Bilastine compound and preparation method thereof
A bilastine and compound technology, which is applied in the field of bilastine compounds and their preparation, can solve the problems of poor stability and hygroscopicity, no detailed research and disclosure of the bilastine compound purification method, light sensitivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
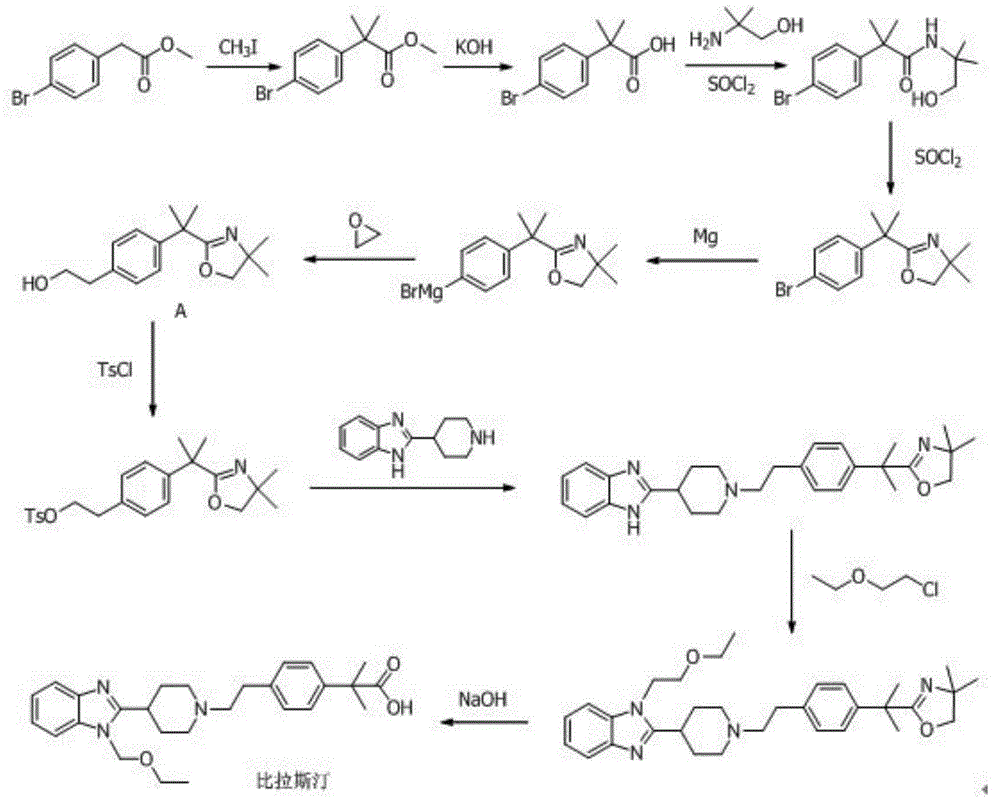
[0111] Synthesis of p-methylphenylethanol p-toluenesulfonate (III)
[0112] A mixture of 13.6g (0.1mol) of p-methylphenylethanol (II), 21.0g (0.11mol) of p-toluenesulfonate, 11.1g (0.11mol) of triethylamine and 80ml of dichloromethane was reacted at room temperature for 1h. Add 80ml of water, stir, separate layers, the organic layer is washed with saturated NaHCO3, washed with water, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain 26.7g of p-methylphenylethanol p-toluenesulfonate (III). 92%, content 93.6% (HPLC normalized).
