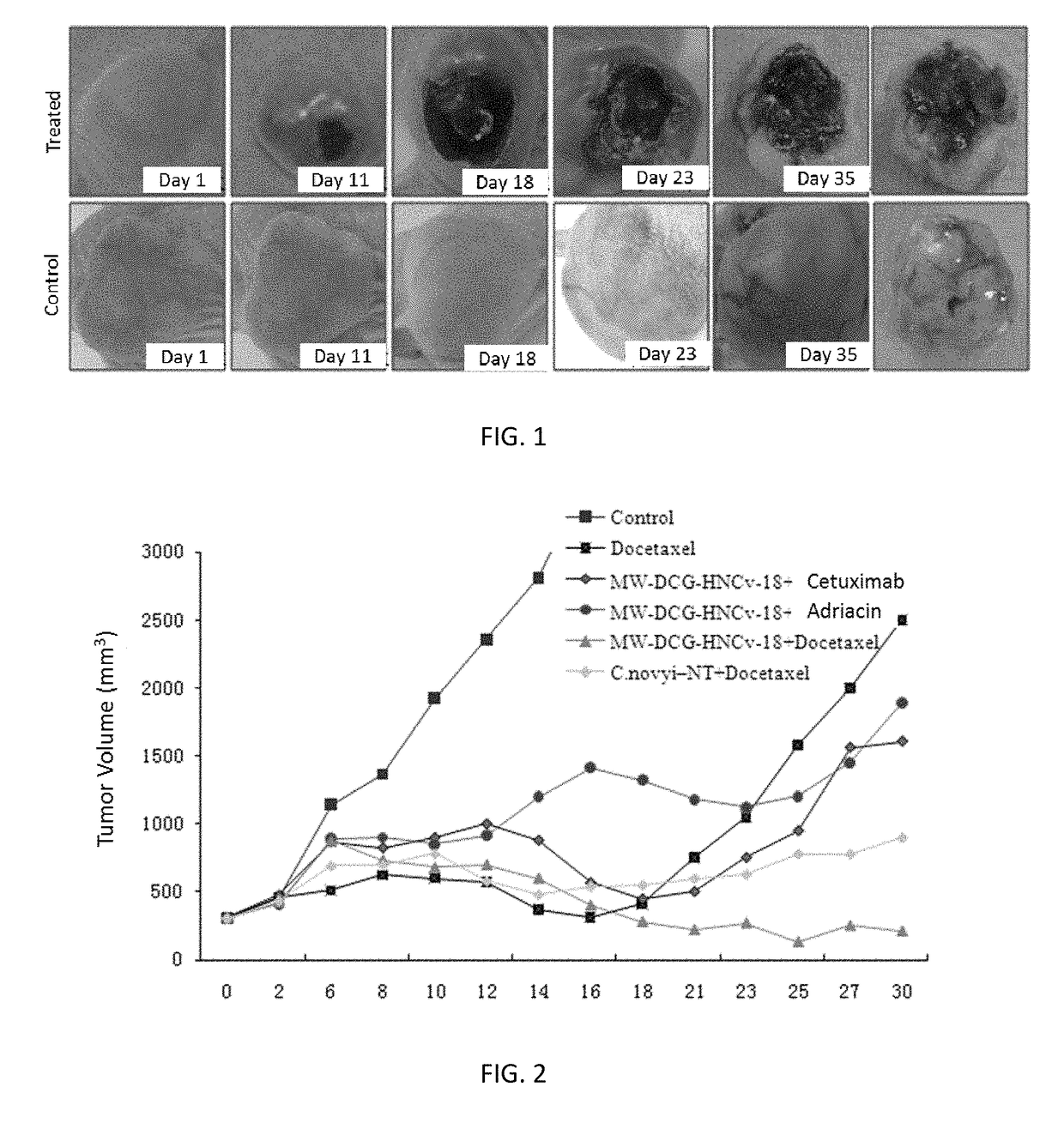
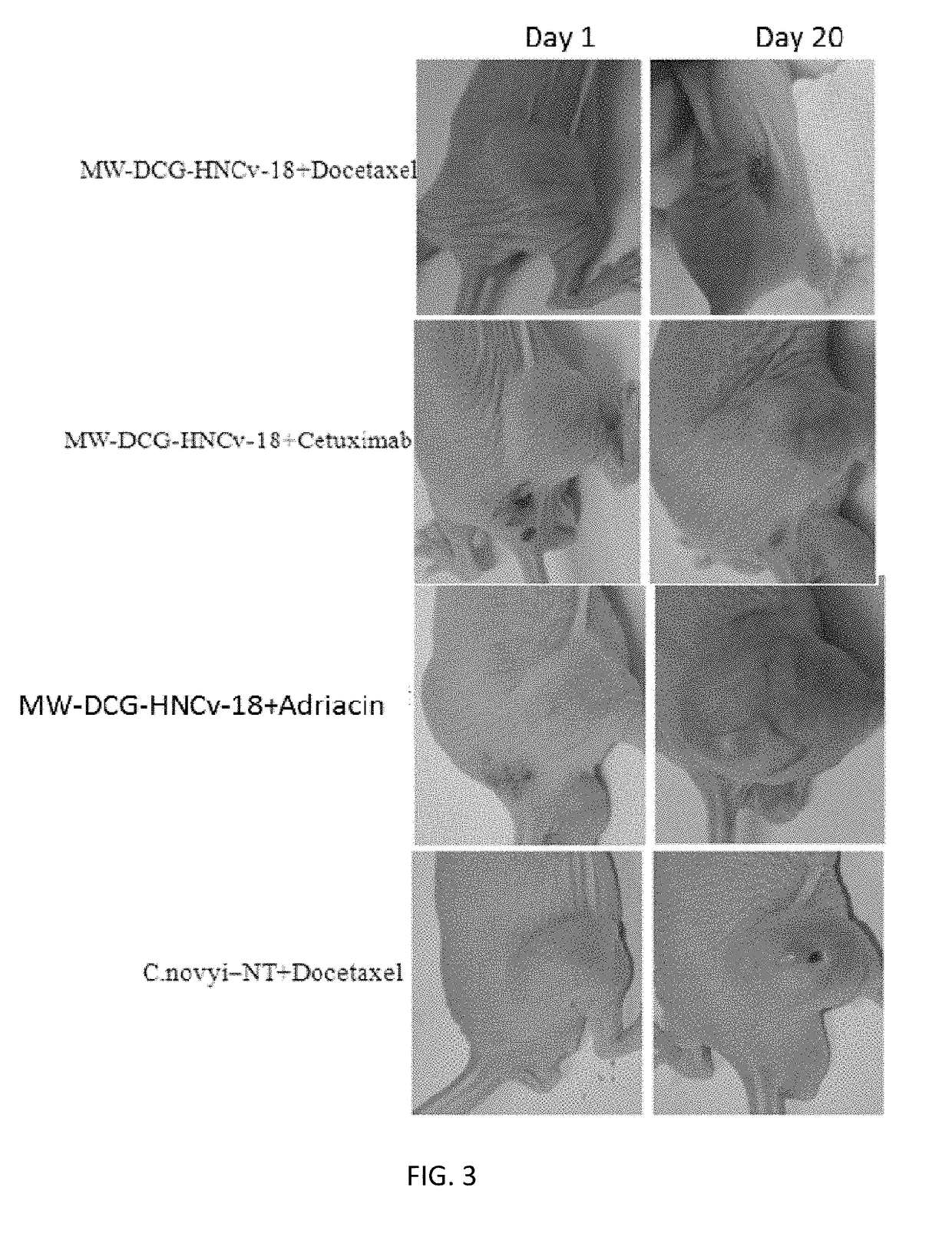
Application of derivative of clostridium ghonii
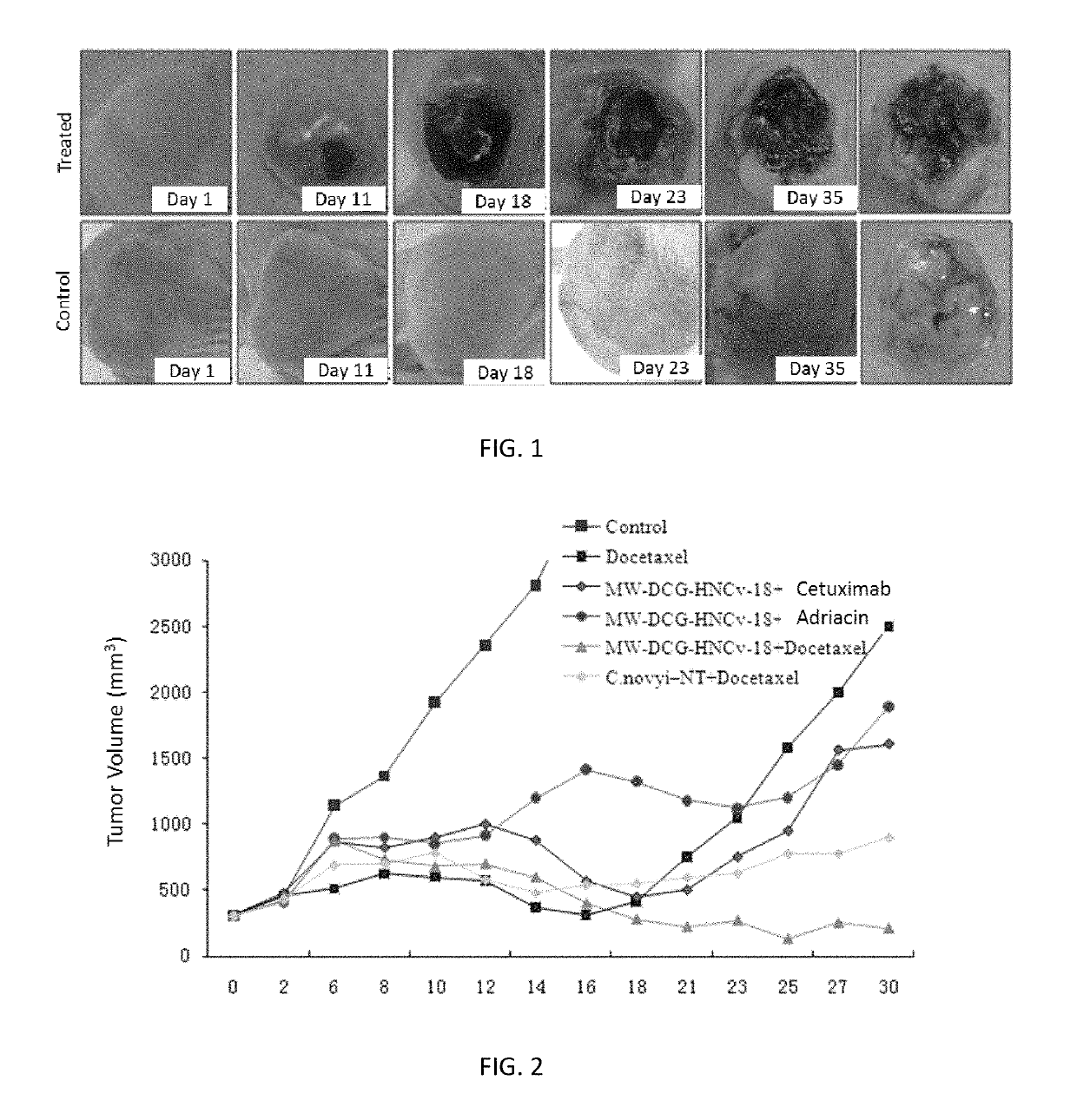
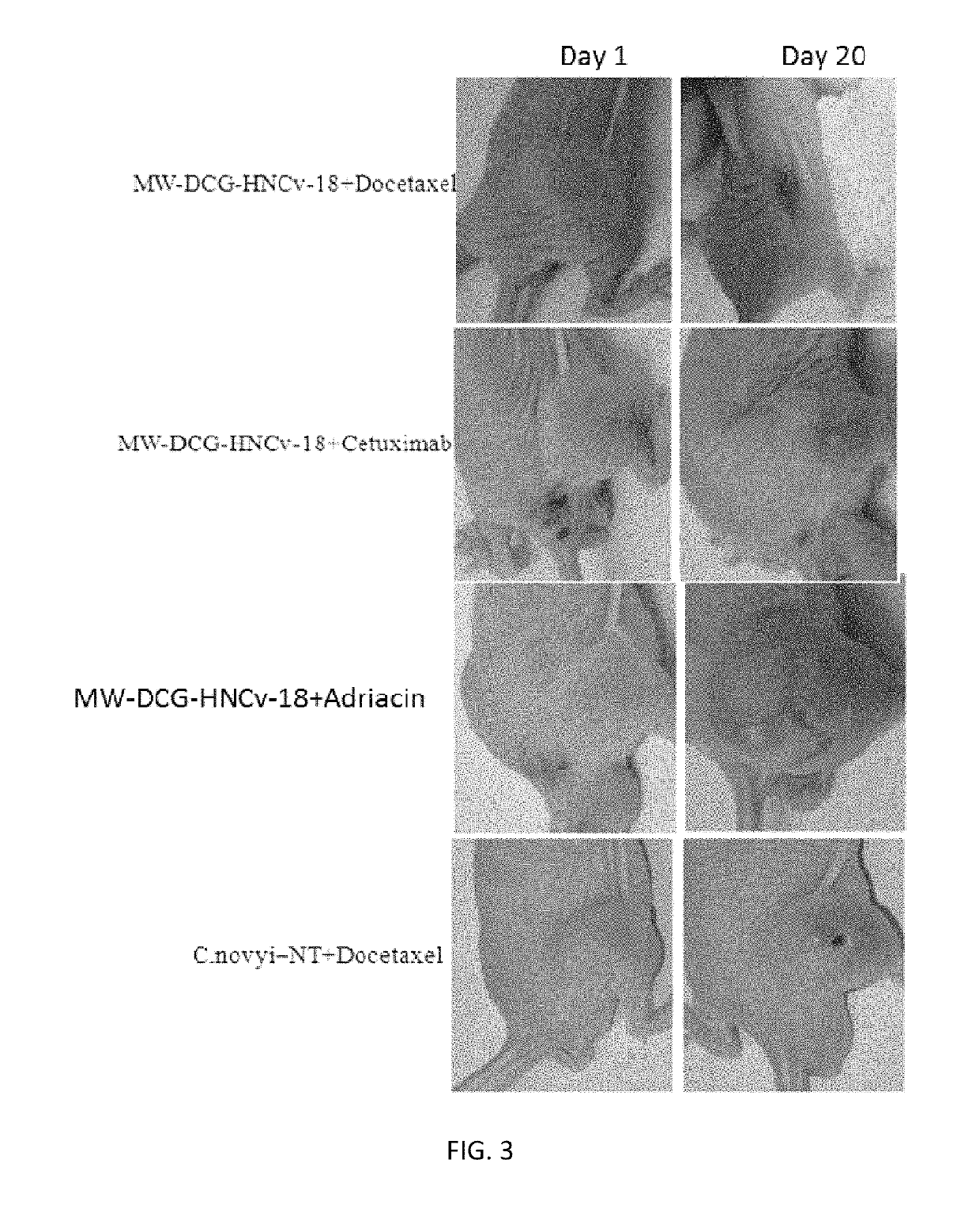
ActiveUS20170354633A1InhibitionEnhanced inhibitory effectPowder deliveryOrganic active ingredientsDocetaxel-PNPMedicine
Application of Derivatives of Clostridium ghonii, especially in the application of Derivatives of Clostridium ghonii MW-DCG-HNCv-18 in