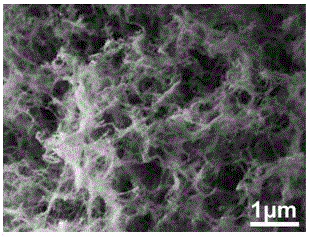
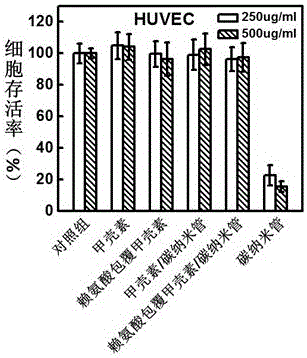
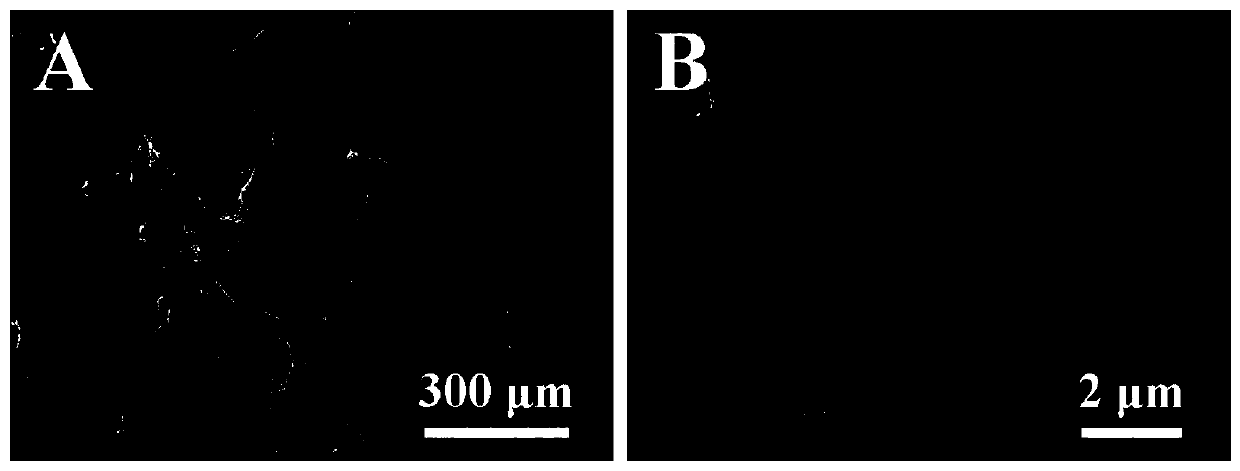
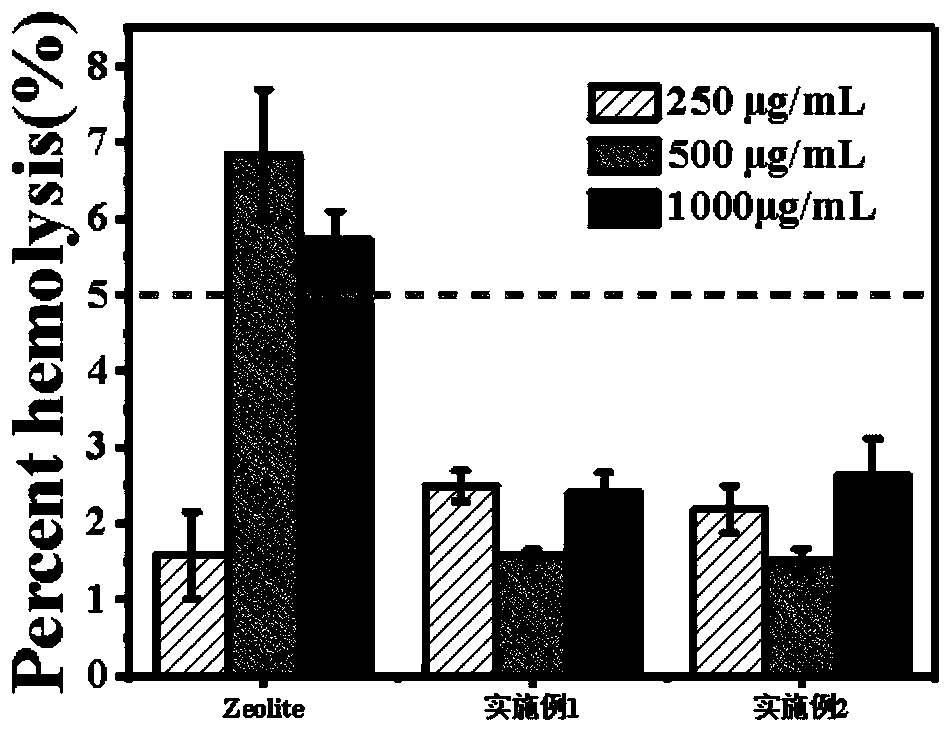
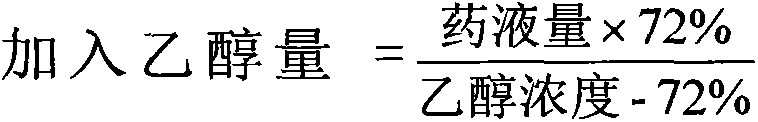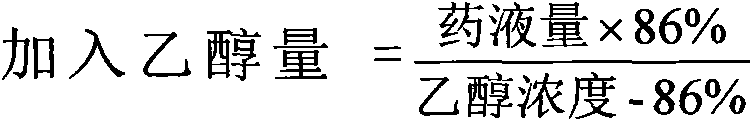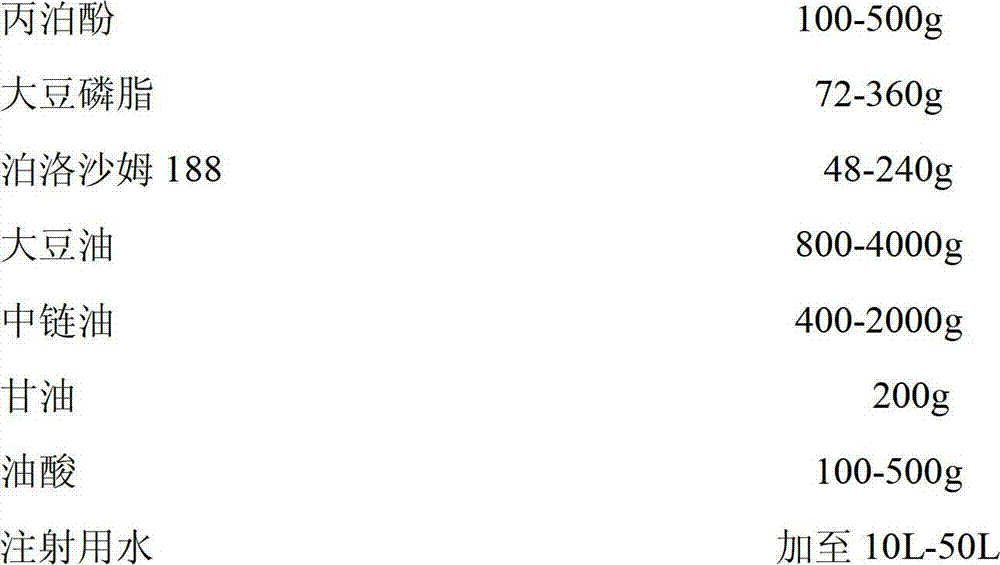Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
141results about How to "No hemolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fasudil hydrochloride pharmaceutical composition for injection
InactiveCN102266343AFix stability issuesHigh yieldOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical drugMedicinal chemistry
The invention discloses a pharmaceutical composition of fasudil hydrochloride for injection. The fasudil hydrochloride injection is composed of fasudil hydrochloride, cysteine hydrochloride and sodium chloride, each containing hydrochloric acid method Sudil 15-60mg, cysteine hydrochloride mg, sodium chloride mg. The preparation method is as follows: take 90% of the prescribed amount of water for injection, at a temperature of 55-65°C, add the prescribed amount of cysteine hydrochloride, stir to dissolve; add the prescribed amount of fasudil hydrochloride, stir until dissolved, and pour into Then add the sodium chloride of recipe quantity in the solution, stir until dissolving completely; Measure initial pH value, according to initial pH value, adjust pH value range with 4% sodium hydroxide solution and 10% cysteine hydrochloride solution in 5.5- 6.5; add medicinal charcoal to the mixture, stir; suction filter, add water for injection to the full amount, mix evenly; fine filter; fill; sterilize; The pharmaceutical composition of fasudil hydrochloride has good light stability, no crystallization and clarity, and good stability. The invention has the advantages of improving product yield, reducing cost, realizing industrialization, and better clinical application. more obvious advantages.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for preparing anticoagulant vascular stent
InactiveCN101927037AImprove bindingNot easy to fall offPharmaceutical containersMedical packagingMedicineDistilled water
The invention discloses a method for preparing an anticoagulant vascular stent, which comprises the following steps of: A, depositing a plasma-polymerized allyl amine functional film on the surface of the vascular stent by using a pulse plasma polymerization method; B, preparing heparin sodium mixed solution; and C, precipitating heparin sodium, namely soaking the vascular stent on which the plasma-polymerized allyl amine functional film is deposited prepared in the step A into the heparin sodium mixed solution prepared in the step B, reacting at the temperature of between 4 and 20 DEG C for 12 to 48 hours, and after the reaction, fully rinsing by using distilled water, and drying to obtain the anticoagulant vascular stent. The vascular stent prepared by the method has the advantages of high binding force between an anticoagulant film layer on the surface of the vascular stent and the vascular stent, and excellent tissue compatibility and blood compatibility.
