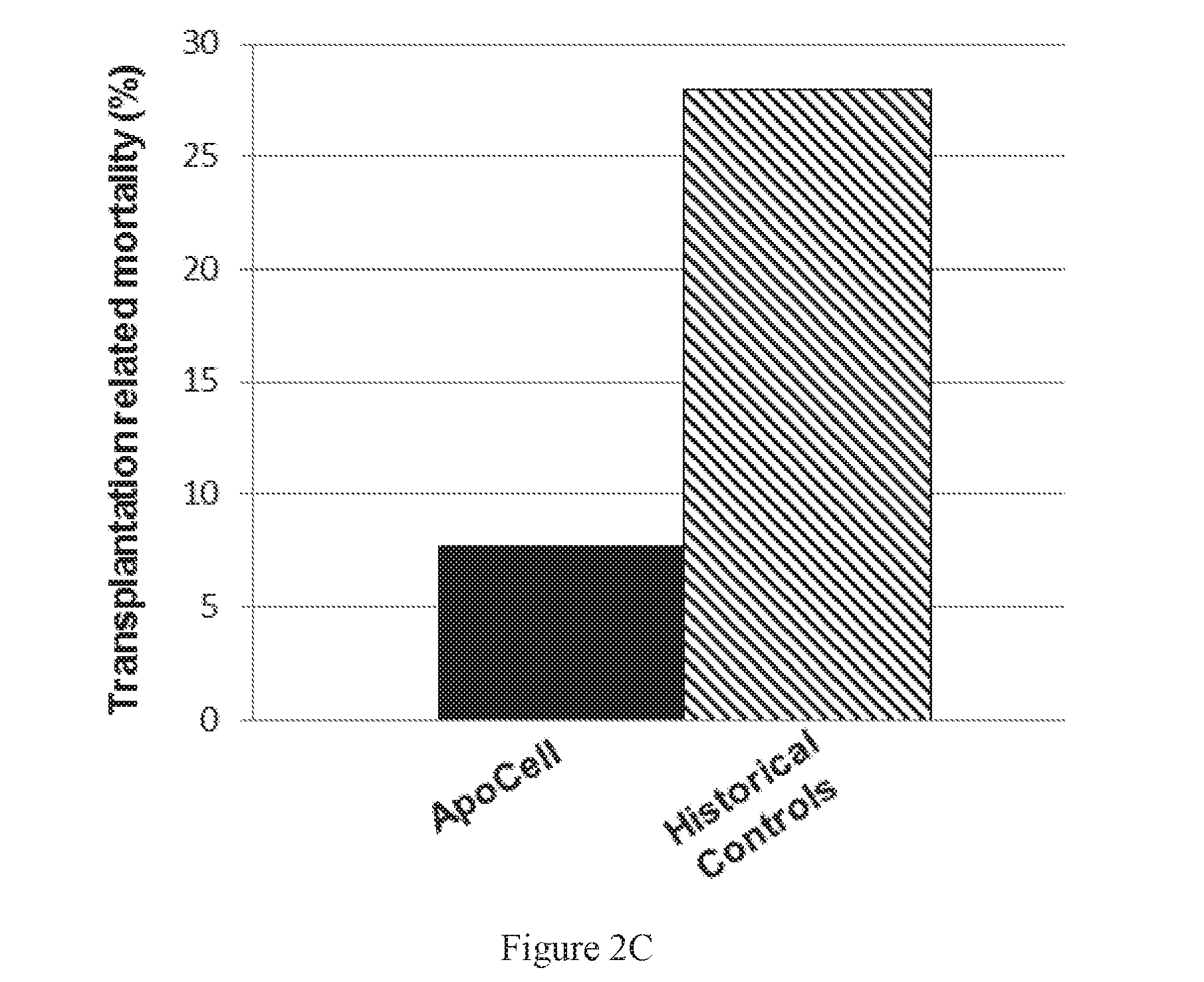
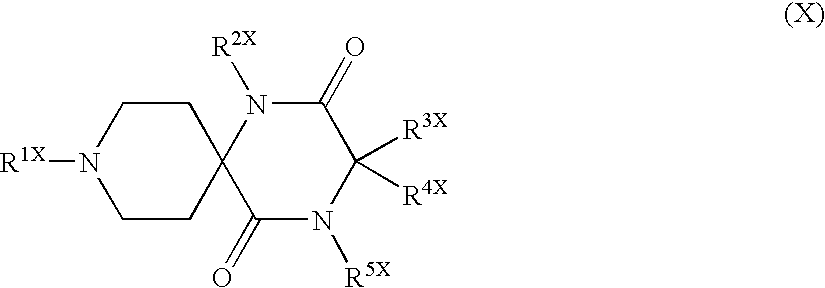
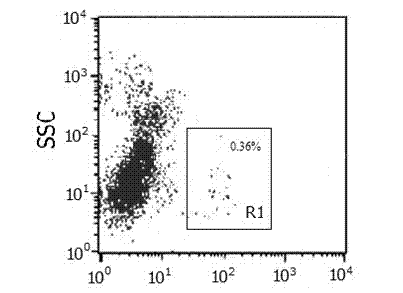