preparation of medicines for treating non-small cell lung carcinoma. It also discloses a medicine combining the Derivatives of Clostridium ghonii MW-DCG-HNCv-18 strain with Docetaxel as the active ingredients. According to the invention, the MW-DCG-HNCv-18 strain is found to have specific inhibition effect on non-small cell lung carcinoma for the first time, the inhibition effect on the non-small cell lung carcinoma is significantly superior to that of other known similar strains, and through screening, the MW-DCG-HNCv-18 strain is found to have more prominent inhibition effect on non-small cell lung carcinoma when combined with Docetaxel injection.
Owner:SHANDONG XINCHUANG BIOLOGICAL TECH CO LTD
Docetaxel injection composition and preparation method thereof
ActiveCN102988285BOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPolyethylene glycol
The invention provides a docetaxel injection composition and preparation method thereof. The components of the docetaxel injection are as follows: the concentration of a main drug docetaxel ranges from 20 mg / mL to 60 mg / mL; the proportional range of the main drug to absolute ethyl alcohol is 1 : 1-1 : 50; the proportion of the main drug to poly(ethylene glycol stearate) 15 ranges from 1 : 1 to 1 : 50; anhydrous citric acid is used as a pH conditioning agent; and the pH range adjusted by anhydrous citric acid is from 3 to 4.5.