Owner:CHENGDU SOUTHWEST JIAOTONG UNIV SCI & TECH GARDEN MANAGEMENT
Reagent for processing trace whole blood and application thereof
ActiveCN103323308AAvoid inactivationInhibit bacterial growthPreparing sample for investigationBiological testingBlood serumBlood plasma
The invention relates to a reagent for processing trace whole blood and an application thereof. The reagent comprises the following components: a buffer solution, an osmotic pressure retaining agent, a protective agent, an anticoagulant and a preservative. By adopting the reagent, a trace whole blood sample can be diluted by optional times (2-1000 times) and treated to obtain dilution of serum or plasma without damaging the sample and causing erythrocyte hematolysis.
Owner:BEIJING BOHUI INNOVATION TECH
Intravenous nanometer suspension injection contg. oxaliplatin platinum phospholipid compound
InactiveCN1868455AHigh drug loadingImprove stabilityAntineoplastic agentsOil/fats/waxes non-active ingredientsFreeze-dryingPhospholipid complex
A nano-class suspension injection of oxaliplatin-phoshpatide composition for intravenous injection is proportionally prepared from oxaliplatin, surfactant, pH regulator, isotonic agent, antioxidant, water for injection, and optional excipient (for the freeze-dried powder injection).
Owner:SHENYANG PHARMA UNIVERSITY
Self-emulsifying preparation of taxane compound and preparation method thereof
ActiveCN101829052ASimple production processEase of industrial productionOrganic active ingredientsEmulsion deliveryPhospholipidSurface-active agents
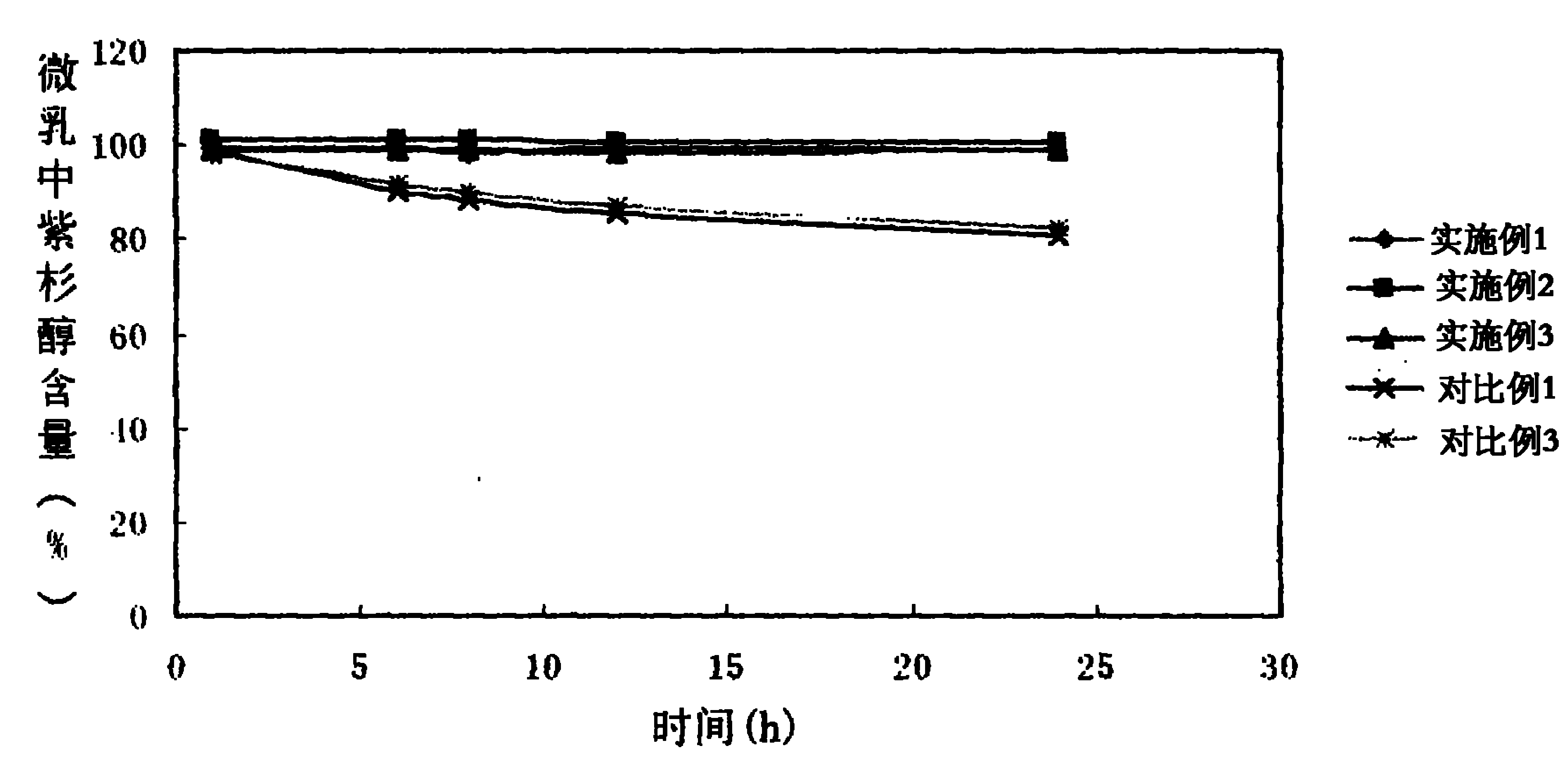
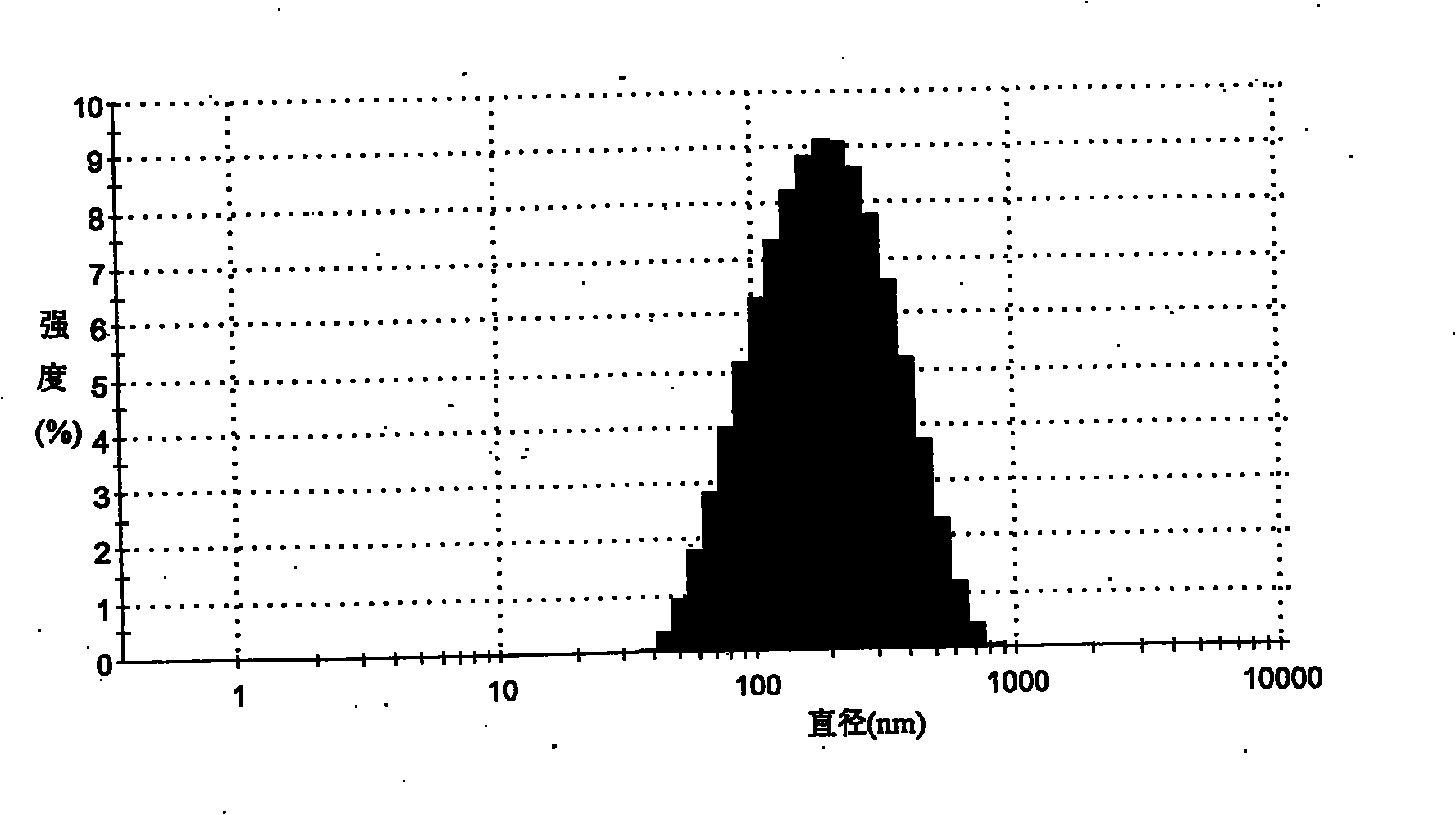
