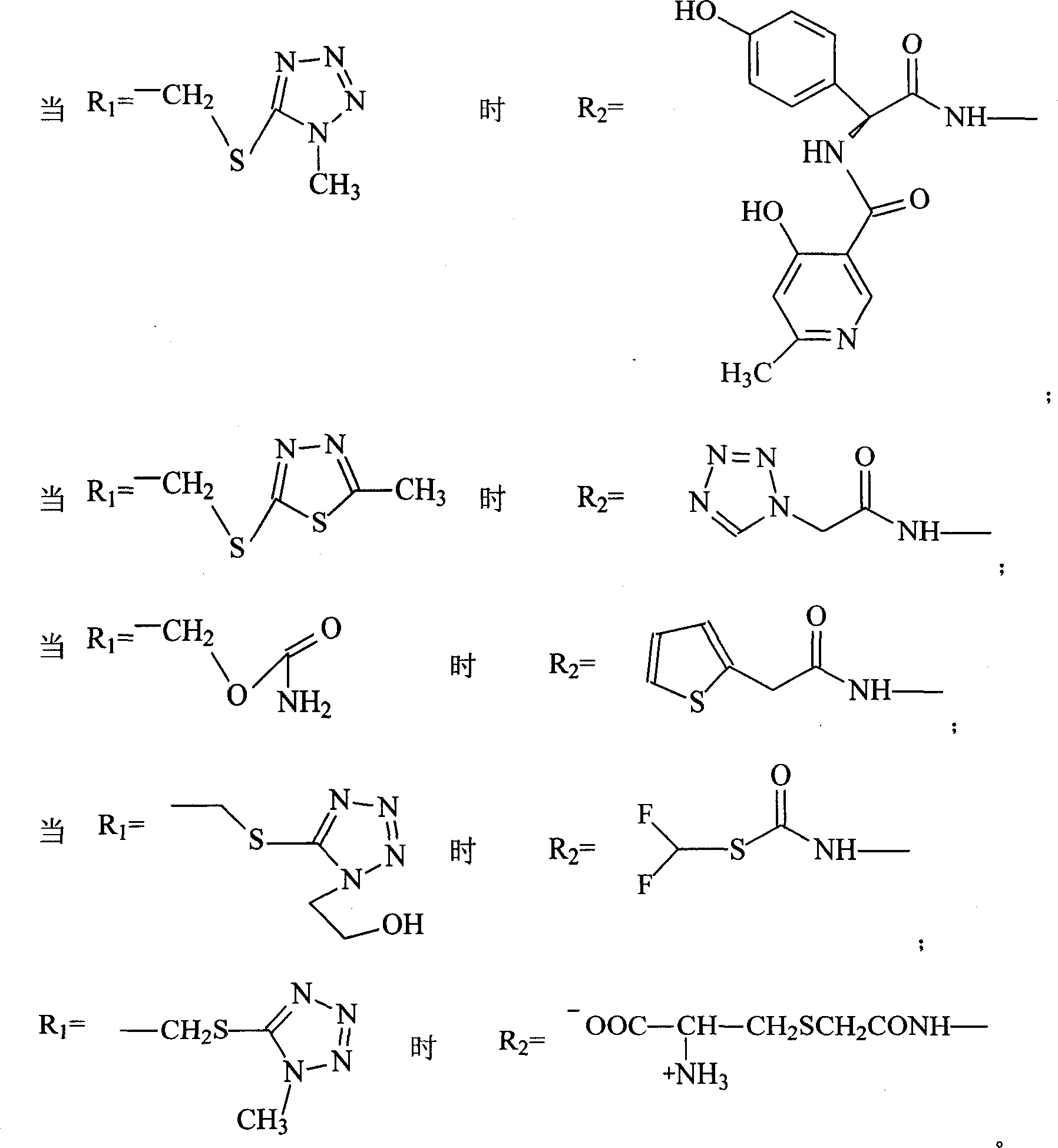
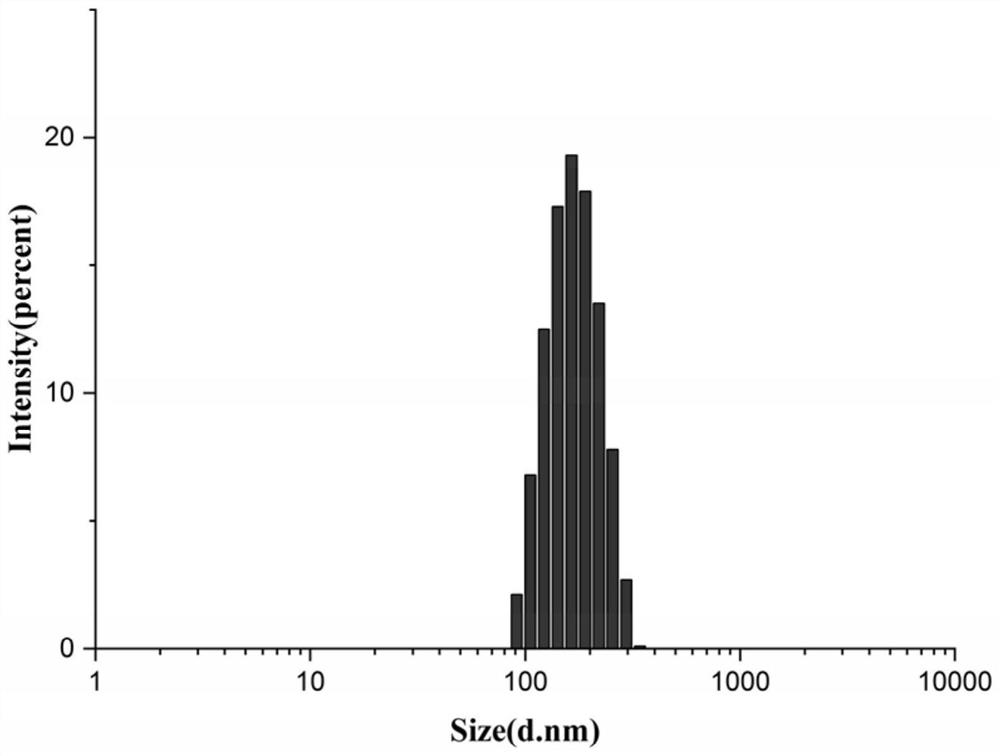
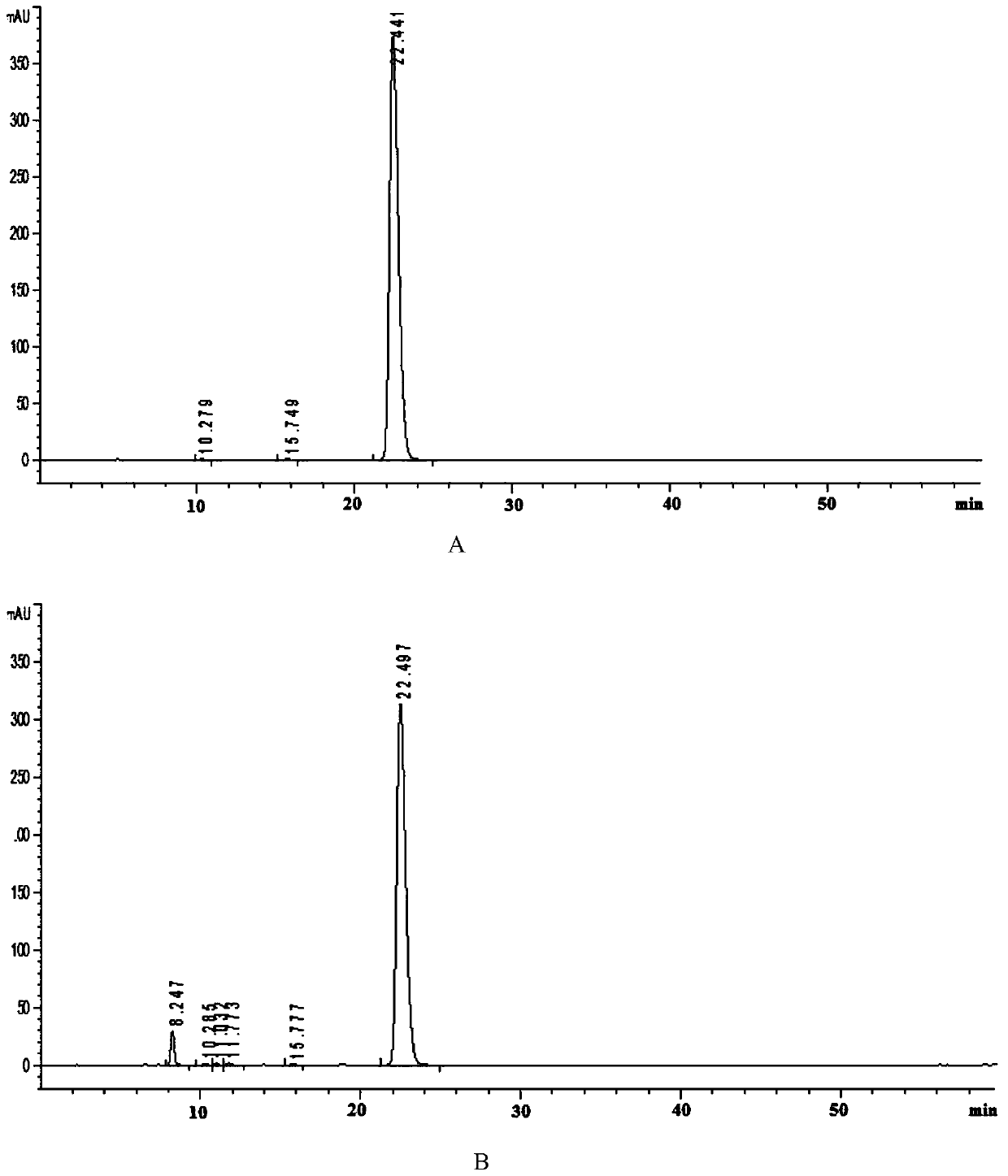
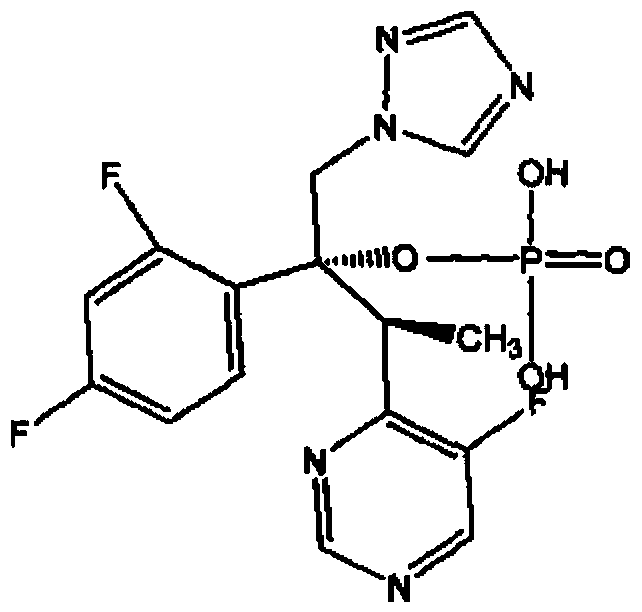
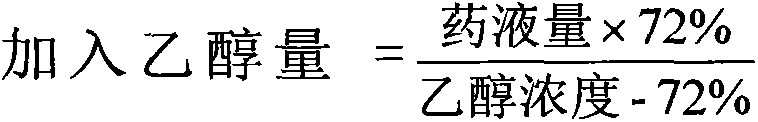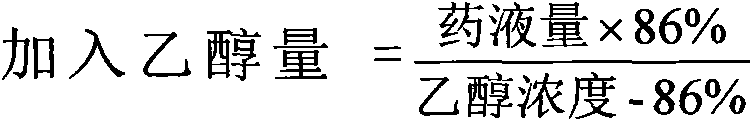Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "No vascular irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A stable torasemide injection and its preparation method
InactiveCN101007003AReduced dosing timeNo hemolyticPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMaterial growthActivated carbon
There is provided an injection with torasemide as the main active ingredient in the invention, the preparing method of the injection is also included in the invention. The stability of the injection in the invention has been greatly improved. There is solubilizing agent and stabilizer in the preparation, the pH value is 9.0 or larger. The preparing method includes the following steps: adding 1-50% (by weight) of solubilizing agent to certain amount of injecting water; adding torasemide to the water, after it is completely dissolved, adding 0.1-5% part of stabilizer; adding water to requested amount and stirring uniformly; adding activated char to adsorb for 30min at high temperature; decarbonizing and filtering; adding medically used alkali to regulate the ph value to 9.0 or larger; aseptically filtering; after the assay is approved, packaging, degerming and stocking. The advantages of the invention include: high stability, short preparing time, low cost, no haemolyticus, blood vessel stimulus and allerfic response, low growing speed of relative substances.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
