Freeze-drying composition of posaconazole prodrug and preparation method and application of freeze-drying composition of posaconazole prodrug
A prodrug, posaconazole technology, applied in the direction of freeze-drying delivery, powder delivery, antifungal agents, etc., to achieve stable performance, easy large-scale industrial production, and high quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
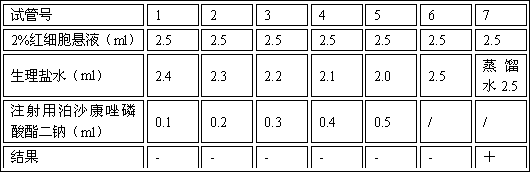
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of posaconazole phosphate for injection
[0023] Prescription: posaconazole phosphate 200g
[0024] Water for injection 2000ml
[0025]
[0026] Makes 1000 bottles
[0027] Add 1500ml of water for injection into the liquid preparation container, add posaconazole phosphate in the exact prescription amount, stir to dissolve, slowly add sodium hydroxide, adjust the pH to 8.0, add water to the full amount, and then add 0.05% (W / V) medicinal charcoal, stirred for 20 minutes, decarbonized by coarse filtration with a sand filter, and finely filtered with a 0.22 μm microporous membrane until the clarity was qualified; Put a half stopper in the bottle, pre-freeze the sample at -40°C for 4 hours, vacuumize, keep the vacuum at 10-30Pa, slowly raise the temperature to -20°C, and keep it for 12 hours; then slowly raise the temperature to 20°C, keep it for 4 hours, control the moisture 2%, cork and cap.
Embodiment 2
[0028] Embodiment 2: the preparation of posaconazole phosphate disodium for injection
[0029] Prescription: Posaconazole Phosphate Disodium 200g
[0030] Water for injection 2000ml
[0031]
[0032] Makes 1000 bottles
[0033] Add 1500ml of water for injection into the liquid preparation container, add posaconazole disodium phosphate disodium phosphate in the exact prescription amount, stir to dissolve, slowly add acetic acid, adjust the pH to 8.5, add water to the full amount, and then add 0.05% (W / V) medicinal charcoal, stirred for 60 minutes, decarbonized by coarse filtration with a sand filter rod, and finely filtered with a 0.22 μm microporous membrane until the clarity was qualified; Put a half stopper in the bottle, pre-freeze the sample at -45°C for 4 hours, vacuumize, keep the vacuum at 10-30Pa, slowly raise the temperature to -20°C, and keep it for 12 hours; then slowly raise the temperature to 0°C, keep it for 6 hours, and then slowly Raise the temperature t...
Embodiment 3
[0034] Embodiment 3: the preparation of posaconazole phosphate disodium pentahydrate for injection
[0035] Prescription: Posaconazole Phosphate Disodium Pentahydrate 200g
[0036] Mannitol 2g
[0037] Water for injection 2500ml
[0038]
[0039] Makes 1000 bottles
[0040] Add 2000ml of water for injection into the liquid preparation container, add the exact prescription amount of posaconazole phosphate disodium pentahydrate, 2g of mannitol, stir, slowly add sodium carbonate, adjust the pH to 9.0, add water to the full amount, Then add 0.1% (W / V) medicinal charcoal, stir for 10 minutes, decarbonize by coarse filtration with a sand filter, and fine filter with a 0.22 μm microporous membrane until the clarity is qualified; after the determination of the intermediate content is qualified, set the Measure and pack in vials, add half stopper, pre-freeze the sample at -40°C for 4 hours, vacuumize, keep the vacuum at 10-30Pa, slowly raise the temperature to -20°C, and keep it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com