The invention provides a self-emulsifying medical composition for delivering taxane medicaments and a preparation method thereof. The self-emulsifying medical composition is transparent liquid, comprises an effective dose of taxane compound, an oil component, a surface active agent and alcohol and preferentially comprises lactic acid, wherein the surface active agent consists of phospholipid and polyoxyethylenated castor oil. After the self-emulsifying composition is added to a glucose injection, the composition can self-emulsify into micro emulsion the mean grain size of which is 50-300nm, and the micro emulsion can stabilize for more than 12 hours at room temperature. The preferential taxane compound in the invention is taxol.
Owner:YINGU PHARMA
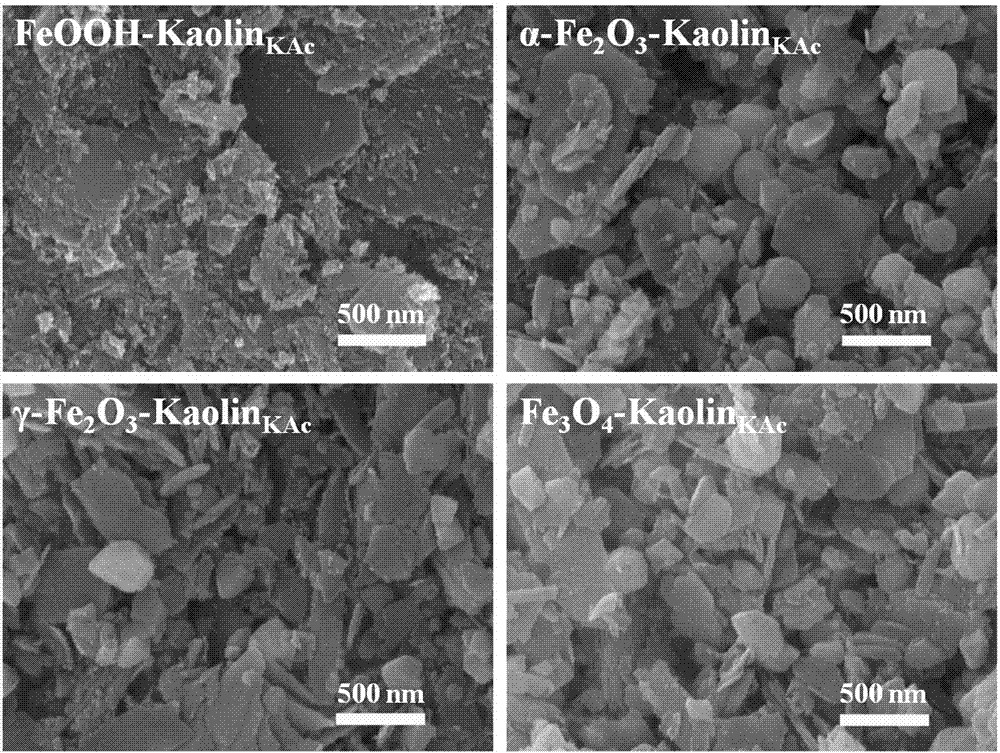
Iron oxide/nano kaolin-containing composite hemostatic and preparation method thereof
ActiveCN107213508AGood hemostatic effectFast hemostasisHeavy metal active ingredientsSurgical adhesivesBiocompatibility TestingIron oxide
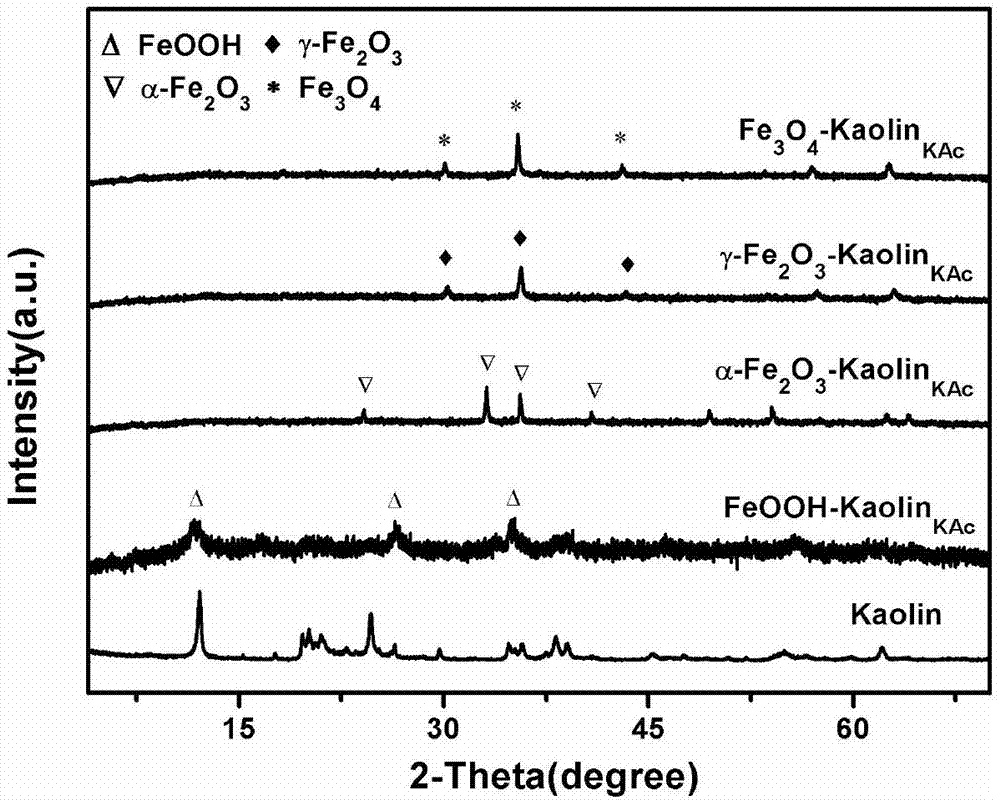
The invention discloses an iron oxide / nano kaolin-containing composite hemostatic and a preparation method thereof. The composite hemostatic consists of nano kaolin and iron oxide, wherein the nano kaolin is adopted as a carrier; and the iron oxide is loaded on the surface of the nano kaolin. The composite hemostatic is prepared through a precipitation method, and has the advantages of being good in hemostatic effect, rapid in wound heal speed, free of conspicuous cytotoxicity, free of hemolysis, high in biocompatibility and the like.