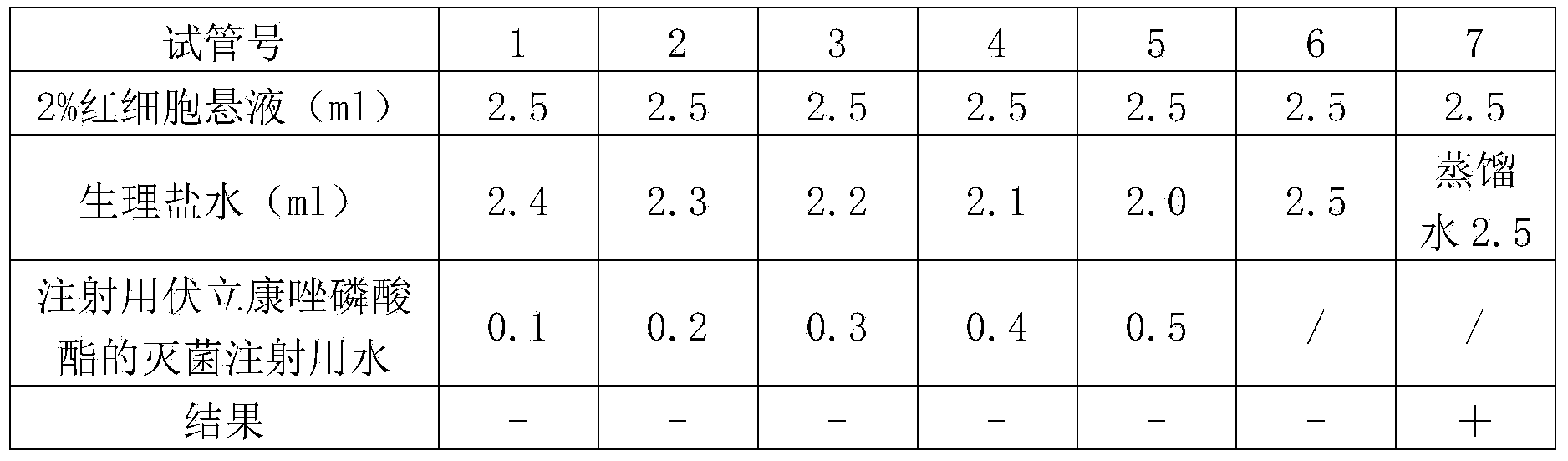
Voriconazole phosphate ester for injection and preparation method thereof
ActiveCN101744778AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliveryPhosphateMedical prescription
The invention provides voriconazole phosphate ester for injection and a medicinal salt thereof and a preparation method for the voriconazole phosphate ester for injection and the medicinal salt thereof. The preparation method comprises the following steps: adding 5 to 98 percent water for injection in a liquid preparation container; adding 90 to 110 percent of the accurate formula dosage of voriconazole phosphate ester and the medicinal salt thereof in the container; stirring the mixture; slowly dropwise adding a pH value regulator; regulating pH to between 6.0 and 11; supplementing water to the full dosage and then adding 0.01 to 1.0 percent (weight in volume) medicinal carbon into the product; stirring the mixture for 15 to 60 minutes; using a sand filter stick to carry out rough filtration and decarburization on the obtained product, and using a 0.22mum millipore filter to carry out fine filtration on the product until the clarity is qualified; after determining that the content of the midbody is qualified, determining the filling quantity and subpackaging the finished product in the vial; adding the semi-plug; carrying out freezing and drying on the sample; controlling the moisture content between 1 and 8 percent; pressing the plug; and carrying out capping.
