Preparation of docetaxel long-circulating liposome and freeze-dried powder injection thereof
A technology of long-circulating liposomes and docetaxel, which is applied in the directions of liposome delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. It can reduce the interaction between liposomes and cells, and achieve the effect of improving drug loading and stability, reducing unsafe factors, and strong tumor inhibition in vitro.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation prescription (100mL capacity)
[0055] Docetaxel 100mg
[0056] Soy Phospholipids (SPC) 1.5g
[0057] Distearoylphosphatidylglycerol (DSPG) 150mg
[0058] Cholesterol 600mg
[0059] a-Tocopherol 17mg
[0060] Sucrose about 10g
[0061] Sodium citrate about 294mg
[0062] Citric acid amount
[0063] Water for injection to the required volume
[0064] The preparation process is as follows:
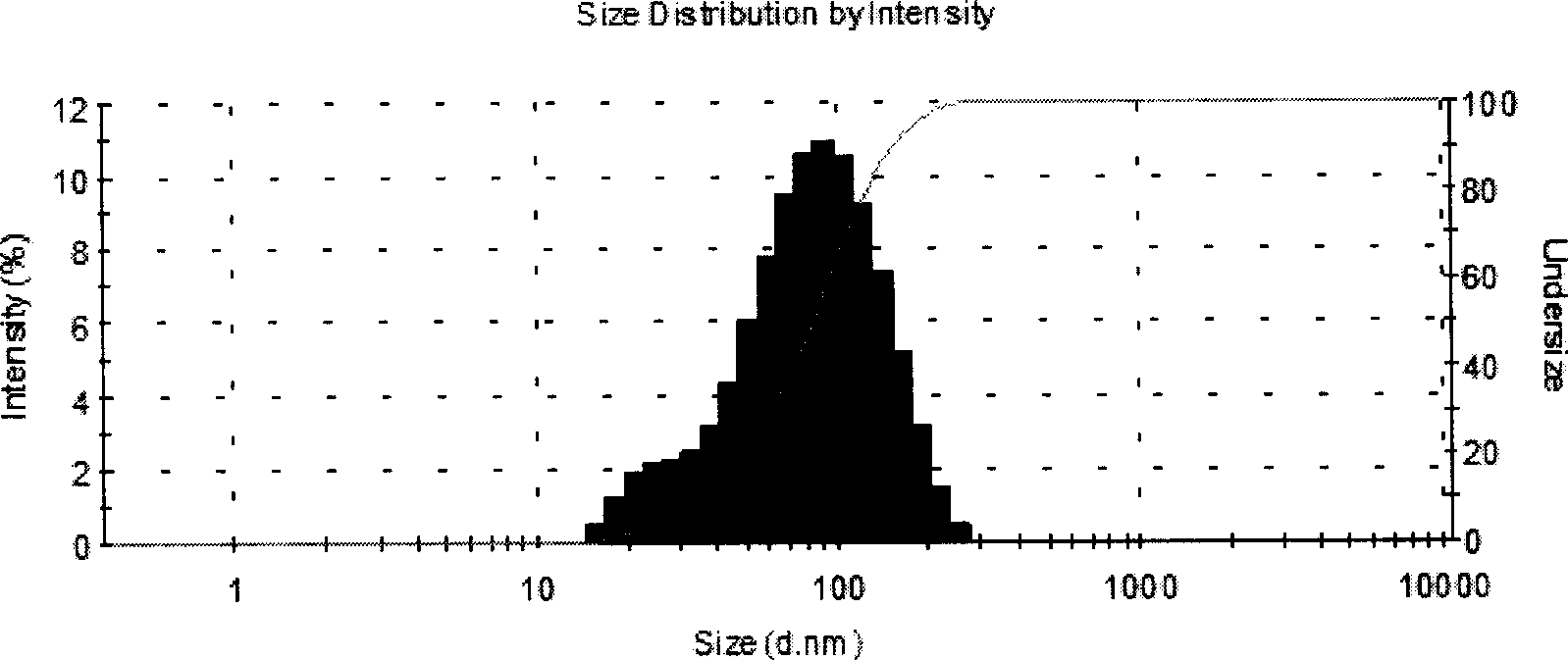
[0065] Select soybean lecithin, distearoylphosphatidylglycerol, cholesterol and a-tocopherol in chloroform solution and mix uniformly according to the formula; use spray drying method to remove chloroform under reduced pressure to form lipid mixture; prepare 0.01M sodium citrate solution , Dissolving sucrose in sodium citrate solution as a hydration solution, the hydration temperature is generally between 55°C±5°C, multi-lamellar liposome suspension; after hydration is complete, use a high-pressure homogenizer to homogenize to an average The particle size is 100±1...