Owner:CENT SOUTH UNIV
Red cell membrane encapsulated polyester-type As2O3-supported nano particles and preparation method thereof
ActiveCN106943378AEvenly distributedGood sustained releaseInorganic active ingredientsPharmaceutical non-active ingredientsRed cell membraneDrug delivery
The invention discloses a red cell membrane encapsulated polyester-type As2O3-supported nano particles and a preparation method thereof. The nano particle employs the red cell membrane as shell and a polyester-type material as a core. The polyester-type material is supported with the As2O3. The particle size of the nano particle is about 200 nm. The nano particle has a round shape and is uniformly distributed, has a clear core-shell structure and good stability, is free of causing hemolysis and agglutination of red cells and can be used in intravenous injection. The drug delivery system, compared with a free medicine, has significantly sustained releasing effect, and can solve the problem of quick increase of blood concentration after intravenous injection of the As2O3. Because that substances, being 3-400 nm in size, are generally not discharged from kidney or a rejection system, so that the drug delivery system has advantages in circulation in blood system and achieves passive targeted effects; the blood concentration of a toxic medicine is reduced and further toxicity is reduced, so that a new strategy for development of toxic antitumor medicine preparations is provided.
Owner:SHANGHAI JIAO TONG UNIV
Preparation technology of nanometer aluminum hydroxide adjuvant
InactiveCN101991850AHigh densityGood dispersionImmunological disordersAntibody medical ingredientsAdjuvantAluminium hydroxide
The invention discloses a preparation technology of nanometer aluminum hydroxide adjuvant, comprising the following steps: taking benzalkonium bromide, n-caprylic alcohol and cyclohexane at equal ratio; stirring mixture at high speed, and pouring the mixture into a triangular flask; putting in a stir bar; putting the triangular flask on a magnetic stirrer, adding a proper quantity of AlCl3 solution, and stirring; slowing dropwise adding ammonia water into a separating funnel to regulate dripping rate; causing a reaction system to react for 2 hours at the pH of 10; after the reaction ends, adding acetone for demulsification and centrifugation; abandoning supernatant; respectively adding absolute ethyl alcohol and deionized water; evenly stirring by a glass rod; washing for three times, and decentralizing; collecting decentralized sediment into a beaker; drying for 12 hours in a drying oven to obtain fluffy Al(OH)3 adjuvant. The prepared nanometer aluminum hydroxide adjuvant has small grain diameter, good dispersibility and stability and favourable biocompatibility. In an animal experiment, the anti-tumor effect of nanometer aluminium adjuvant adsorption autovaccine is obviously superior to the curative effect obtained by using common aluminium adjuvant adsorption autovaccine.
Owner:SOUTHEAST UNIV
Disposable soft connecting band collection container and anti-freezing vacuum blood taking needle of bleeding opening
InactiveCN101695446AThe test results are accurateImprove the level of clinical treatmentCatheterDiagnostic recording/measuringCavity wallBiomedical engineering
The invention belongs to the technical field of medical instruments, and relates to a disposable soft connecting band collection container and an anti-freezing vacuum blood taking needle of a bleeding opening. The disposable soft connecting band collection container is formed in the following mode that: a biological product anticoagulant coating layer is arranged on an inner cavity wall of a circular connector; one end of the connector is provided with a blood taking plastic duct; a vacuum valve is placed on the plastic duct; the other end of the plastic duct is provided with the circular connector; the inner cavity wall of the circular connector is provided with the biological product anticoagulant coating layer; one end of the connector is provided with a venous blood taking needle, while the other end is provided with a blood collection sample bottle needle; the blood collection sample bottle needle is connected with a blood collection sample bottle cap; the blood collection sample bottle cap is connected with a rubber plug; the rubber plug is connected with a blood collection sample bottle; and one side on the blood collection sample bottle close to the blood collection sample bottle cap is provided with a secondary bleeding opening. The disposable soft connecting band collection container and the anti-freezing vacuum blood taking needle of the bleeding opening can ensure that a blood sample generates no cruor and hemolysis so as to ensure the accuracy of the test result. The blood dosage during the test can be controlled flexibly so as to ensure repeated tests at any time or the addition of new testing items.
Owner:天津百新生物技术研发有限公司
Curcumin compound injection and intravenous injection preparation thereof
InactiveCN102225048AComply with clinical drug requirementsHigh drug loadingAntipyreticAnalgesicsSolventAdverse effect
The invention relates to the technical field of medicine, and particularly relates to a curcumin compound injection and an intravenous injection preparation thereof. The curcumin compound injection mainly comprises a curcumin compound, a solvent for injection and a small amount of pH regulator. The curcumin compound intravenous injection preparation is used only by mixing the curcumin compound injection and an emulsifier in a volume ratio of (1:5)-(1:250). After the curcumin compound injection and the emulsifier are mixed, the drug loading rate of the curcumin compound in the intravenous injection preparation is up to 1.5 mg / ml, and no crystal is separated out within 18 hours. The curcumin compound injection provided by the invention has a simple and safe preparation method and good stability, and is convenient for storage and transportation. The curcumin compound intravenous injection preparation has good stability due to the use of the emulsifier as a carrier solvent, and has no toxic and adverse effects. The invention solves the problem that the curcumin compound can not be prepared into the intravenous injection preparation and applied in clinics for a long period of time.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Stable parecoxib sodium pharmaceutical composition for injection