Owner:HC SYNTHETIC PHARMA CO LTD
Organic amine salt of cephalosporin compound and its preparation method
InactiveCN101007811AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefodizimeCefpiramide
The invention relates to the organic amine salt or hydrate for ceph compound used for treating bacteria infection, and the chemical formula is demonstrated in (I).Said organic amine can be lycine, arginine, tert-butylamine, diethanolamine, triethanolamine, diethylamine or meglumine. It comprises cefuroxime organic amine salt, ceftriaxone organic amine salt, ceftezole organic amine salt, cefoperazone organic amine salt, cephalothin organic amine salt, cefotaxime organic amine salt, cefradine organic amine salt, cefonicid organic amine salt, cefmetazole organic amine salt, cefodizime organic amine salt, cefmenoxime organic amine salt, ceftizoxime organic amine salt, cefpiramide organic amine salt, cefazolin organic amine salt, cefoxitinorganic amine salt and flomoxef organic amine salr. The invention provides the medical compound taking compound in formula (I) as active element and its application to preparation of medicine for treating bacteria infection.
Owner:陈文展
Moxifloxacin hydrochloride glucose injection and preparation method and use thereof
InactiveCN101836950APrecipitation does not occurReduce solubilityAntibacterial agentsOrganic active ingredientsAdditive ingredientMoxifloxacin hydrochloride
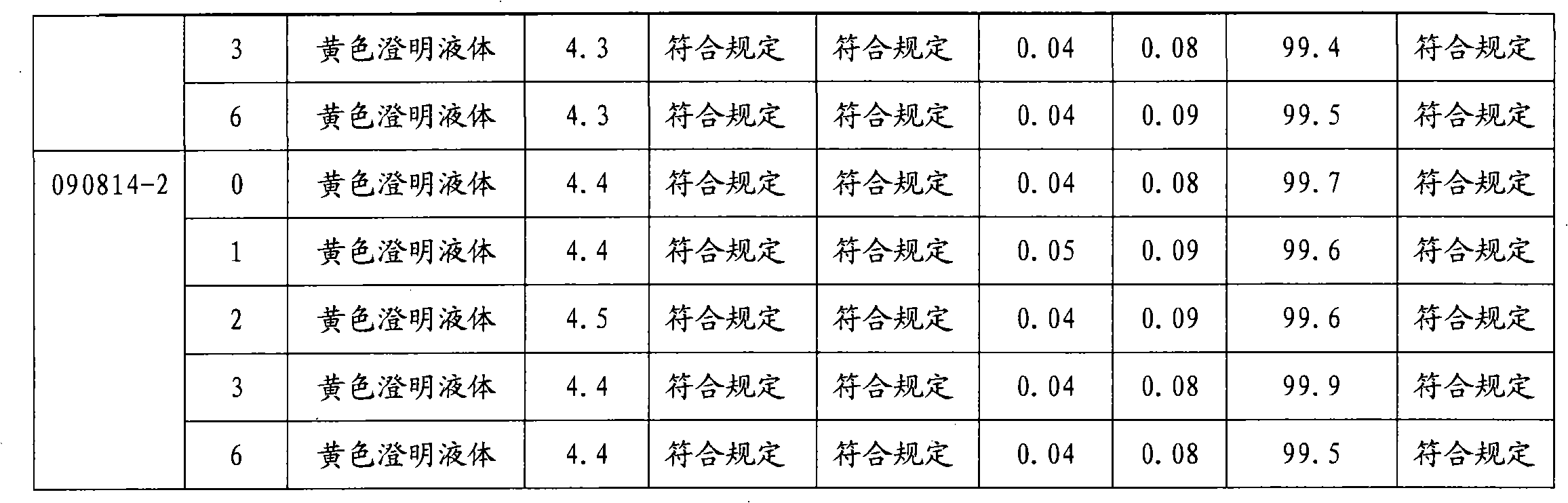
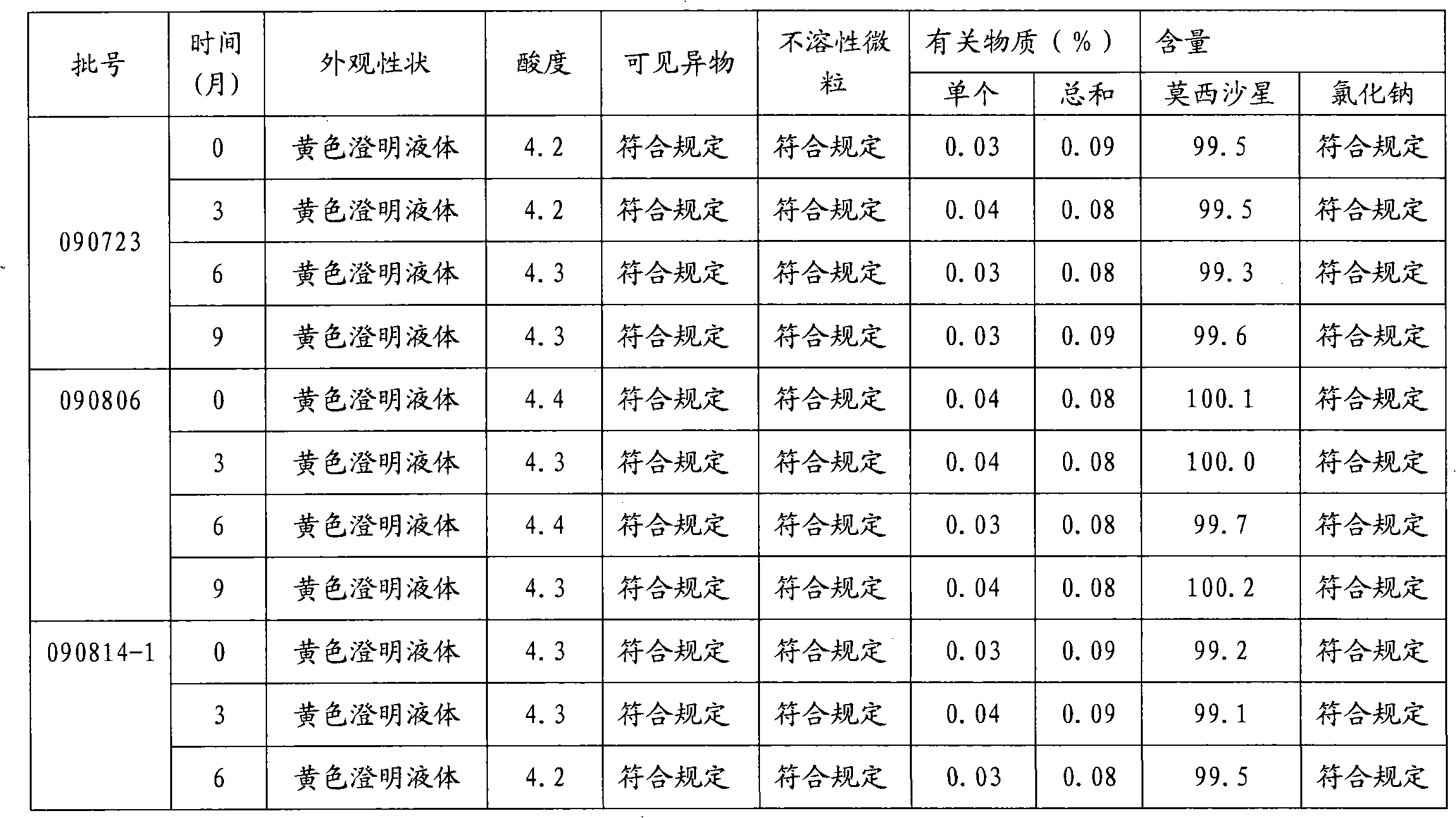
The invention provides moxifloxacin hydrochloride glucose injection and a preparation method and use thereof. The method for preparing the injection comprises the following steps of: adding water for injection accounting for 20 to 98 percent of the batch volume into an ingredient tank, and adding glucose, a metal complexing agent and the moxifloxacin hydrochloride in a ratio; after stirring to fully dissolve the components, regulating the pH value to between 4.0 and 4.5 by using 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide, adding medicinal carbon accounting for 0.05 percent (W / V) of the total volume, uniformly stirring, maintaining the temperature of between 70 and 80 DEG C for 20 minutes, and performing circular filtering for over 20 minutes; replenishing the water for injection to the batch scale, stirring for 5 to 10 minutes, and detecting the pH value of the prepared solution (controlling to between 4.0 and 4.5); after determining that no residual water is present in an elevated tank and a pipeline, opening a valve of the elevated tank, and sampling liquid medicament at a self-circulation pipeline sampling port after the liquid medicament circulates for 20 minutes through a filter element and the elevated tank; detecting according to the intermediate quality standard, requiring that the content of the moxifloxacin hydrochloride is between 1.52 and 1.68 mg / ml, the glucose content is between 47.5 and 52.5 mg / ml, and the pH value is between 4.0 and 4.5; after the intermediate is detected to be qualified, beginning to fill; and conveying the filled semi-finished products into a sterilizing cabinet for sterilization, wherein the sterilization condition is to sterilize for 8 to 30 minutes at 121 DEG C through thermal pressure steam.