Owner:哈药集团股份有限公司 +1
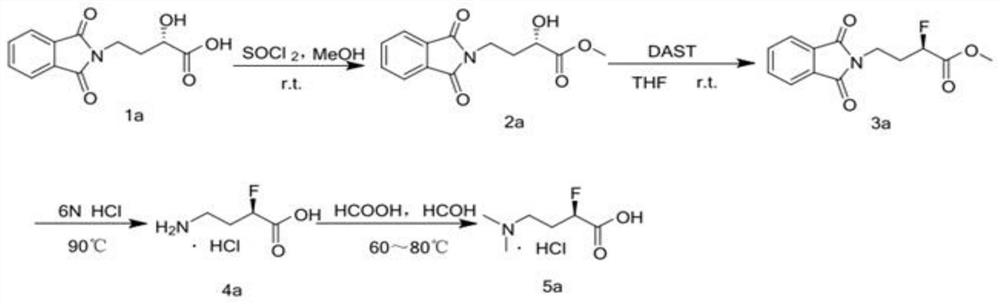
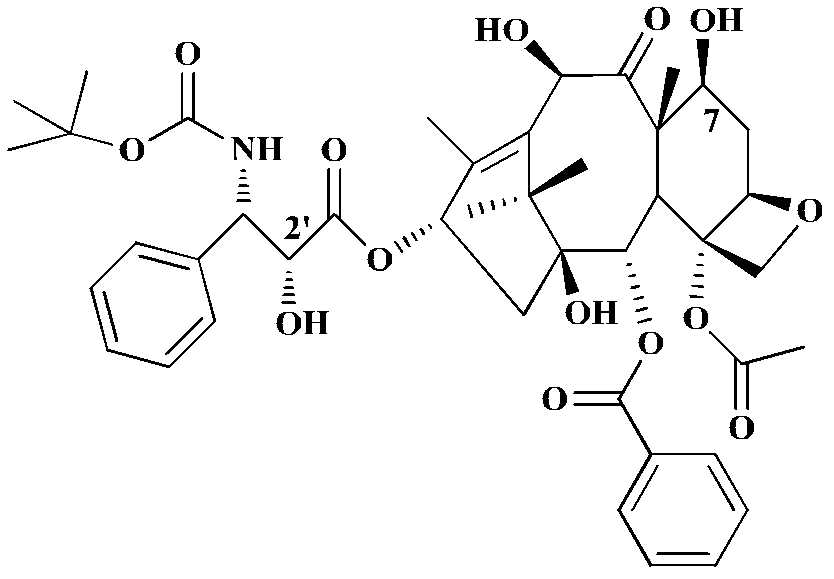
Industrial synthesis method of water-soluble docetaxel derivative
The invention provides an industrial synthesis method of a water-soluble docetaxel derivative. The method is characterized in that 10-deacetylbaccatin III is used as a substrate, and the 10-deacetylbaccatin III and (R)-4-dimethylamino-2-fluoro-butyric acid hydrochloride are synthesized into the water-soluble docetaxel derivative. According to the method, a two-step synthesis technology, a centrifugal extraction separation technology, a recrystallization technology, a supercritical CO2 fluid drying technology and a full-automatic control technology are combined, synthesis and purification are carried out under the synergistic effect of the technologies, the water solubility of the water-soluble docetaxel derivative produced by the combined technology can reach 12 mg / ml or above, and the the water-soluble docetaxel derivative has about 2000 times higher solubility in water than docetaxel. A docetaxel injection prepared by the method does not use absolute ethyl alcohol or Tween-80 hydrotropy, and the docetaxel derivative is a water-soluble docetaxel derivative which is efficient, free of anaphylactic reaction and small in toxic and side effect. Therefore, the product has the characteristics of small toxic and side effects, low production cost, high product purity, high product yield and stable quality, and is suitable for industrial large-scale production.
Owner:陈开云
Segmented copolymer - docetaxel combination, preparation thereof and preparation method thereof
InactiveCN102626521BBiologically activeInhibition of effluxOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention belongs to the technical field of pharmaceutical chemicals and discloses segmented copolymer - docetaxel combination, a preparation thereof and a preparation method thereof. The combination is combined by bonding polyoxyethylene - polypropylene oxide - polyoxyethylene (PEO- b - PPO - b - PEO with a product name as Pluronics (BASF company)) triblock copolymer and docetaxel through ester bond, and the Pluronics - docetaxel combination is obtained. By utilizing amphipathy of the combination and adopting physical methods to prepare micelle aqueous solution, a freeze-dried preparation can also be prepared. Nanomicelle is capable of gathering at tumor parts through 'enhanced permeation and retention effects (EPR effects)', and tumor targeting of docetaxel is enhanced. Besides, an active targeting factor is connected on Pluronics polymer so as to have the function of active targeting. The segmented copolymer - docetaxel combination overcomes the defects that existing docetaxel injection is poor in water-solubility and severe in allergic reaction and the like.