InactiveCN104434815AHigh yieldReduce market riskPowder deliveryAntipyreticParecoxib sodiumPharmaceutical drug
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The pharmaceutical composition specifically includes parecoxib sodium and injection additives. Disodium hydrogen phosphate and sodium calcium edentate are adopted as the buffering agent, and sodium hydroxide is taken as the pH regulator. The production process is feasible, the quality is stable and controllable, the skin irritation is small, and at the same time the composition is convenient for transportation and storage. The invention provides the reasonable preparation prescription and preparation technology for clinical medication, and industrialization is easy to realize.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Bilastine compound and preparation method thereof
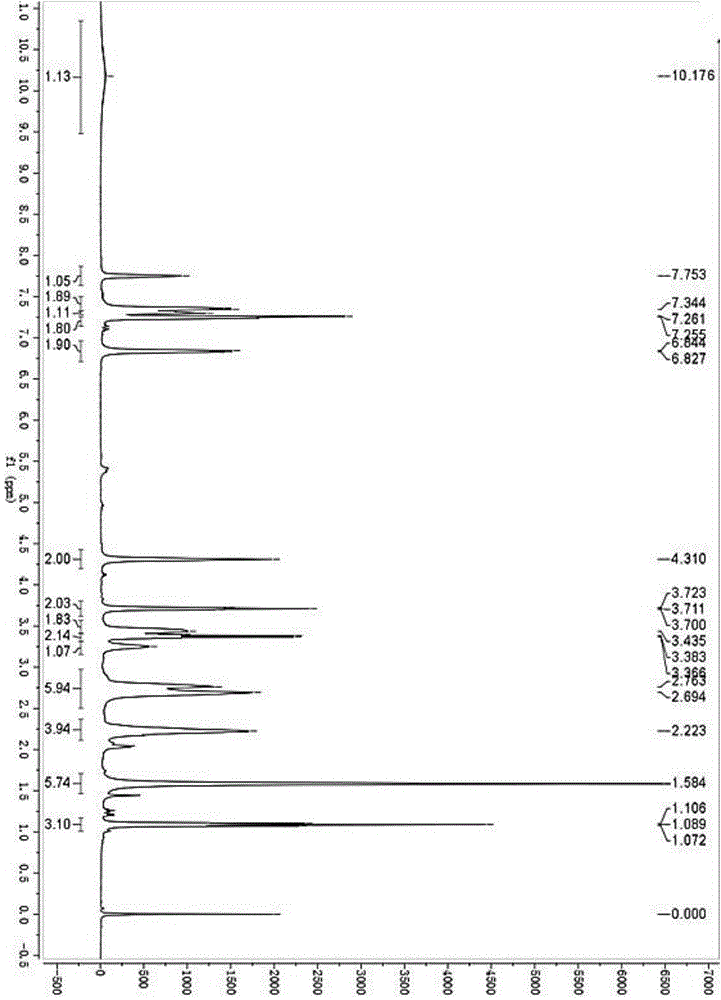
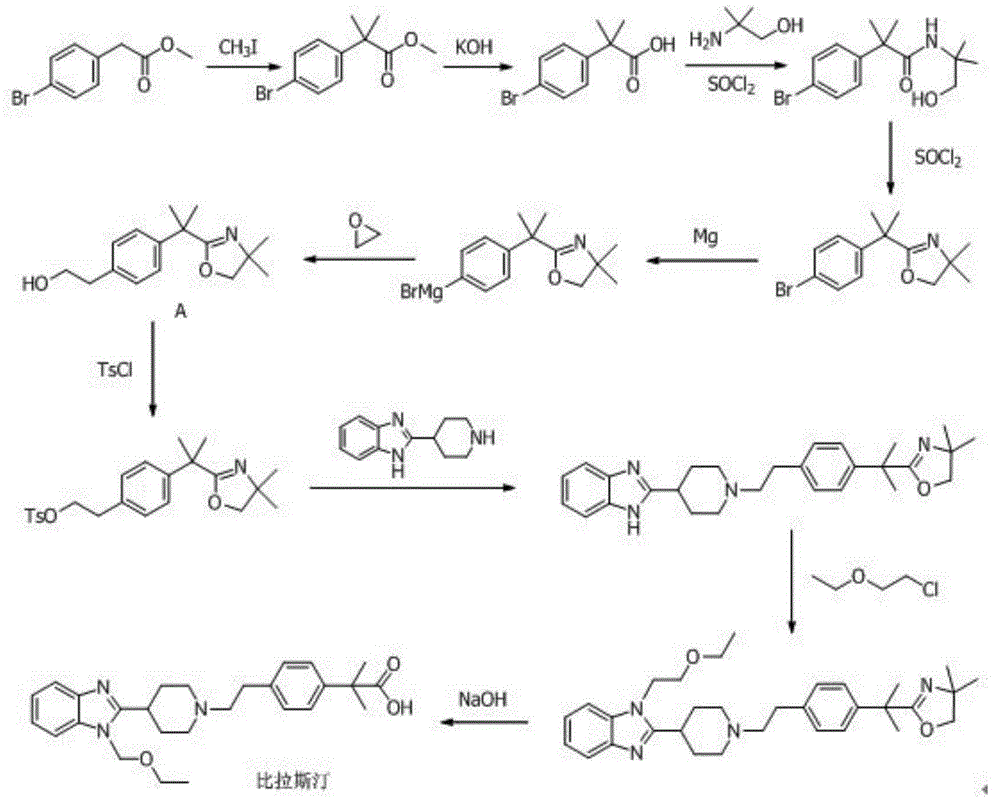
ActiveCN104530002AImprove stabilityHigh purityOrganic active ingredientsSenses disorderSolubilitySulfonyl chloride
The invention belongs to the technical field of medicines, and specifically relates to a bilastine compound and a preparation method of the compound. The bilastine compound is high in stability and not obvious in moisture-absorption weight gain under a high-humidity condition, and related substances are not increased; compared with bilastine in other crystal forms, the bilastine compound is high in solubility and outstanding in physical and chemical performances. The preparation method comprises the following steps: by taking p-methyl phenethyl alcohol as a starting raw material, performing the sulfonylation reaction for hydroxy through sulfonyl chloride to obtain sulfonate; condensing sulfonate with 1-ethoxyethyl-2-piperidyl benzoglioxaline; performing bromination for benzyl; carrying out grignard reaction to introduce carboxyl to the benzyl, and then converting benzyl into ester; performing dimethylation for benzyl through iodomethane; finally hydrolyzing to obtain bilastine. The method involves seven synthesis reactions, and has the advantages that few synthesis steps are carried out, the reaction conditions are mild, the raw materials are easily obtained, the reaction process is simple, the yield is high, the cost is relatively low, the industrialization is easily carried out, and three-waste pollution is less.
Owner:天津梅花生物医药科技有限公司
Rana nigromaculata antibacterial peptide and gene and application thereof
InactiveCN102229659ABroad-spectrum antimicrobial activitySimple structurePeptide/protein ingredientsPeptidesAntibacterial actionSugar nucleotide
The invention relates to a rana nigromaculata antibacterial peptide and a gene and application thereof, belonging to the technical field of medical technology. The rana nigromaculata antibacterial peptide is a single-chain polypeptide coded by a gene of a rana nigromaculata amphibious animal in China and has molecular weight of 1495.85D and isoelectric point of 9.0; and complete sequence of the rana nigromaculata antibacterial peptide is valine-isoleucine-proline-isoleucine-valine-serin-glycine-leucine-leucine-serin-serin-leucine-leucine-glycine-lysine. A coding gene of the rana nigromaculata antibacterial peptide is composed of 333 nucleotides, wherein nucleotides No 142-186 are used for coding a mature part. The artificially synthesized rana nigromaculata antibacterial peptide has strong antibacterial action and high safety, also has the advantages of simple sequence and convenience for synthesis and can be applied to a novel antiinfection medicine.
Owner:KUNMING UNIV OF SCI & TECH
Pidotimod injection and the producing method thereof
InactiveCN101062404ANo vascular irritationReactivity NoneSenses disorderDipeptide ingredientsSolubilityMedicine
The invention discloses a Pidumode injection, which is characterized by the following: producing solution agent with Pidumode medicine and medicinal carrier; adjusting pH value at 6. 0-8. 0; setting volume of each packing unit at 2-500ml; incorporating 100-800mg Pidumode; forming salt with Pidumode and caustic soda; possessing good water-solubility and medicinal crop with the same as Pidumode; easy-degrading to aminoglutaric acid and thiazolidinecarboxylic acid with Pidumode; adjusting the pH value of the solution to near neutral; stabilizing the quality of product. This invention can be used to intravenous injection.
Owner:沈阳双鼎制药有限公司 +1
Chitin/carbon nanotube composite adsorbent for blood perfusion and preparation method thereof
ActiveCN106378101ASimple preparation processLow costOther chemical processesAlkali metal oxides/hydroxidesFiberMicrosphere
The invention discloses a chitin / carbon nanotube composite adsorbent for blood perfusion and a preparation method thereof. The preparation method comprises the following steps of: by taking a natural macromolecular material chitin as a carrier, under a low-temperature stirring condition, performing freezing-unfreezing circulation so as to combine the chitin dissolved in an alkali / urea water solvent system with carbon nanotubes and form a uniform chitin / carbon nanotube composite solution, performing emulsification and thermal induction self-assembling process, thereby forming chitin / carbon nanotube composite nano fiber microspheres. The prepared microspheres have through hole canals applicable to free diffusion of bilirubin, have a good adsorption effect on combined bilirubin, and moreover are excellent in biocompatibility and blood compatibility. The composite nano fiber microspheres can be also fixed with an effective amount of high-activity ligand through covalent bonds, and the cleaning property and biocompatibility of the bilirubin of the microspheres can be further improved.