Owner:HC SYNTHETIC PHARMA CO LTD
Tirofiban powder injection and its preparing method
InactiveCN1820751ANo vascular irritationNo hemolyticPowder deliveryOrganic active ingredientsPh bufferingActive component
The present invention relates to new preparation form of Tirofiban, especially Tirofiban powder for injection, as antiplatelet medicine and its preparation process. The powder for injection consists of Tirofiban as active component, freeze drying support agent and pH buffering agent. The present invention expands the administration range of Tirofiban and raises the clinical administration level of Tirofiban.
Owner:HUAZHONG UNIV OF SCI & TECH
Freeze-drying composition of posaconazole prodrug and preparation method and application of freeze-drying composition of posaconazole prodrug
InactiveCN105287403AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliverySolubilityCase fatality rate
The invention relates to a freeze-drying composition of a posaconazole prodrug and a preparation method and application of the freeze-drying composition of the posaconazole prodrug. The freeze-drying composition has the advantages that the freeze-drying composition is high in water solubility, and safety of the freeze-drying composition is guaranteed due to the fact that cyclodextrins auxiliary materials need not to be added during the preparation of the freeze-drying composition; the freeze-drying composition is suitable for being used for treating various amphotericin-intolerant or refractory adult invasive fungal infections; the freeze-drying composition is used as a preventive drug for high-risk patients, the freeze-drying composition is applicable to patients above 13 years old and with impaired immunity and especially applicable to patients who have graft versus host disease (GVHD) after hematopoietic stem cell transplant, patients with leukemia and patients with long-term leukopenia due to chemotherapy; compared with control drugs such as fluconazole and itraconazole, the freeze-drying composition can effectively prevent invasive aspergillosis and can lower the mortality related to the invasive fungal infections.
Owner:HC SYNTHETIC PHARMA CO LTD
Polyvinyl-phosphorylcholine elaioplast preparation and its making method
InactiveCN1895224ANo vascular irritationEasy to useDigestive systemPhosphorous compound active ingredientsVitamin CCholesterol
A polyene phosphatidylcholine liposome with high curative effect is prepared through dissolving polyene phosphatifylcholine, phosphatide and cholesterol in organic solvent, constant-temp drying for removing solvent, filming, adding vitamin, stabilizer and mannitol or glucose solution, dissolving, ultrasonic vibration or homogenizing, filtering by membrane for removing bacteria, loading in containers, filling N2 or H2 and sealing.
Owner:裴泽军
Highly concentration water-soluble vitamin for intravenous injection and preparation and use
InactiveCN1543978AImprove the immunityImprove immunityOrganic active ingredientsMetabolism disorderHigh concentrationVitamin C
The present invention relates to a high-concentration-type water soluble vitamin used for intravenous injections, with each bottle containing 6.2 +-1.6 mg aneurine mononitrate, 9.8+-2.5 mg lactoflavin sodium phosphate, 80+-20 mg nicotinic amide, 9.8+-2.5 mg phosphopyridoxamine, 33.0+-8.3 mg panthoject, 200+-50 mg vitamin, 120 +-30 mu g biotin, 0.8+-0.2 mg acidum folicum, 10.0+-2.5 mu g vitamin B12 and 1-6ml water for injection. The preparing method is as follows: stirring the components mentioned above with water for injection until they are entirety dissolved, freezing-drying them in a freezing-drying machine, pressing them to prepare the water soluble vitamins used for intravenous injection. The water soluble vitamins used for intravenous injection in accordance with the present invention can be used for guarding against and curing cacotrophia caused by deficiency of water soluble vitamins and for auxiliary cure of chronic diseases such as chronic hepatic disease and the like; the water soluble vitamins used for intravenous injection possesses advantages of rapid vitamin supplying, medicament curative effect enhancement, reduced side-effects and the like, can satisfy clinical require for supplying of serious water soluble vitamins deficiency caused by diseases.
Owner:吴良信 +1
Pidotimod injection and the producing method thereof
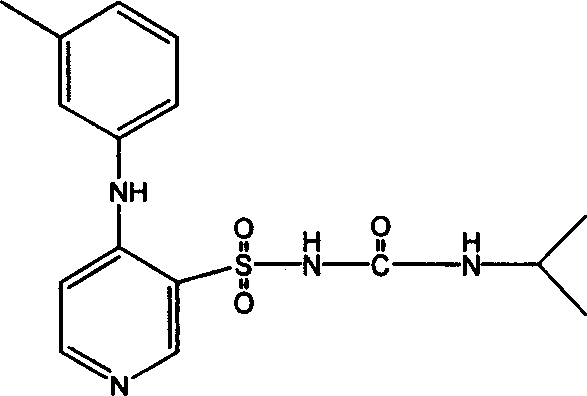
InactiveCN101062404ANo vascular irritationReactivity NoneSenses disorderDipeptide ingredientsSolubilityMedicine
The invention discloses a Pidumode injection, which is characterized by the following: producing solution agent with Pidumode medicine and medicinal carrier; adjusting pH value at 6. 0-8. 0; setting volume of each packing unit at 2-500ml; incorporating 100-800mg Pidumode; forming salt with Pidumode and caustic soda; possessing good water-solubility and medicinal crop with the same as Pidumode; easy-degrading to aminoglutaric acid and thiazolidinecarboxylic acid with Pidumode; adjusting the pH value of the solution to near neutral; stabilizing the quality of product. This invention can be used to intravenous injection.
Owner:沈阳双鼎制药有限公司 +1
Moxifloxacinhydrochloride sodium chloride injection, preparation method thereof and use thereof
InactiveCN101884613AWill not crystallizePrecipitation does not occurAntibacterial agentsOrganic active ingredientsPhosphateSodium Chloride Injection
The invention relates to moxifloxacinhydrochloride sodium chloride injection. The injection comprises the following components in percentage by weight / volume: 0.03 to 1 percent of moxifloxacinhydrochloride, 0.01 to 3 percent of weak acid, 0.01 to 3 percent of salt of weak acid, 0.01 to 3 percent of phosphoric acid, 0.01 to 3 percent of amino acid or phosphate and 0.65 to 0.95 percent of sodium chloride.
Owner:HC SYNTHETIC PHARMA CO LTD
Antihypertensive drug fat milk injection and preparation method thereof
ActiveCN107661294AQuality improvementImprove securityOrganic active ingredientsEmulsion deliveryClevidipineOil phase
The invention discloses an antihypertensive drug fat milk injection and a preparation method thereof. The antihypertensive drug fat milk injection is a clevidipine butyrate fat milk injection and contains clevidipine butyrate and a pharmacologically acceptable medical excipient; the medical excipient comprises an oil-phase medium, an emulsifier, an osmotic pressure regulator, a stabilizer, a metalcheating agent, a pH value regulator and injection water. The antihypertensive drug fat milk injection has stable quality and high safety and is not irritant to blood vessels.