Owner:SHANDONG UNIV
Method for improving stability of docetaxel injection
PendingCN113520866AReduce direct contactAvoid degradationOrganic active ingredientsPharmaceutical delivery mechanismPenicillinEngineering
The invention relates to a method for improving the stability of a docetaxel injection. The method comprises the following steps: adding part of a first auxiliary material into a mixing tank and stirring; adding a second auxiliary material into the mixing tank, stirring and dissolving, vacuumizing, and introducing nitrogen; adding the docetaxel raw material, the remaining first auxiliary material and other additives into a mixing tank, and stirring for dissolving; carrying out vacuum-pumping treatment on the mixing tank, and filling nitrogen after vacuum degassing is finished; continuously stirring to uniformly mix the liquid medicine; applying nitrogen pressure into the mixing tank, and filtering the liquid medicine into a sterile storage tank; and filling the liquid medicine in the sterile storage tank into a penicillin bottle, pressing a plug, and rolling a cover. Compared with the prior art, the nitrogen is filled in the liquid preparation and / or filling stage, and the nitrogen filling pressure is strictly controlled, so that the direct contact between the medicine and oxygen can be well reduced, the medicine is prevented from being degraded, the medicine stability is improved, and the quality and effectiveness of the medicine are ensured.
Owner:LUOXIN PHARM SHANGHAI CO LTD +1
Docetaxel injection storage device and preparation method thereof
PendingCN111603387AFast dissolutionAffect the efficacyShaking/oscillating/vibrating mixersPharmaceutical containersPenicillinChemical reaction
The invention discloses a docetaxel injection storage device, which comprises a penicillin bottle body, a butyl rubber plug is plugged into the inner wall of an opening of the penicillin bottle body,and the penicillin bottle body is filled with oily docetaxel injection and a butyl rubber vibrator. According to the storage device and the preparation method thereof, the penicillin bottle space needs to be fully filled with a solvent by preparation personnel in the preparation process; therefore, bubbles can be prevented from being generated during oscillation, the dissolving speed of the docetaxel injection in the solvent can be increased, and the situation that the drug effect of the docetaxel injection is affected due to the fact that the drug and the butyl rubber vibrator carry out physical and chemical reactions is prevented.
Owner:WUHU NO 2 PEOPLES HOSPITAL
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021BImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
A kind of preparation method of docetaxel injection
ActiveCN107157926BImprove solubilitySimple manufacturing methodOrganic active ingredientsPharmaceutical delivery mechanismDrugs preparationsBiochemistry
The invention relates to the field of drug preparations, in particular to a docetaxel injection and a preparation method thereof. The docetaxel injection diluted to the clinical application concentration with a 5% glucose solution or a 0.9% sodium chloride solution can be stored for a long time without separating out precipitations. With the adoption of the preparation method, the defects that docetaxel diluted to the clinical application concentration with the 5% glucose solution or the 0.9% sodium chloride solution is poor in stability and likely to separate out precipitations and cannot be stored for a long time are overcome, and the medicinal safety is improved.