Owner:WUHAN UNIV
Riboflavine sodium phosphate composition injection and preparation method thereof
ActiveCN101601647ANo stimulationAvoid harmOrganic active ingredientsMetabolism disorderWater useSodium phosphates
The invention discloses a riboflavine sodium phosphate composition injection and a preparation method thereof. The riboflavine sodium phosphate composition injection comprises riboflavine sodium phosphate, citric acid, sodium hydroxide and sodium chloride. Each injection contains 5 to 15mg of riboflavine sodium phosphate, 2.1mg of citric acid, 2 to 4mg of sodium hydroxide and 0.6 to 0.8mg of sodium chloride. The preparation method comprises the following steps: adding water used for injection into the citric acid for stirring and dissolving; adjusting the pH to about 6.2 with sodium hydroxide; adding sodium chloride for stirring to dissolve the sodium chloride; adding the water used for injection and 1g of active carbon; adding riboflavine sodium phosphate while stirring; after the riboflavine sodium phosphate is completely dissolved, complementing the water for injection until the total amount is completely complemented; performing filtering, refluxing, filling, nitrogen charge, melting to seal, sterilizing at a high temperature; and after reducing the temperature, detecting with lamps, packing and checking, and obtaining the finished product. The riboflavine sodium phosphate composition injection does not crystallize, has good clarity and stability, and is helpful for improving the yield of the product, reducing the market rick of the product and is better applied to clinical treatment.
Owner:江西制药有限责任公司
Trauma hemostatic sponge and preparing method and application thereof
ActiveCN109999216AGood hemostatic effectFast hemostasisSurgical adhesivesPharmaceutical delivery mechanismGrapheneExothermic reaction
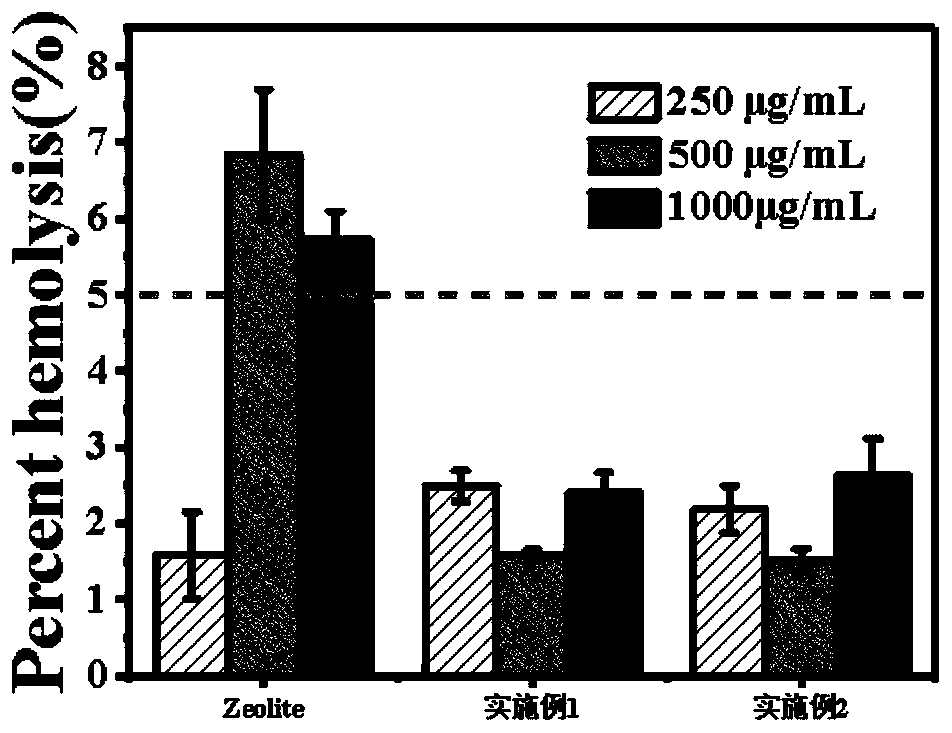
The invention relates to the technical field of hemostasis, in particular to a trauma hemostatic sponge and a preparing method thereof. The trauma hemostatic sponge comprises zeolite and graphene; thegraphene is of a three-dimensional crosslinking structure; the zeolite is dispersed in the three-dimensional crosslinking structure; the mass ratio of the zeolite to the graphene is 1:(0.2-5). The trauma hemostatic sponge has a distinctive hemostasis mechanism and excellent hemostasis performance. According to records of the embodiment, the trauma hemostatic sponge has a high hemostasis speed, amild exothermic reaction and high biological safety.
Owner:BEIJING UNIV OF CHEM TECH
Nanometer lipid ultrasonic contrast agent and preparation method thereof
InactiveCN103638534AGood biocompatibilityNon-cytotoxicEchographic/ultrasound-imaging preparationsLipid filmSolubility
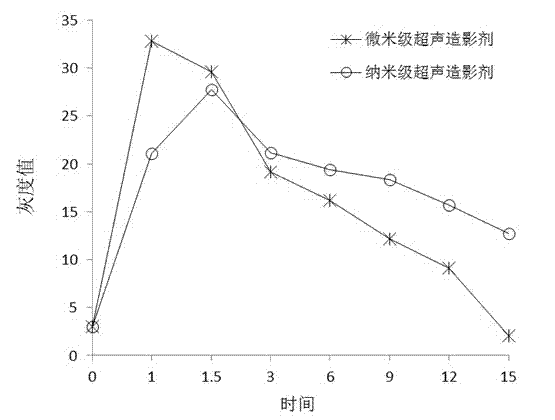
The invention discloses a nanometer lipid ultrasonic contrast agent and a preparation method thereof. According to the method, the nanometer lipid ultrasonic contrast agent which is small in particle size and can develop stably is prepared by adopting lipid film dispersion and mechanical oscillation methods, is good in outer shell crushing resistance, is difficult to crack under the condition of low mechanical index, is extremely low in solubility and diffusion rate in a solution, can perform microcirculation pouring well, can last longer time in blood, has favorable acoustic property and stability, can obviously enhance the heart, kidney, liver and tumor development by in-vivo radiography, and long in radiography time which is more than 30 minutes.
Owner:SOUTHEAST UNIV
Ozagrel sodium drug combination for injection
InactiveCN102846561AFix stability issuesHigh yieldPowder deliveryOrganic active ingredientsProduction rateMANNITOL/SORBITOL
The invention discloses an ozagrel sodium drug combination for injection. The ozagrel sodium injection solution consists of ozagrel sodium, mannitol and anhydrous sodium carbonate, wherein each piece contains 20-80 mg of ozagrel sodium, 20-80 mg of mannitol and 5-15 mg of anhydrous sodium carbonate. The drug combination is prepared by a method comprising the following steps: taking a recipe quantity of injection water, adding the mannitol and the anhydrous sodium carbonate and stirring until the mannitol and the anhydrous sodium carbonate are dissolved; adding the ozagrel sodium, and stirring until the ozagrel sodium is fully dissolved; regulating a pH value between 7.7 and 8.7; adding medicinal carbon and stirring; leaching, replenishing the injection water to a full quantity and uniformly mixing; finely filtering; encapsulating; freeze-drying; inspecting with a light; and warehousing to obtain the ozagrel sodium drug combination. The ozagrel sodium drug combination has good stability. The production rate of the product is increased, the cost is lowered, and industrialization is realized. The drug combination can be better applied in clinic and has more remarkable advantages.