Owner:WUHAN CONFORM PHARMA CO LTD
Pharmaceutical composition for treating bone diseases, injection thereof and preparation methods thereof
ActiveCN104127473AIncreased effect on bone diseaseGood treatment effectNervous disorderAntipyreticSide effectTreatment effect
The invention relates to a pharmaceutical composition for treating bone diseases, an injection thereof and preparation methods thereof. The pharmaceutical composition is composed of an extraction liquid of pig four limbs and a melon seed extraction liquid. Further, the pharmaceutical composition is added with mixed extraction liquids respectively prepared from commen bomhax flower, flos buddlejae and herba eupatorii. The prepared pharmaceutical composition has substantially improved treatment effect on rheumatoid arthritis, degenerative osteoarthritis, ankylosing spondylitis, sciatica, lumbar disc herniation and other bone diseases, is improved in treatment effect on gout and has no toxic and side effects. Additionally, the provided injection has extremely high stability and is substantial in effect.
Owner:哈尔滨圣泰生物制药有限公司
Docetaxel freeze-dried microemulsion preparation and preparation method thereof
ActiveCN103301061AGood dispersionImprove stabilityPowder deliveryOrganic active ingredientsPolyoxyethylene castor oilHemolysis
The invention discloses a docetaxel freeze-dried microemulsion preparation and a preparation method thereof. The docetaxel freeze-dried microemulsion preparation comprises the following raw materials by weight: 0.05-5 parts of docetaxel, 0.1-40 parts of an oil phase, 5-30 parts of a surfactant, 0-40 parts of a cosurfactant, 5-85 parts of a hydrophilic phase, 0-15 parts of a cosolvent, 0-5 parts of an antioxidant, and 1-40 parts of a freeze-drying protective agent. Specifically, the surfactant is one or several of polyethylene glycol-8-caprylin / caprin, polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil, poloxamer, polyethylene glycol-12-hydroxystearate, polyethylene glycol stearate 15 and sorbitan monooleate. The preparation method includes: preparing the raw materials into a microemulsion according to the ratio, and then conducting freeze-drying. Before clinical use, the docetaxel freeze-dried microemulsion preparation has no need for a tedious two-step dilution process, after redissolving, the docetaxel freeze-dried microemulsion preparation can be subjected to intravenous injection, and has no vascular irritation and small allergic reaction. Hemolytic experiments show that the docetaxel freeze-dried microemulsion preparation does not generate hemolysis.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
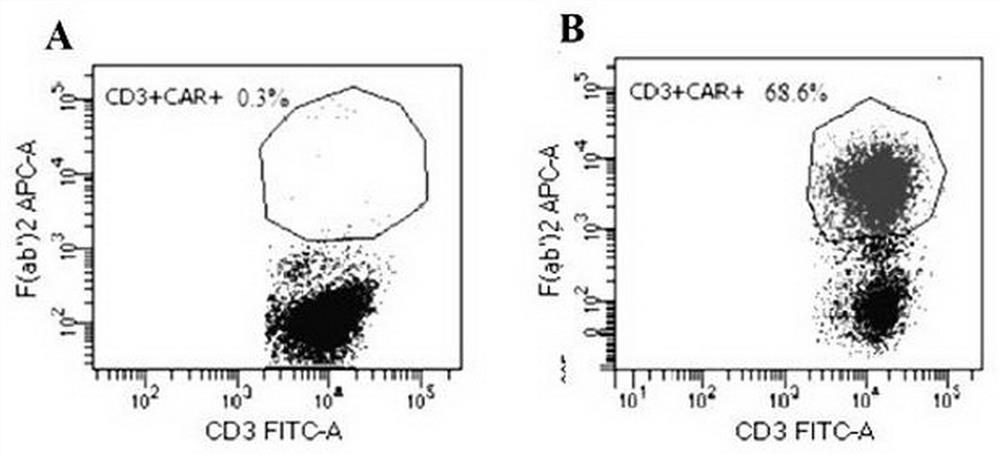
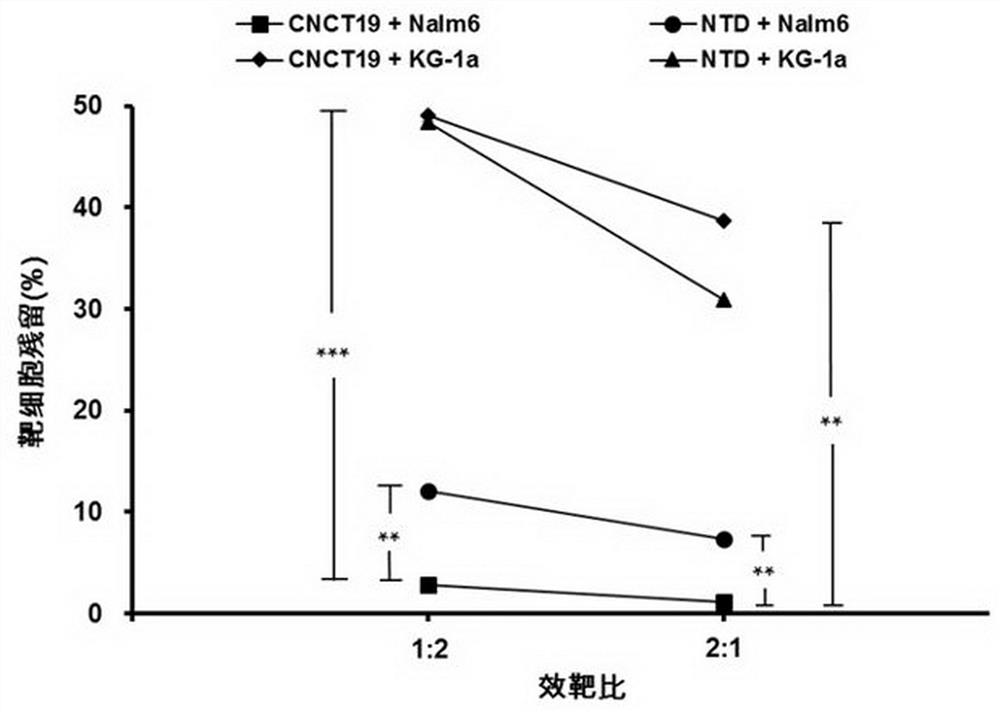
CD19 targeting chimeric antigen receptor and application thereof
ActiveCN112079934APromote secretionProlong survival timeNGF-receptor/TNF-receptor superfamilyImmunoglobulinsAntigen receptorImmune effector cell
The invention provides a chimeric antigen receptor. The chimeric antigen receptor comprises an amino acid sequence shown in SEQ ID NO. 1. The application also provides nucleic acids encoding the chimeric antigen receptor, vectors comprising the nucleic acids, immune effector cells comprising the chimeric antigen receptor, the nucleic acid molecules and / or the vectors, methods of preparing the immune effector cells, compositions comprising the immune effector cells, and uses of the chimeric antigen receptor.