Embodiment 2
[0067] Preparation prescription (100mL capacity)
[0068] Docetaxel 200mg
[0069] Egg Yolk Lecithin (EPC) 4g
[0070] Dimyristoylphosphatidylglycerol (DMPG) 1g
[0071] Cholesterol 1g
[0072] a-tocopheryl succinate 90mg
[0073] Lactose about 10g
[0074] Sodium succinate about 800mg
[0075] Succinic acid amount
[0076] Water for injection to the required volume
[0077] The preparation process is as follows:
[0078] Select egg yolk lecithin, dimyristoyl phosphatidylglycerol, cholesterol and a-tocopheryl succinate in chloroform-methanol (2:1) solution and mix well according to the formula; remove the organic solvent under reduced pressure by evaporation under reduced pressure , to form a lipid mixture; prepare 0.03M sodium succinate solution, dissolve lactose in sodium succinate solution as a hydration solution, the hydration temperature is generally 65 ° C ± 5 ° C, multi-lamellar liposome suspension; After the hydration is complete, use a high-pressure homogeniz...
Embodiment 3
[0080] Preparation prescription (100mL capacity)
[0081] Docetaxel 400mg
[0082] Distearoylphosphatidylcholine (DSPC) 12g
[0083] Distearoylphosphatidylglycerol (DMPG) 2.4g
[0084] Cholesterol 6g
[0085] a-Tocopherol 100mg
[0086] Trehalose about 20mg
[0087] Phosphate about 1.5g
[0088] Water for injection to the required volume
[0089] The preparation process is as follows:
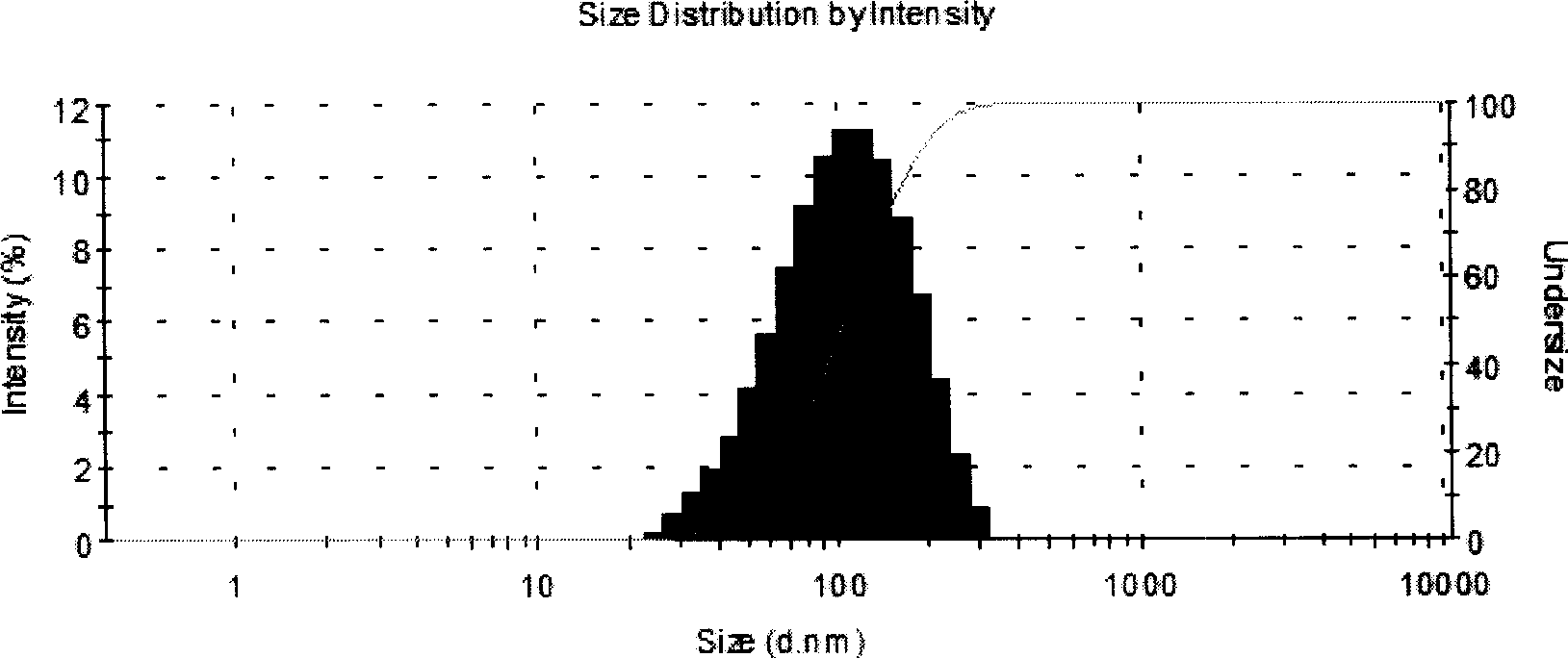
[0090] Distearoylphosphatidylcholine, distearoylphosphatidylglycerol, cholesterol and a-tocopherol were selected according to the formula and dissolved in chloroform-methanol (2:1) and mixed uniformly; the solvent was removed by spray drying to form lipid quality mixture; prepare 0.05M phosphate buffer solution, dissolve trehalose in phosphate solution as a hydration solution, the hydration temperature is generally 45°C±5°C, multi-lamellar liposome suspension; after complete hydration Use a high-pressure homogenizer to homogenize to an average particle size of 80 ± 10nm, dilute to the v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com