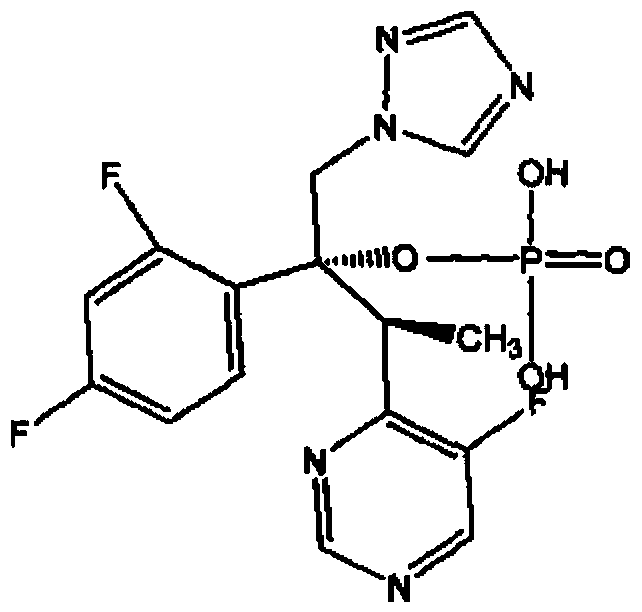
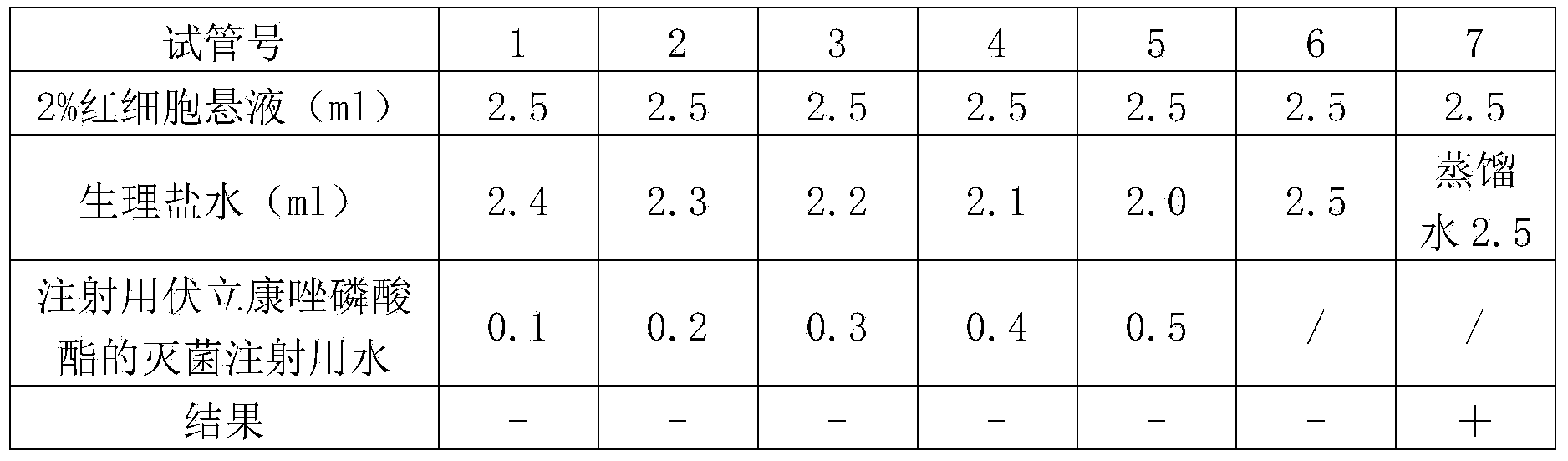
Composition of voriconazole phosphate for injection or pharmaceutically acceptable salt thereof and preparation method thereof
A technology for voriconazole phosphate and medicinal salts, applied in the field of medicine, can solve problems such as loss, fracture, renal dysfunction, etc., and achieve the effects of easy operation, good appearance and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of voriconazole phosphate for injection
[0020] Prescription: Voriconazole Phosphate 200g
[0021] Water for injection 2000ml
[0022] __________________________________
[0023] Makes 1000 bottles
[0024] Add 1500ml of water for injection into the liquid preparation container, add the exact prescription amount of voriconazole phosphate, stir to dissolve, slowly add sodium hydroxide, adjust the pH to 8.0, add water to the full amount, and then add 0.05% (W / V) The medicinal charcoal was stirred for 60 minutes, decarbonized by coarse filtration with a sand filter rod, and finely filtered with a 0.22 μm microporous membrane until the clarity was qualified; after the content of the intermediate was determined to be qualified, the fixed amount was packed in vials, Add half stopper, decarburize the sample by coarse filtration with a sand filter rod, and fine filter it with a 0.22 μm microporous membrane until the clarity is qualified; after ...
Embodiment 2
[0025] Embodiment 2: the preparation of voriconazole disodium phosphate for injection
[0026] Prescription: Voriconazole Disodium Phosphate 200g
[0027] Water for injection 2000ml
[0028] __________________________________
[0029] Makes 1000 bottles
[0030] Add 1500ml of water for injection into the liquid preparation container, add voriconazole phosphate disodium phosphate in the exact prescribed amount, stir to dissolve, slowly add acetic acid, adjust the pH to 8.5, add water to the full amount, and then add 0.05% (W / V) The medicinal charcoal was stirred for 60 minutes, decarbonized by coarse filtration with a sand filter rod, and finely filtered with a 0.22 μm microporous membrane until the clarity was qualified; after the content of the intermediate was determined to be qualified, the fixed amount was packed in vials, Add a half stopper, decarburize the sample by coarse filtration with a sand filter rod, and fine filter with a 0.22 μm microporous membrane until the...
Embodiment 3
[0031] Embodiment 3: the preparation of voriconazole phosphate for injection
[0032] Prescription: Voriconazole Phosphate 200g
[0033] Mannitol 2g
[0034] Water for injection 2500ml
[0035] __________________________________
[0036] Makes 1000 bottles
[0037] Add 2000ml of water for injection in the liquid preparation container, add voriconazole phosphate of accurate prescription amount, under stirring of 2 g of mannitol, slowly add sodium bicarbonate, adjust pH to 7.8, add water to full amount, then add 0.05% (W / V) medicinal charcoal, stirred for 60 minutes, decarbonized by coarse filtration with a sand filter rod, and finely filtered with a 0.22 μm microporous membrane until the clarity is qualified; after the determination of the intermediate content is qualified, the fixed amount is packed in vials In the medium, add half stopper, decarburize the sample by coarse filtration with a sand filter rod, and fine filter with a 0.22 μm microporous membrane until the cla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com