Owner:SICHUAN HUIYU PHARMA
Non-predissolved Docetaxel injection
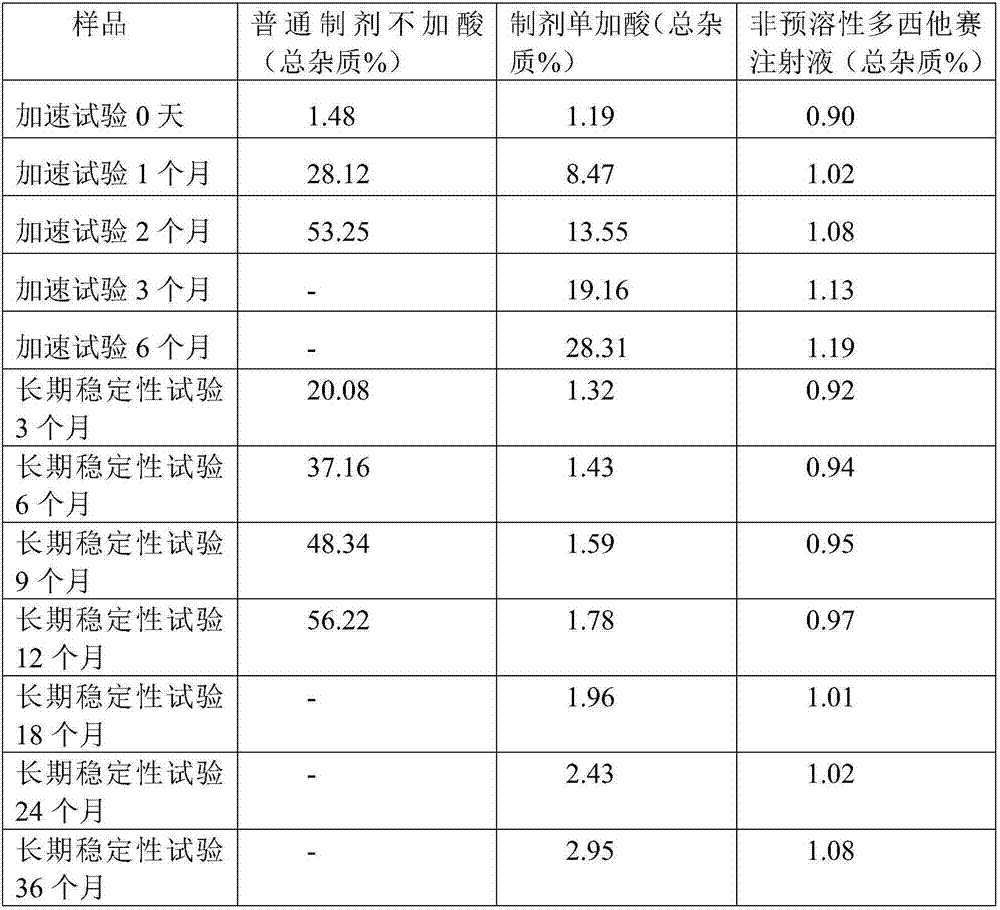
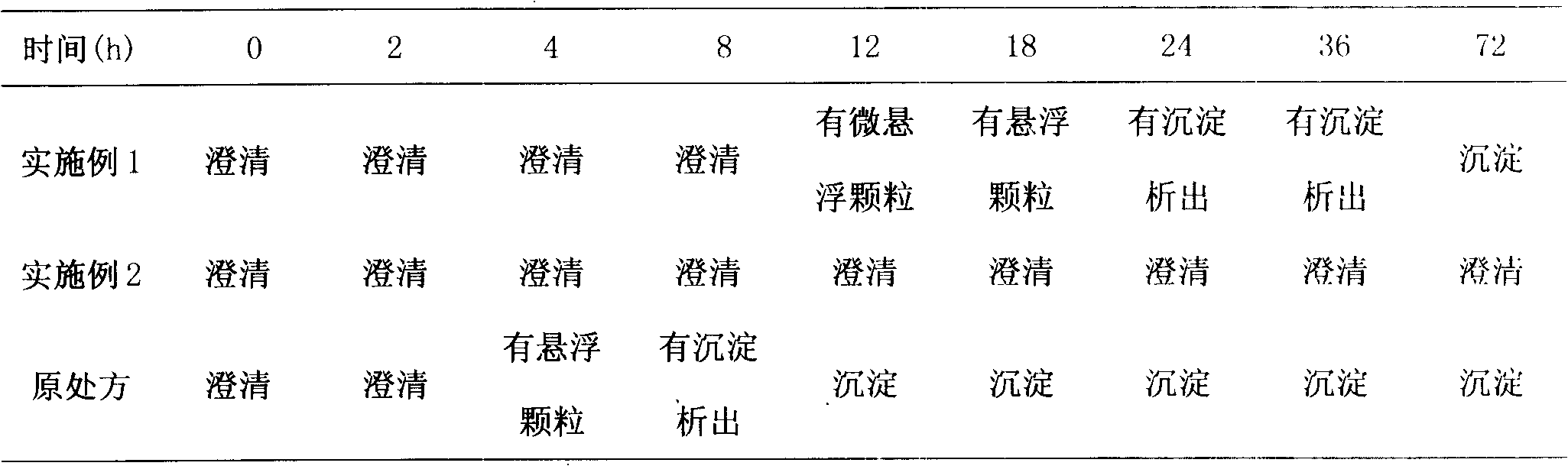
InactiveCN107970210AImprove stabilityHigh speedOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPenicillin
The invention relates to a non-predissolved Docetaxel injection. The non-predissolved Docetaxel injection is prepared by the following method comprising the steps of dissolving citric acid and sodiumcitrate in anhydrous ethanol, sequentially adding Docetaxel, polysorbate 80 for injection and activated carbon for injection with stirring, carrying out stirring and mixing to achieve thorough dissolving after charging is completed, carrying out decarbonization filtration with a titanium bar filter, sterilization filtration with a polytetrafluoroethylene microporous filtration film and filtrationwith a common microporous filtration film sequentially, then, loading filtrate into penicillin bottles, and carrying out cap pressing, capping, labeling and packaging. According to the non-predissolved Docetaxel injection, a proton supplying buffer agent anhydrous ethanol solution prepared from citric acid and sodium citrate is adopted, thus, the solution can basically keep a constant pH(power ofhydrogen) value, and the change of related substances is not obvious along with variance in temperature and time; the problem that Docetaxel injections are poor in solubility and poor in stability issolved, and the non-predissolved Docetaxel injection can be preserved and transported at normal temperature, has no need of predissolving during use and can be prepared instantly; and the number of procedures is few, and the process flow is short.