Owner:TIANJIN SONGRUI MEDICAL TECH
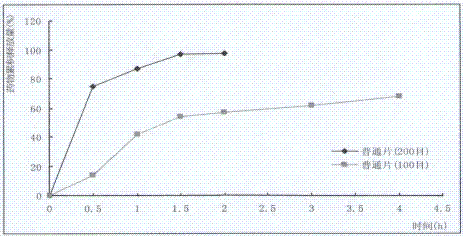
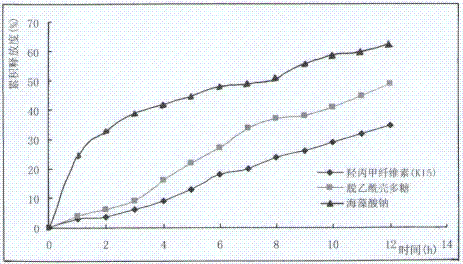
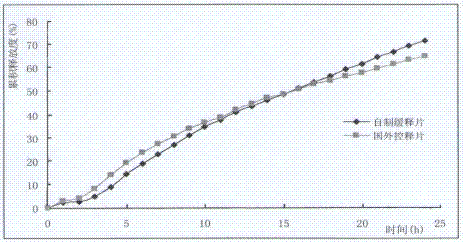
Nifedipine sustained release tablets and preparation process thereof
ActiveCN102512394AReduce solubilitySlow dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsSolventFilm coating
The invention discloses nifedipine sustained release tablets and a preparation process thereof. The nifedipine sustained release tablets are prepared from nifedipine, a sustained release agent, a bulking agent, a bonding agent, a solubilizer and a lubricant by the steps of pulverizing, sieving, mixing, granulating, drying, tabletting and film coating. The sustained release agent adopts chitosan, and weight percentages of the components are as follows: nifedipine 20%, chitosan 14-24%, bulking agent 50-60%, bonding agent 4%, solubilizer 1%, and lubricant 1%. The nifedipine sustained release tablets of the invention are simple in production process and long in sustained release action, the adverse reactions after administration are less and mild, and the curative effect on mild and moderate hypertension is definite. The nifedipine sustained release tablets can uniformly release nifedipine within 24 hours, can last the effective blood concentration over 24 hours, and only need to be administered once everyday.
Owner:浙江泰利森药业有限公司
Pharmaceutical composition for treating bone diseases, injection thereof and preparation methods thereof
ActiveCN104127473AIncreased effect on bone diseaseGood treatment effectNervous disorderAntipyreticSide effectTreatment effect
The invention relates to a pharmaceutical composition for treating bone diseases, an injection thereof and preparation methods thereof. The pharmaceutical composition is composed of an extraction liquid of pig four limbs and a melon seed extraction liquid. Further, the pharmaceutical composition is added with mixed extraction liquids respectively prepared from commen bomhax flower, flos buddlejae and herba eupatorii. The prepared pharmaceutical composition has substantially improved treatment effect on rheumatoid arthritis, degenerative osteoarthritis, ankylosing spondylitis, sciatica, lumbar disc herniation and other bone diseases, is improved in treatment effect on gout and has no toxic and side effects. Additionally, the provided injection has extremely high stability and is substantial in effect.
Owner:哈尔滨圣泰生物制药有限公司
Whole blood perfusion adsorbent as well as preparation method and application thereof
ActiveCN112871139AImprove adsorption capacityReduce the amount of adsorptionOther blood circulation devicesOther chemical processesMicrospherePerfusion
The invention belongs to the field of natural polymer biomedical materials, and particularly relates to a whole blood perfusion adsorbent as well as a preparation method and application thereof; the adsorbent is large-size nanofiber porous network microspheres compounded by natural polymer nanofibers, an inorganic functional filler, an organic functional filler and / or an organic coating material, wherein the inorganic functional filler and the organic functional filler are wrapped in the natural polymer nanofiber microspheres, and the organic coating material coats the surfaces of the natural polymer nanofiber microspheres. The whole blood perfusion adsorbent has a microsphere size suitable for whole blood perfusion, has good blood compatibility, has a nanofiber porous network structure, is an inorganic and organic functional material capable of specifically adsorbing toxins, can be designed for specific toxins, and is high in adsorption efficiency, high in selectivity and easy to popularize and apply.
Owner:WUHAN UNIV
Preparation method for hydrophilic trauma dressing made from polyurethane
InactiveCN1202785CImprove hydrophilicityNo foreign body reactionAdhesive dressingsAbsorbent padsWound dressingPolyethylene glycol
A hydrophilic polyurethane wound dressing is composed of hydrophilic polyurethane soft foam slices and a moisture-permeable, breathable, waterproof and bacteria-isolating polyurethane film. Preparation method: Stir polyethylene glycol, toluene diisocyanate (TDI-80) and glycerol evenly at room temperature, then raise the temperature to 80-90°C and react for 90-120 minutes to prepare a polyurethane prepolymer; stir the polyurethane prepolymer at room temperature. Mix polyether, organotin catalyst, tertiary amine catalyst, pore opening agent, stabilizer and water evenly, then add polyurethane prepolymer, stir evenly, foam at room temperature, and mature for at least 24 hours into slices to obtain polyurethane soft foam slices; Prepare the polyurethane prepolymer into an acetone or / and N,N-dimethylformamide solution with a concentration of 40.0 to 55.0%, then add water, cast it to form a film, then remove the solvent at 40 to 80°C, and freeze at room temperature. The polyurethane film can be obtained after curing reaction for 16 to 24 hours.
Owner:SICHUAN UNIV +1
Docetaxel freeze-dried microemulsion preparation and preparation method thereof
ActiveCN103301061AGood dispersionImprove stabilityPowder deliveryOrganic active ingredientsPolyoxyethylene castor oilHemolysis
The invention discloses a docetaxel freeze-dried microemulsion preparation and a preparation method thereof. The docetaxel freeze-dried microemulsion preparation comprises the following raw materials by weight: 0.05-5 parts of docetaxel, 0.1-40 parts of an oil phase, 5-30 parts of a surfactant, 0-40 parts of a cosurfactant, 5-85 parts of a hydrophilic phase, 0-15 parts of a cosolvent, 0-5 parts of an antioxidant, and 1-40 parts of a freeze-drying protective agent. Specifically, the surfactant is one or several of polyethylene glycol-8-caprylin / caprin, polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil, poloxamer, polyethylene glycol-12-hydroxystearate, polyethylene glycol stearate 15 and sorbitan monooleate. The preparation method includes: preparing the raw materials into a microemulsion according to the ratio, and then conducting freeze-drying. Before clinical use, the docetaxel freeze-dried microemulsion preparation has no need for a tedious two-step dilution process, after redissolving, the docetaxel freeze-dried microemulsion preparation can be subjected to intravenous injection, and has no vascular irritation and small allergic reaction. Hemolytic experiments show that the docetaxel freeze-dried microemulsion preparation does not generate hemolysis.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Antibacterial peptide secreted by clostridium butyricum as well as preparation method and application thereof
ActiveCN103772494AHigh antibacterial activityNo hemolysisMicroorganism based processesAnimal feeding stuffClostridialesMolecular sieve
The invention discloses an antibacterial peptide secreted by clostridium butyricum as well as an amino acid sequence and an application thereof. The amino acid sequence of the antibacterial peptide is represented by a sequence table SEQ ID NO:1, the molecular weight is 2264.6 Daltons and the isoelectric point is 8.64. A preparation method comprises the following steps: carrying out cation exchange, molecular sieve chromatography and reverse high performance liquid chromatography on the antibacterial peptide secreted by the clostridium butyricum in an ammonium sulfate precipitation culture medium to purify the antibacterial peptide. The antibacterial peptide has obvious antibacterial activity, does not have hemolysis effect on erythrocytes of pigs and can be used for developing and preparing an antibacterial medicine.
Owner:ZHEJIANG UNIV
Melittin liposome nanometer preparation, and preparation method and applications thereof
ActiveCN109288794AHigh drug loadingEasy to storeAntibacterial agentsAntimycoticsLipid formationHemolysis
The invention belongs to the technical field of novel nanometer medicinal preparation, and more specifically relates to a melittin liposome nanometer preparation, and a preparation method and applications thereof. The melittin liposome nanometer preparation contains melittin liposome which at least contains melittin, an anionic polymer, and a cationic lipid carrier. The raw materials are safe, andare easily available; the preparation method is simple in operation, and is high in repeatability; the particle size and uniformity of the liposome preparation are ideal, drug loading amount is large, preparation stability is excellent, storage is convenient, melittin leakage amount is low, no hemolysis is caused, the toxicity is low, biodegradable characteristic is achieved, functionalization modification can be realized, long circulation in a circulation system and tumor active and passive targeting distribution function are achieved, and large scale production can be realized.