Owner:JUVENTAS CELL THERAPY LTD
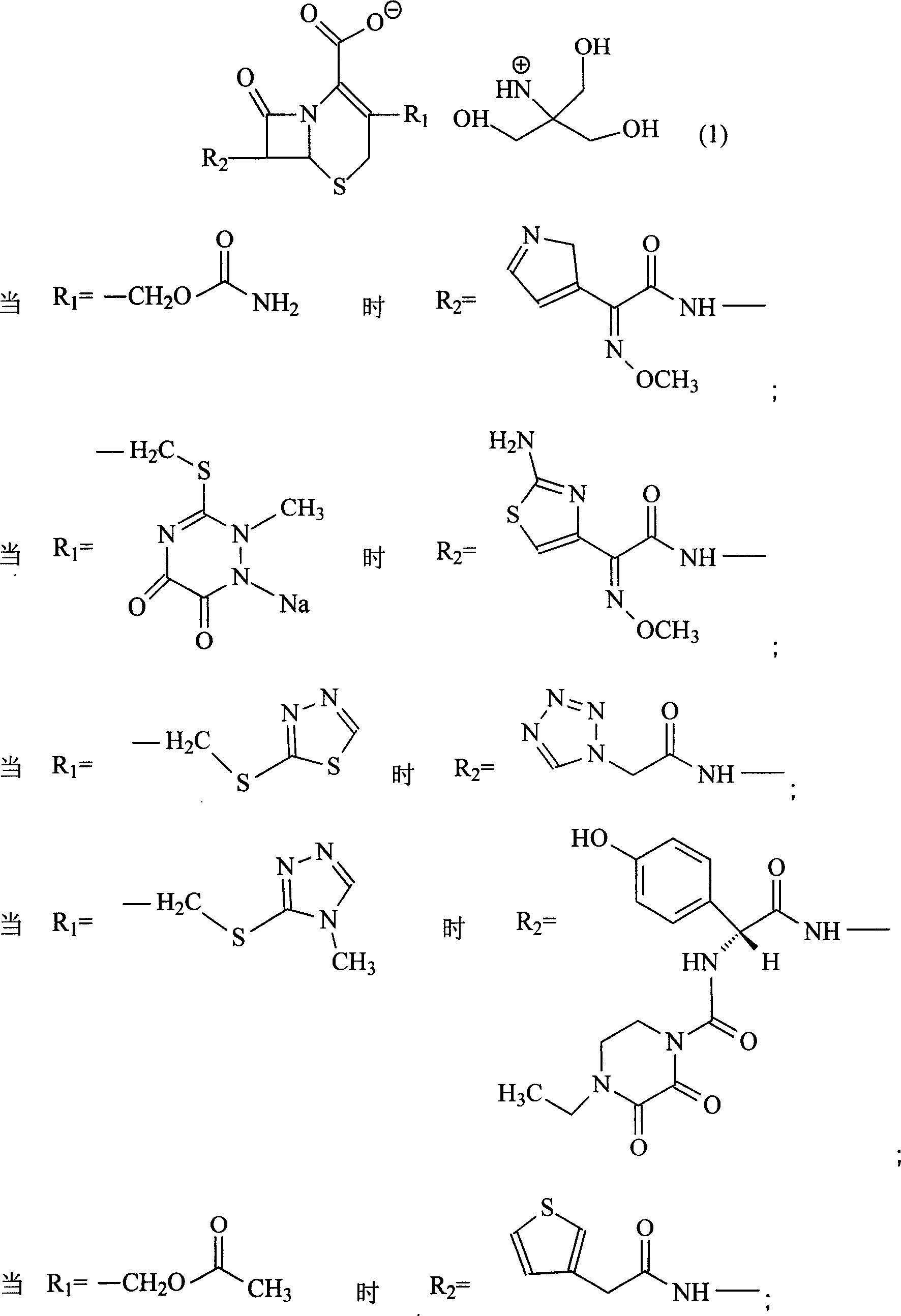
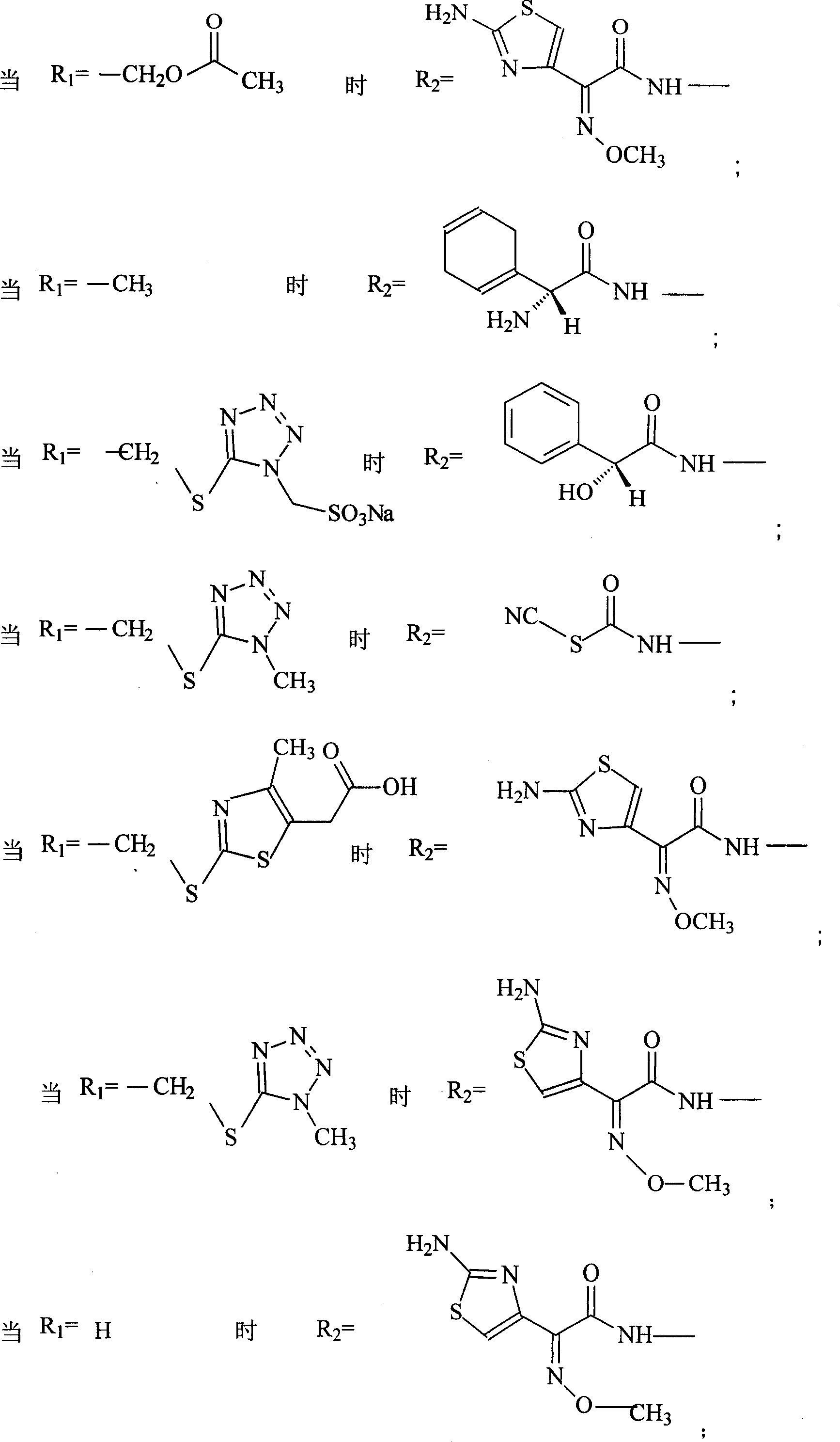
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
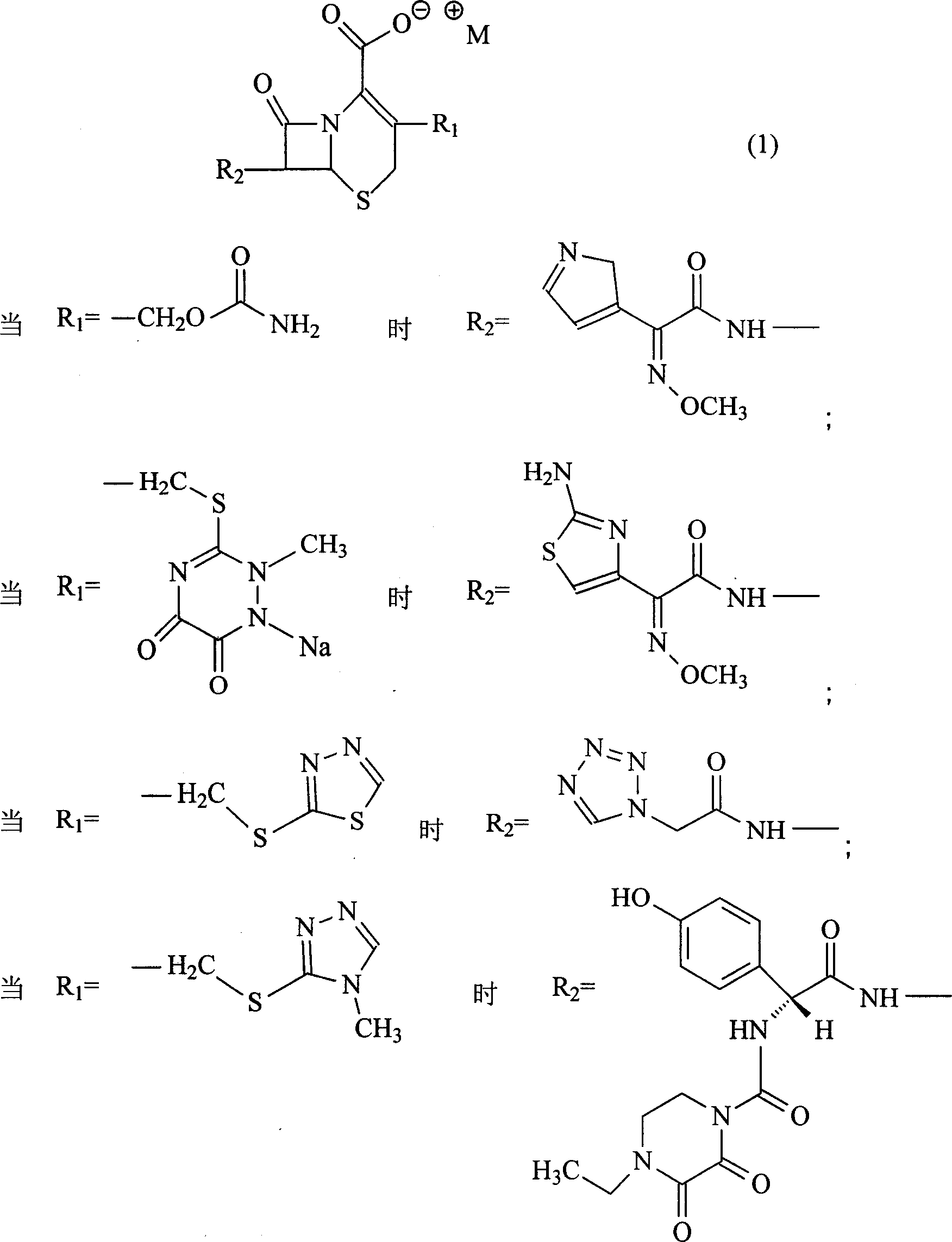
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
Ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and preparation method thereof
InactiveCN103565733AReduce dosageSolve the problem of opalescence opacityOrganic active ingredientsAntipyreticActivated carbon filtrationMicrofiltration membrane
The invention provides an ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and a preparation method thereof. The injection contains 1-10 mg / ml of ibuprofen, and injection water, wherein the pH is adjusted to 6.0-6.5 by an acid-base modifier; the dosage of the required acid-base modifier accounts for about 2-6% by weight / volume of the injection; the sodium chloride content of an isoosmotic adjusting agent is 0.70-0.90% by weight / volume. The preparation technology comprises the following steps: firstly, dissolving the acid-base modifier and the ibuprofen; adding the injection water to 20% of total mass; adding sodium chloride to stir and dissolve; adding activated carbon, filtering and decarburizing; adding water to 90% of total mass, adjusting the pH to 6.0-6.5, and adding water to total mass; filtering by 0.45 microns and 0.22 microns of microfiltration membranes; and filling and sealing and sterilizing at 121 DEG C for 8-20 minutes.
Owner:南京帝易医药科技有限公司
Medicine composition of rhodiola root and puerarin
InactiveCN1931214AAvoid the shortcomings of large differences in quality of different productsHigh purityOrganic active ingredientsPharmaceutical delivery mechanismVascular diseasePuerarin
The present invention belongs to the field of medicine technology, and is especially one kind of medicine composition for treating cardiac and cerebral vascular diseases and its preparations and preparation process. The medicine composition consists of rhodiola root in 300-1800 weight portions, preferably 1000 weight portions, or its extract, and puerarin in 30-500 weight portions, preferably150 weight portions. The composition may be prepared into different pharmaceutically acceptable forms, preferably injection. The composition has synergistic effect and high stability.
Owner:海安江理工技术转移中心有限公司
Ketoprofen intravenous administration preparation and preparation method thereof
InactiveCN102895220AAvoid secondary pollutionConvenient for clinical operationOrganic active ingredientsAntipyreticKetoprofenInjection solution
The invention provides a ketoprofen intravenous administration preparation and a preparation method thereof. The injection contains 0.4mg / ml-4mg / ml ibuprofen, an alkali modifier in the amount required for adjusting pH to 6.5-9.0, 0.70%-0.90% w / v sodium chloride and water for injection.
