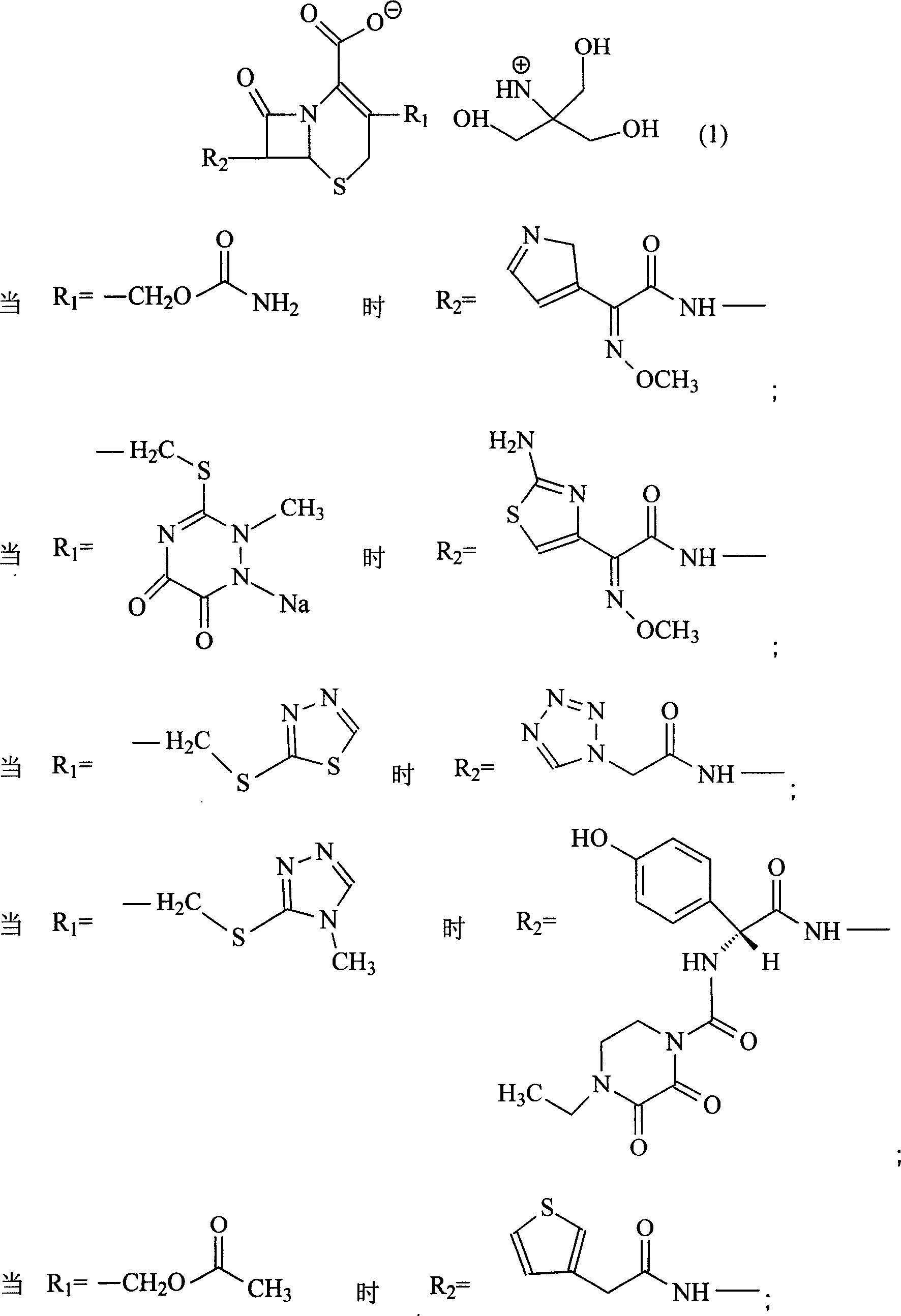
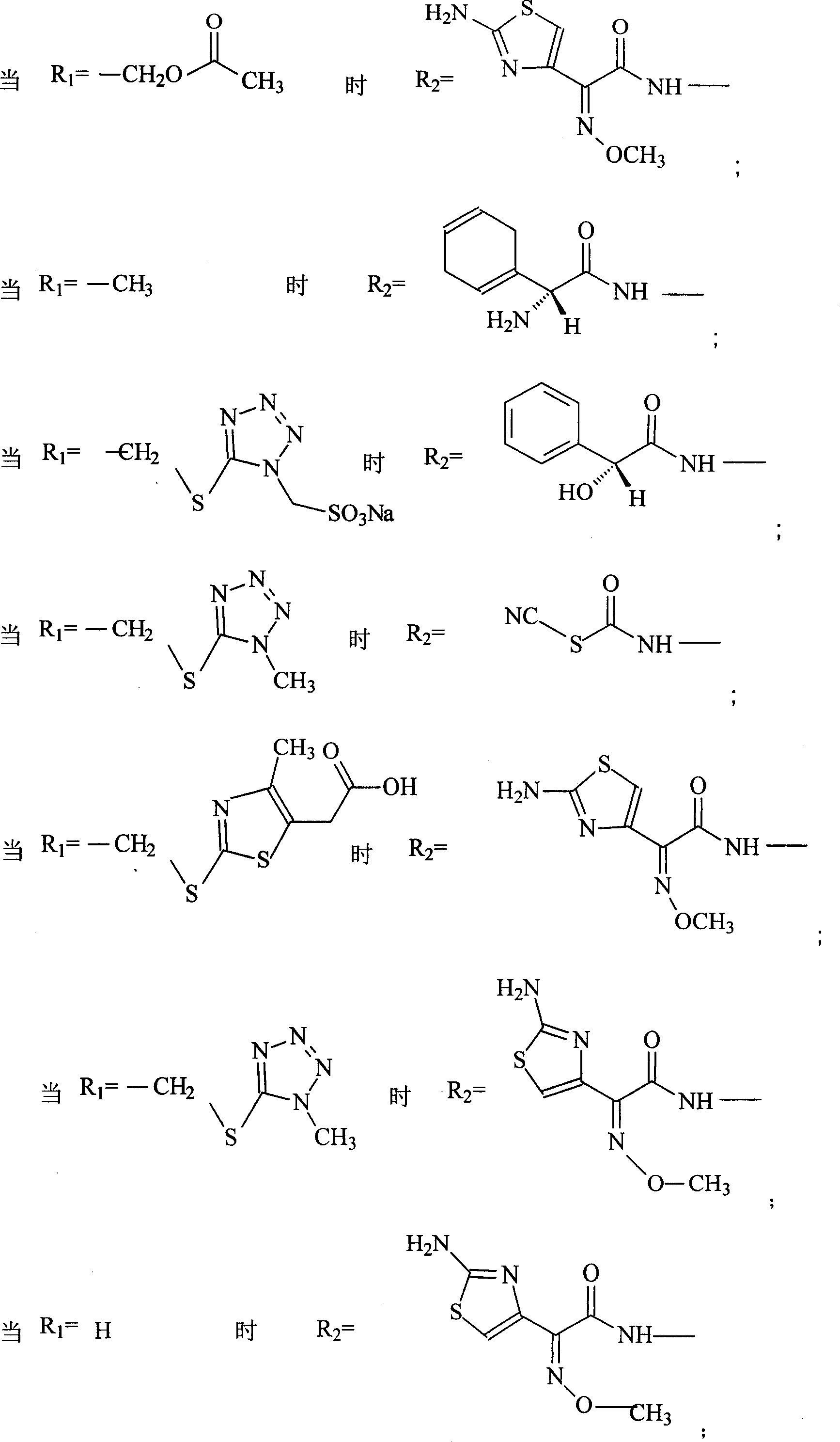
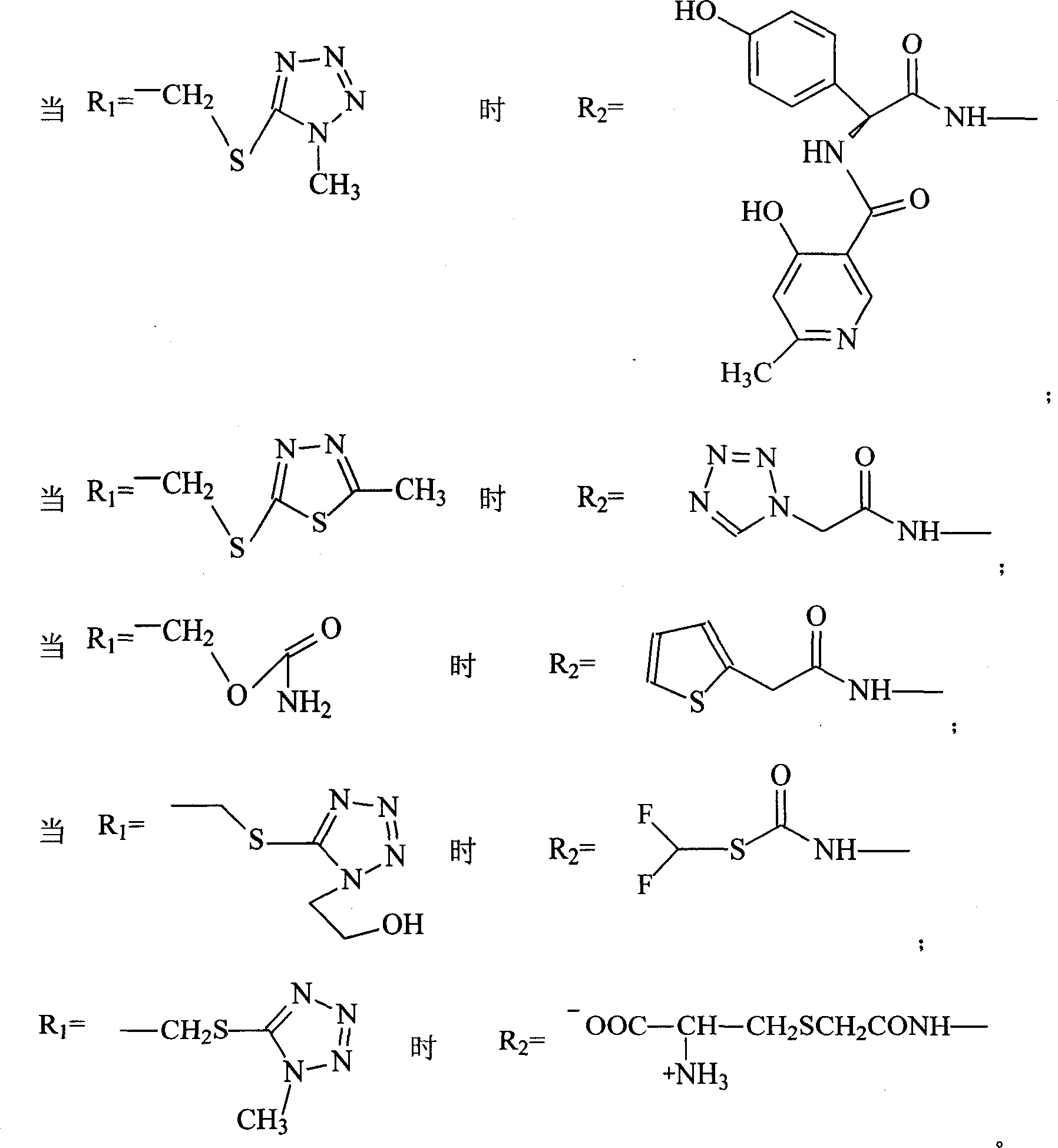
Amino butanetriol salt of cephalosporin compounds and preparing method
A technology of tromethamine and pine tromethamine is applied in the field of derivatives of cephalosporins and their preparation, and can solve the problems of vascular irritation, high risk, incompatibility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the preparation of cefuroxime tromethamine
[0067] In a 250ml three-necked flask, add 12.1g (0.1mol) of tromethamine and 20ml of 60% ethanol, and drop into 20ml of 80% ethanol solution containing 42.4g (0.1mol) of cefuroxime acid under high temperature stirring, and stir until the mixture is Clarify, then stir at room temperature for 2 hours, drop the mixed reactants into 300ml (5-10°C) acetone solution to obtain white powder crystals, filter the solid powder with suction, wash the solid with a small amount of acetone, and pentoxide at high temperature Vacuum drying under the condition of diphosphorus for 24 hours yielded 48 g of powdery solid with a yield of 88%, mp: 121-123° C., content analysis: (HPLC method) containing cefuroxime acid 77.6-77.8%. The product was analyzed by elements (theoretical value: C44.0%H5.0%N12.8%032.3%S5.9%; measured value: C44.9%H5.2%N12.6%032.0%S5.3%), According to NMR, MS, UV, IR and HPLC analysis, the product is cefuroxime...
Embodiment 2
[0068] Embodiment 2: the preparation of ceftriaxone trometamol
[0069] In a 250ml three-neck flask, add 12.1g (0.1mol) of tromethamine, 4ml of methanol, and 2ml of water, stir at high temperature, add 57.7g (0.1mol) of ceftriaxone, stir to completely dissolve, then stir for 2 hours, and mix the mixture Dropped into 500ml of acetone at high temperature, a large amount of white crystalline powder was precipitated, the solid was filtered by suction, washed with a small amount of acetone, and dried at high temperature under phosphorus pentoxide for 24 hours to obtain 62.3g of white crystalline ceftriaxone tromethamine salt. Yield: 89.2%, mp: 93-95°C, content analysis (HPLC method): contains 82.1-84.1% of ceftriaxone acid. The product was analyzed by elements (theoretical value: C37.8%H4.0%N18.1%022.9%S13.8%Na3.4%; measured value: C38.0%H4.2%N17.9%022.7%S13. 7%Na3.5%), NMR, MS, UV, IR and HPLC analysis, the product is ceftriaxone tromethamine salt, and the purity of ceftriaxone tro...
Embodiment 3
[0070] Embodiment 3: the preparation of ceftezole trometamol
[0071] In a 250ml three-neck flask, add 12.1g (0.1mol) of tromethamine, 4ml of methanol, and 2ml of water, stir at high temperature, add 44g (0.1mol) of ceftezole, stir to make it completely dissolve, then stir for 1 hour, and put the mixture in Drop into 500ml of acetone at high temperature, a large amount of white crystalline powder is precipitated, the solid is suction filtered, washed with a small amount of acetone, and dried at high temperature under phosphorus pentoxide for 24 hours to obtain 49.8g of white crystalline ceftezole tromethamine salt. Yield: 88.8%, content analysis (HPLC method): it contains 77.8-79.0% of ceftezole acid. The product was analyzed by elements (theoretical value: C36.4%H4.1%N22.5%020.0%S17.0%; measured value: C36.2%H4.3%N 22.3%020.2%S17.0%), NMR , MS, UV, IR and HPLC analysis, the product is ceftezole tromethamine salt, and the purity of ceftizoxime tromethamine is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com