Owner:SHANGHAI JIAO TONG UNIV
Disposable hard connecting band collection container and anti-freezing vacuum blood taking needle of bleeding opening
The invention belongs to the technical field of medical instruments, and particularly relates to a disposable hard connecting band collection container and an anti-freezing vacuum blood taking needle of a bleeding opening. The disposable hard connecting band collection container is formed in the following mode that: an anticoagulant coating layer is arranged on an inner cavity wall of a circular connector; one end of the connector is provided with a venous blood taking needle; the rear part of the connector is provided with a vacuum valve; the other end of the connector is provided with the blood collection sample bottle needle; the blood collection sample bottle needle is connected with a blood collection sample bottle cap; the blood collection sample bottle cap is connected with a rubber plug; the rubber plug is connected with a blood collection sample bottle; and one side on the blood collection sample bottle close to the blood collection sample bottle cap is provided with one secondary bleeding opening. The disposable hard connecting band collection container and the anti-freezing vacuum blood taking needle of the bleeding opening have the advantages that: the anticoagulant coating layer is added on the basis of the conventional hard connection type blood taking needle so that blood flowing out of a body can contact an anticoagulant; a blood sample is ensured to generate no cruor and hemolysis so as to ensure the accuracy of the test result; and the secondary bleeding opening is added so that a clinical inspector can flexibly control the blood dosage during the test to ensure repeated tests at any time or add new testing items.
Owner:天津百新生物技术研发有限公司
Fondaparinux sodium composition injection and preparation method thereof
InactiveCN106727289AEffective pH adjustmentHigh clarityOrganic active ingredientsInorganic non-active ingredientsSodium hydroxideChemistry
The invention relates to a Fondaparinux sodium composition injection and a preparation method thereof. The injection is prepared from raw materials as follows: 5-10 parts by mass of Fondaparinux sodium, 6-12 parts by mass of sodium chloride, 2.6-3 parts by mass of a buffer solution and 3-6 parts by volume of water for injection, wherein the buffer solution is a mixture of citric acid, sodium hydroxide and hydrochloric acid, and a mass ratio of citric acid, sodium hydroxide and hydrochloric acid is (2.4-2.6):1:(1.3-1.9); the raw materials are subjected to orderly feeding, stirring, filtering, reflowing, filling, nitrogen charge, fused sealing, high-temperature sterilization, light inspection after cooling, packaging and checking, and the Fondaparinux sodium composition injection is obtained. The Fondaparinux sodium composition injection prepared with the method cannot produce crystals, is good in clarity, greatly improves the stability and is better applied to clinical treatment.
Owner:ABA CHEM SHANGHAI
Propofol medicinal composition for injection
InactiveCN102824307AEfficient emulsifying abilityChemically stableHydroxy compound active ingredientsAnaestheticsMedicineGlycerol
The invention discloses a propofol medicinal composition for injection, which consists of propofol used as a raw material and soybean lecithin, poloxamer 188, soybean oil, medium chain oil, glycerol, oleic acid and water which are used as auxiliary materials. The propofol medicinal composition for injection has high stability and has more obvious advantages of improving the yield of a product, reducing the cost, implementing the industrialization and further clinically applying the propofol medicinal composition.
Owner:TIANJIN SONGRUI MEDICAL TECH
Method for modifying leukocyte filtration membrane
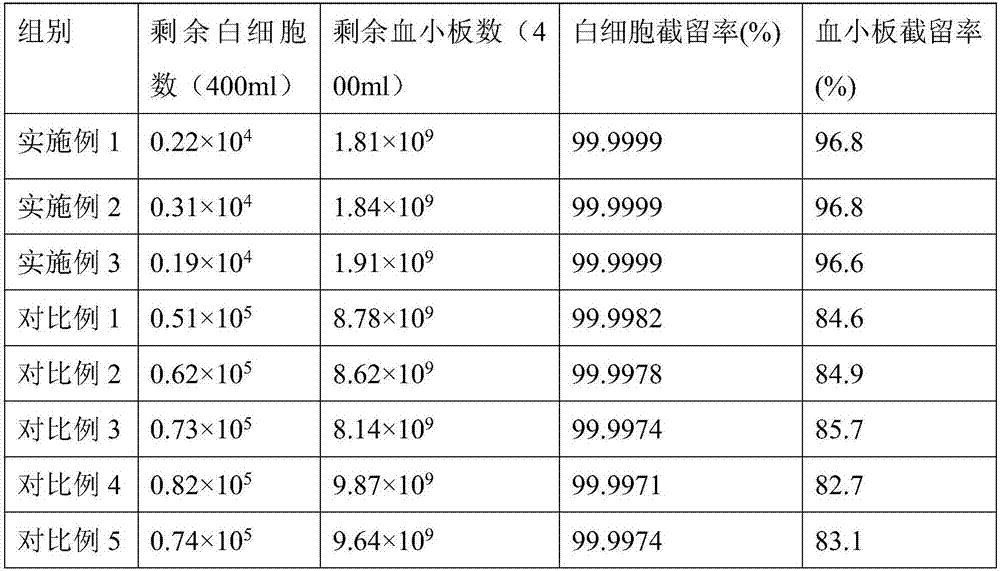
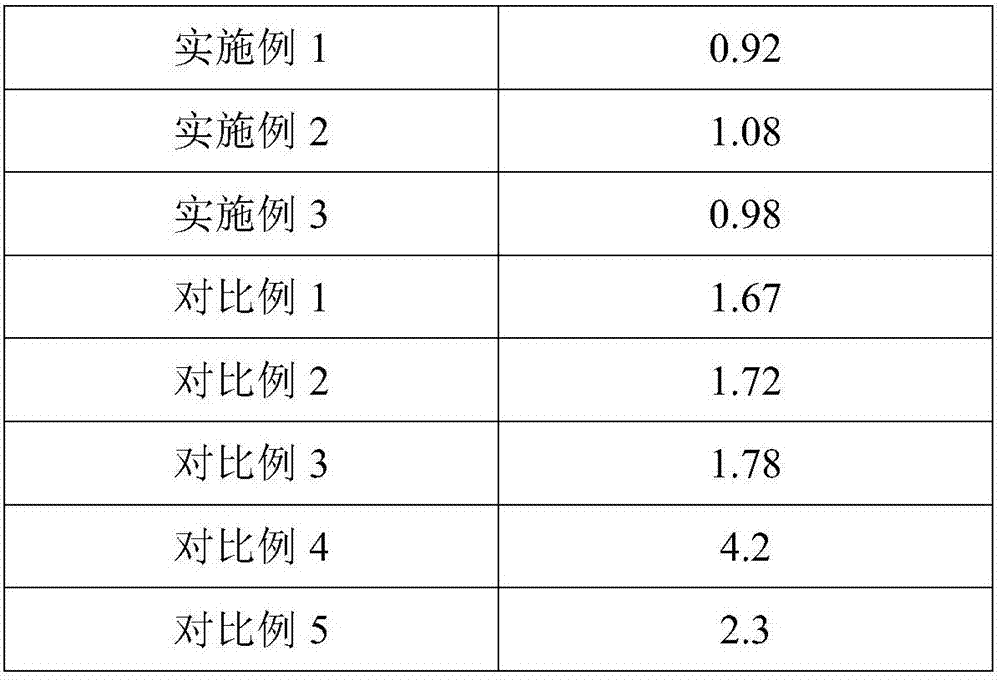
ActiveCN107138058AFully moistenedFully infiltratedSemi-permeable membranesBlood transfusionPolymer scienceHemolysis
The invention belongs to the technical field of leukocyte filtration and relates to a method for modifying a leukocyte filtration membrane. A cheap nonwoven fabric having acid and base resistance, corrosion resistance and high chemical stability is used as a base film material and is orderly subjected to two pretreatment processes, a double-component system of acrylic acid and sodium soyate is co-grafted to the treated fabric, and the double-component system undergoes a polymerization reaction under UV irradiation to produce a polymer containing a hydrophilic carboxyl group. The method has a high grafting rate, does not produce hemolysis phenomenon and has obvious protective effects on the red blood cells. The modified filter film has high interception effects on leukocytes and platelets in the whole blood.
Owner:广州达济医学科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com