Owner:HC SYNTHETIC PHARMA CO LTD
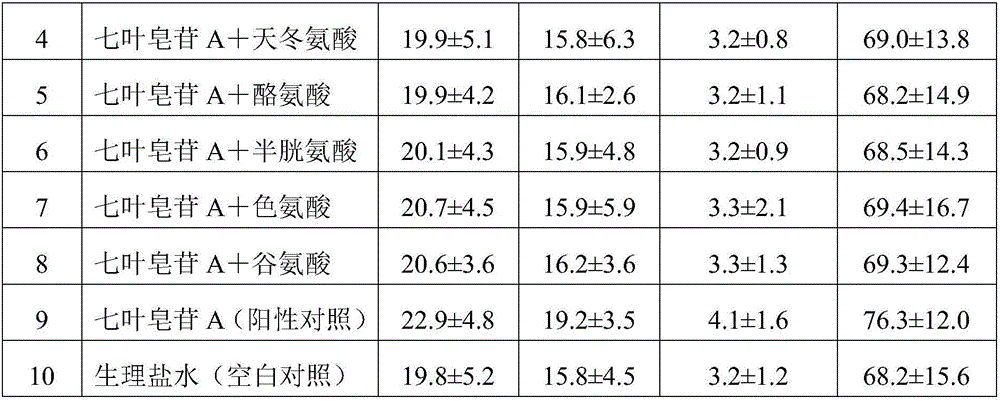
Aescine A injection and preparation method therefor
ActiveCN106511282AStrong anti-inflammatoryHigh activityOrganic active ingredientsPowder deliveryLiver and kidneyIrritation
The invention discloses an aescine A injection. The aescine A injection comprises aescine A and amino acid at the weight ratio of 10-50 to 1-10. The invention also discloses a preparation method for the injection. Compared with the prior art, the aescine A injection has stronger anti-inflammatory effect and anti-effusion activity, and lower vascular irritation and liver and kidney toxicity.
Owner:WUHAN AIMIN PHARMA
Propofol phosphate for injection and preparation method and application thereof
InactiveCN101780096AImprove performanceNo pollution in the processPowder deliveryOrganic active ingredientsPhosphateMedical prescription
The invention relates to propofol phosphate for injection, pharmaceutical salt thereof and a preparation method thereof. The preparation method comprising the following steps: adding 5-98 percent of water for injection into a preparation container, adding propofol phosphate and pharmaceutical salt thereof of 90-110 percent of accurate prescriptions, slowly dropping a pH value regulator while stirring so as to adjust the pH to be 6.0-11, adding water to the full volume, then adding medicinal carbon of 0.01-1.0 percent (W / V), stirring for 15-60 minutes, roughly filtering with a sand filter rod to remove the carbon, and finely filtering with a 0.22mum microporous membrane until the clarity is qualified; and measuring until the intermediate content is acceptable, fixing the filling amount, sub-packaging into cillin bottles, plugging in plugs by half, freezing and drying samples to control the moisture content being 0.1-8 percent, pressing the plugs, and capping.
Owner:HC SYNTHETIC PHARMA CO LTD
Stepronin powder injection and its preparing method
InactiveCN1820746AQuick effectExpansion of drug occasionsOrganic active ingredientsPowder deliveryFreeze-dryingActive component
The present invention is new preparation form of Stepronin, especially Stepronin powder for injection, and its preparation process. The powder for injection consists of Stepronin or its metal salt or amino salt as active component and freeze drying support agent. Compared with available orally taken Stepronin preparation, the present invention has the advantages of high bioavailability, good medicine absorption, fast medicine dispersion, fast acting, etc. The present invention expands the administration range of Stepronin and raises the clinical administration level of Stepronin.