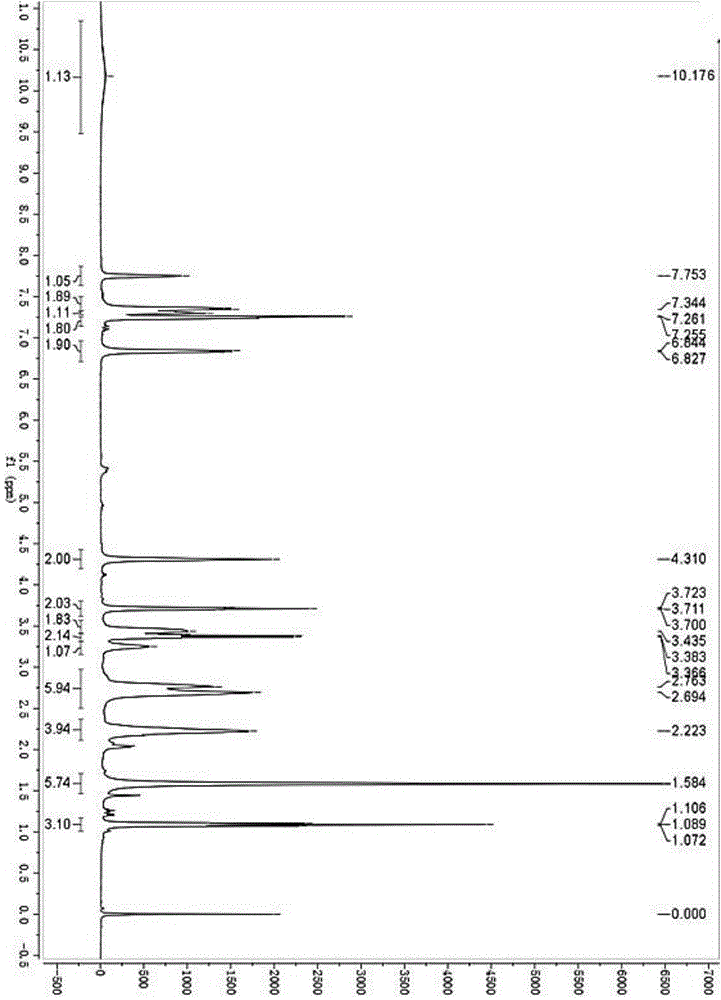
[0113] 1 H NMR (400MHz, CDCl3) δ7.84–7.74 (m, 2H), 7.40–7.30 (m, 2H), 7.16–7.00 (m, 4H), 4.05 (t, J=15.0Hz, 2H), 2.82 ( t,J=15.0Hz,2H), 2.44(s,3H), 2.19(s,3H).
Embodiment 2
[0115] Synthesis of 1-(2-ethoxyethyl)-2-(1-(4-methylphenylethyl)piperidin-4-yl)-1H-benzo[d]imidazole (V)
[0116] In 100ml of dimethylformamide, add 26g (0.0895mol) p-methylphenylethanol p-toluenesulfonate (III), 24.5g (0.0895mol) 1-(2-ethoxyethyl)-2-( Piperidin-4-yl)-1H-benzo[d]imidazole (IV), 18.6g (0.1343mol) anhydrous potassium carbonate, stirred, heated to 80°C for 14h. The reaction solution was concentrated to dryness under reduced pressure, dissolved in chloroform, and the organic layer was washed with water. The organic layer was dried over anhydrous sodium sulfate, filtered, evaporated to dryness under reduced pressure, and recrystallized from ethyl acetate to obtain 30.8 g of (V) white solid. Yield 87.9%, content 96% (HPLC normalization).
[0117] 1 H NMR (400MHz, CDCl3) δ7.34–7.22(m,2H),7.28–7.21(m,2H),7.12–7.05(m,4H),4.34(t,2H),3.74(t,2H), 3.41(d,2H),3.13(d,1H),2.98(t,1H),2.81(t,2H),2.64(t,2H),2.20(m,4H),2.01(m,3H),2.19 (s,3H), 1.05(t,3H).
Embodiment 3
[0119] Synthesis of 1-(2-ethoxyethyl)-2-(1-(4-bromomethylphenylethyl)piperidin-4-yl)-1H-benzo[d]imidazole (VI)
[0120] 30.0g 1-(2-ethoxyethyl)-2-(1-(4-methylphenylethyl)piperidin-4-yl)-1H-benzo[d]imidazole (V) (0.0766 mol), the mixture of N-bromosuccinimide 14.3g (0.0804mol) and AIBN 100mg in carbon tetrachloride 100ml, the solution was warmed to reflux for 8h. After cooling the reaction mixture to room temperature, water was added, the organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain a crude product which was recrystallized from ethanol to obtain 29.9 g of solid, 1-(2-ethoxy Ethyl)-2-(1-(4-bromomethylphenylethyl)piperidin-4-yl)-1H-benzo[d]imidazole (VI), content 90% (HPLC normalized), yield The rate is 83%.
[0121] 1 H NMR (400MHz, CDCl3) δ7.34–7.22(m,2H),7.28–7.21(m,2H),7.12–7.05(m,4H),4.34(t,2H),4.25(s,2H), 3.74(t,2H),3.41(d,2H),3.13(d,1H),2.98(t,1H),2.81(t,2H),2.64(t,2H),2.20(m,4H),2.01 (m,3H), 1.05(t,3H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com