Owner:湖北久安医药集团有限公司
Docetaxel injection and preparation method thereof
InactiveCN101190213BImprove stabilityConvenient for clinical operationOrganic active ingredientsPharmaceutical delivery mechanismDocetaxel-PNPMedical prescription
The invention relates to the technical field of medicine, in particular to a novel preparation method of docetaxel injection. The prescription of the docetaxel injection of the invention consists of docetaxel, stabilizing agent and menstruum used for injection. The preparation method includes the following steps: (1) the stabilizing agent of a prescription amount is dissolved in the menstruum of the prescription amount used for injection; (2) the docetaxel of the prescription amount is dissolved in the solution obtained in the step (1); and then filtering, sub-package and sterilization are carried out to finish the preparation. The docetaxel injection of the invention has better stability and convenient use.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
A kind of preparation method of docetaxel injection not containing Tween
InactiveCN102793678BImprove solubilityExtended stayPowder deliveryOrganic active ingredientsSolubilityChemical industry
The invention relates to a method for preparing a changed formulation of an antitumor medicine sold on the market, in particular to a method for preparing a tween-free docetaxel injection, and belongs to the technical field of medicines and chemical industry. According to the method, poloxamer 407 is used as a carrier of docetaxel, precursor polymer micelles are prepared by a melting method, a powder injection or an injection is prepared, and a stable and clarified docetaxel polymer micelle solution can be obtained by melting the powder injection in water or diluting the injection before the powder injection and the injection are used, so that the solubility of docetaxel is improved, the in-vivo detention time is prolonged, and the adverse reaction caused by using tween-80 is avoided.
Owner:邓世明
Pharmaceutical composition of docetaxel injection
PendingCN114042039ALow toxicityImprove the dilution effectOrganic active ingredientsPharmaceutical delivery mechanismAnaphylaxisDocetaxel-PNP
The invention relates to a pharmaceutical composition of a docetaxel injection, and belongs to the technical field of pharmaceutical preparations. The pharmaceutical composition of the docetaxel injection comprises a medicine, a solubilizer, a solvent, a dispersing agent and a stabilizing agent, wherein the medicine is docetaxel, and the solubilizer is Solutol HS 15 (HS). According to the technical scheme, the novel docetaxel injection is prepared, and the docetaxel injection is good in stability, free of toxicity and free of anaphylaxis.
Owner:SHIJIAZHUANG UNIVERSITY
Application of derivative of clostridium ghonii
ActiveUS10314809B2InhibitionEnhanced inhibitory effectOrganic active ingredientsPowder deliveryDocetaxel-PNPMedicine
Application of Derivatives of Clostridium ghonii, especially in the application of Derivatives of Clostridium ghonii MW-DCG-HNCv-18 in preparation of medicines for treating non-small cell lung carcinoma. It also discloses a medicine combining the Derivatives of Clostridium ghonii MW-DCG-HNCv-18 strain with Docetaxel as the active ingredients. According to the invention, the MW-DCG-HNCv-18 strain is found to have specific inhibition effect on non-small cell lung carcinoma for the first time, the inhibition effect on the non-small cell lung carcinoma is significantly superior to that of other known similar strains, and through screening, the MW-DCG-HNCv-18 strain is found to have more prominent inhibition effect on non-small cell lung carcinoma when combined with Docetaxel injection.
Owner:SHANDONG XINCHUANG BIOLOGICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com