Owner:HUAZHONG UNIV OF SCI & TECH
Medicinal composition
InactiveCN103356619AImprove tumor inhibition rateNo hemolyticPowder deliveryOrganic active ingredientsHemolysisMelanoma
Disclosed in the present invention is a pharmaceutical composition, comprising a weight ratio of 1 : 120 to 1 : 1000 of Camptothecin compounds of formula I and beta-cyclodextrin or derivatives thereof, and an acidic buffer to adjust the pH = 3.5-6.0. The composition can be used to treat solid tumours, such as melanoma, pancreatic cancer, liver cancer, etc. The pharmaceutical composition of the present invention is miscible with a water-miscible co-solvent system in any proportion, and can be used as an intravenous infusion solvent, and has no obvious hemolysis or vascular stimulation; the pharmaceutical composition has a better tumour inhibiting rate than solubilization of surfactants.
Owner:SHANGHAI HUATUO MEDICAL SCI CO LTD
Gambogic acid nano preparation based on hydrophobic prodrug and capable of improving long circulation
ActiveCN112174978AExtend cycle timeGood treatment effectOrganic active ingredientsOrganic chemistryGambogic acidPolyethylene glycol
The invention discloses a gambogic acid nano preparation based on a hydrophobic prodrug and capable of improving long circulation. The nano preparation is a gambogic acid prodrug nano preparation andis gambogic acid oleyl alcohol / polyethylene glycol vitamin E succinate nanoparticles. According to the invention, gambogic acid is modified into an inactive prodrug, and the inactive prodrug is administrated in the form of nanoparticles so that the circulating half-life period of gambogic acid in vivo is improved, the vascular irritation during administration is relieved, and the preparation has important theoretical and practical values for clinical application of gambogic acid.
Owner:CHINA PHARM UNIV
Antiarrhythmic drug fat emulsion injection and preparation method thereof
ActiveCN106137963BSimple preparation processImprove preparation qualityOrganic active ingredientsEmulsion deliveryPharmaceutical medicineOil phase
The invention discloses an anti-arrhythmic drug fat emulsion injection and a preparation method thereof. The anti-arrhythmic drug fat emulsion injection is an amiodarone hydrochloride fat emulsion injection which contains amiodarone hydrochloride and a medicinal excipient acceptable pharmaceutically, wherein the medicinal excipient is prepared from an oil phase, an emulsifier, an osmotic pressure regulator, a stabilizing agent, a pH regulator and water for injection. The anti-arrhythmic drug fat emulsion injection is stable in quality and non-irritant to blood vessels, and the security and compliance of clinical drug application are improved.
Owner:WUHAN CONFORM PHARMA CO LTD
Anti-arrhythmic drug fat emulsion injection and preparation method thereof
ActiveCN106137963AQuality improvementImprove clinical drug safetyOrganic active ingredientsEmulsion deliveryFat emulsionOil phase
The invention discloses an anti-arrhythmic drug fat emulsion injection and a preparation method thereof. The anti-arrhythmic drug fat emulsion injection is an amiodarone hydrochloride fat emulsion injection which contains amiodarone hydrochloride and a medicinal excipient acceptable pharmaceutically, wherein the medicinal excipient is prepared from an oil phase, an emulsifier, an osmotic pressure regulator, a stabilizing agent, a pH regulator and water for injection. The anti-arrhythmic drug fat emulsion injection is stable in quality and non-irritant to blood vessels, and the security and compliance of clinical drug application are improved.
Owner:WUHAN CONFORM PHARMA CO LTD
A kind of succinylcholine chloride injection and preparation method thereof
ActiveCN112402374BLow impurity contentAvoid destructionOrganic active ingredientsMuscular disorderBenzoic acidPh regulation
The invention relates to a succinylcholine chloride injection and a preparation method thereof. Each 1000 L part of the injection contains the following components by weight: 20 parts of succinylcholine chloride, 1-10 parts of bacteriostat, chlorine 5 parts of sodium chloride; the bacteriostatic agent is phenylethyl alcohol, benzoic acid or benzyl alcohol; during its preparation, the pH value is adjusted to 3.0-4.5 with a pH regulator. In the process of preparing succinylcholine chloride injection, the present invention comprehensively controls the temperature of water for injection, the amount of dissolved oxygen, the selection of bacteriostatic agent, pH regulation, continuously feeds nitrogen in the process of filling, and controls the concentration of the drug. The headspace residual oxygen content of the solution and other means have effectively controlled the increase of impurities and the decrease of the effective substance content, improved the utilization rate of the injection, greatly reduced the risk of using the succinylcholine chloride injection, and improved the The effectiveness and application prospect of the injection, the preparation method is simple, and it is easy to produce commercially.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Highly concentration water-soluble vitamin for intravenous injection and preparation and use
InactiveCN1251681CImprove the immunityImprove immunityMetabolism disorderPharmaceutical delivery mechanismHigh concentrationSide effect
The present invention relates to a high-concentration-type water soluble vitamin used for intravenous injections, with each bottle containing 6.2 +-1.6 mg aneurine mononitrate, 9.8+-2.5 mg lactoflavin sodium phosphate, 80+-20 mg nicotinic amide, 9.8+-2.5 mg phosphopyridoxamine, 33.0+-8.3 mg panthoject, 200+-50 mg vitamin, 120 +-30 mu g biotin, 0.8+-0.2 mg acidum folicum, 10.0+-2.5 mu g vitamin B12 and 1-6ml water for injection. The preparing method is as follows: stirring the components mentioned above with water for injection until they are entirety dissolved, freezing-drying them in a freezing-drying machine, pressing them to prepare the water soluble vitamins used for intravenous injection. The water soluble vitamins used for intravenous injection in accordance with the present invention can be used for guarding against and curing cacotrophia caused by deficiency of water soluble vitamins and for auxiliary cure of chronic diseases such as chronic hepatic disease and the like; the water soluble vitamins used for intravenous injection possesses advantages of rapid vitamin supplying, medicament curative effect enhancement, reduced side-effects and the like, can satisfy clinical require for supplying of serious water soluble vitamins deficiency caused by diseases.
Owner:吴良信 +1
Composition of voriconazole phosphate for injection or pharmaceutically acceptable salt thereof and preparation method thereof
ActiveCN101744778BImprove performanceNo pollution in the processPowder deliveryOrganic active ingredientsPhosphateMedical prescription
The invention provides voriconazole phosphate ester for injection and a medicinal salt thereof and a preparation method for the voriconazole phosphate ester for injection and the medicinal salt thereof. The preparation method comprises the following steps: adding 5 to 98 percent water for injection in a liquid preparation container; adding 90 to 110 percent of the accurate formula dosage of voriconazole phosphate ester and the medicinal salt thereof in the container; stirring the mixture; slowly dropwise adding a pH value regulator; regulating pH to between 6.0 and 11; supplementing water to the full dosage and then adding 0.01 to 1.0 percent (weight in volume) medicinal carbon into the product; stirring the mixture for 15 to 60 minutes; using a sand filter stick to carry out rough filtration and decarburization on the obtained product, and using a 0.22mum millipore filter to carry out fine filtration on the product until the clarity is qualified; after determining that the content of the midbody is qualified, determining the filling quantity and subpackaging the finished product in the vial; adding the semi-plug; carrying out freezing and drying on the sample; controlling the moisture content between 1 and 8 percent; pressing the plug; and carrying out capping.
Owner:HC SYNTHETIC PHARMA CO LTD
A Hydrophobic Prodrug-Based Gambogic Acid Nanoformulation with Improved Long Circulation
ActiveCN112174978BExtend cycle timeGood treatment effectOrganic active ingredientsOrganic chemistryGambogic acidNanoparticle
Owner:CHINA PHARM UNIV
Medicine composition of rhodiola root and puerarin
InactiveCN1931214BSynergisticNon-allergenicOrganic active ingredientsPharmaceutical delivery mechanismVascular diseasePuerarin
The present invention belongs to the field of medicine technology, and is especially one kind of medicine composition for treating cardiac and cerebral vascular diseases and its preparations and preparation process. The medicine composition consists of rhodiola root in 300-1800 weight portions, preferably 1000 weight portions, or its extract, and puerarin in 30-500 weight portions, preferably150 weight portions. The composition may be prepared into different pharmaceutically acceptable forms, preferably injection. The composition has synergistic effect and high stability.
Owner:海安江理工技术转移中心有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com