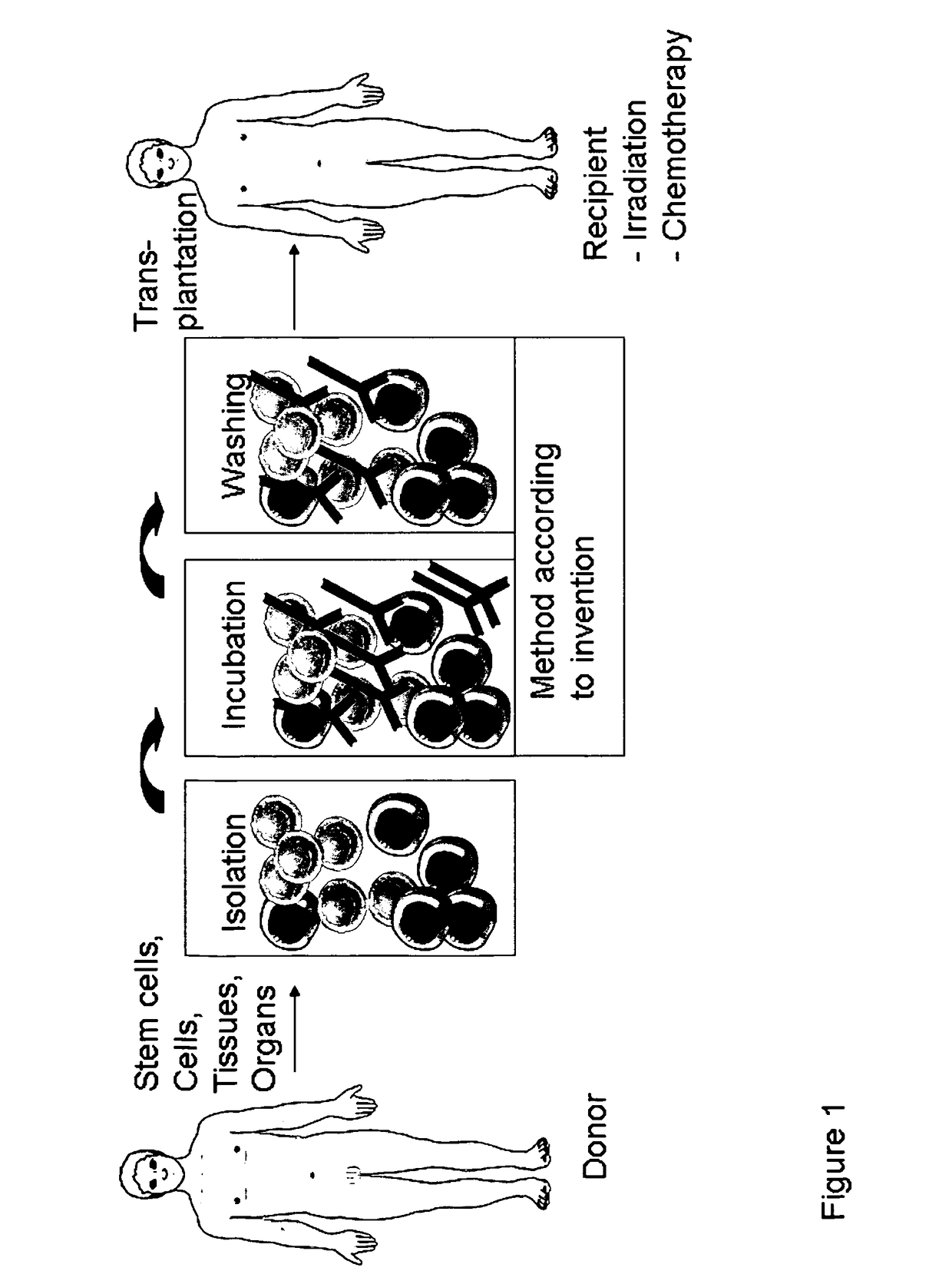
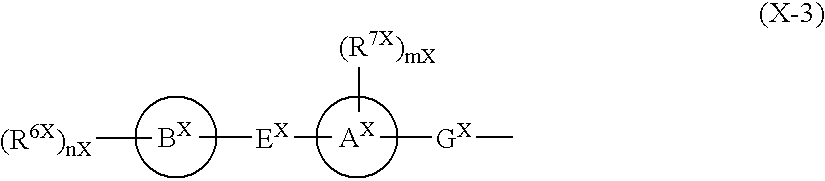Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
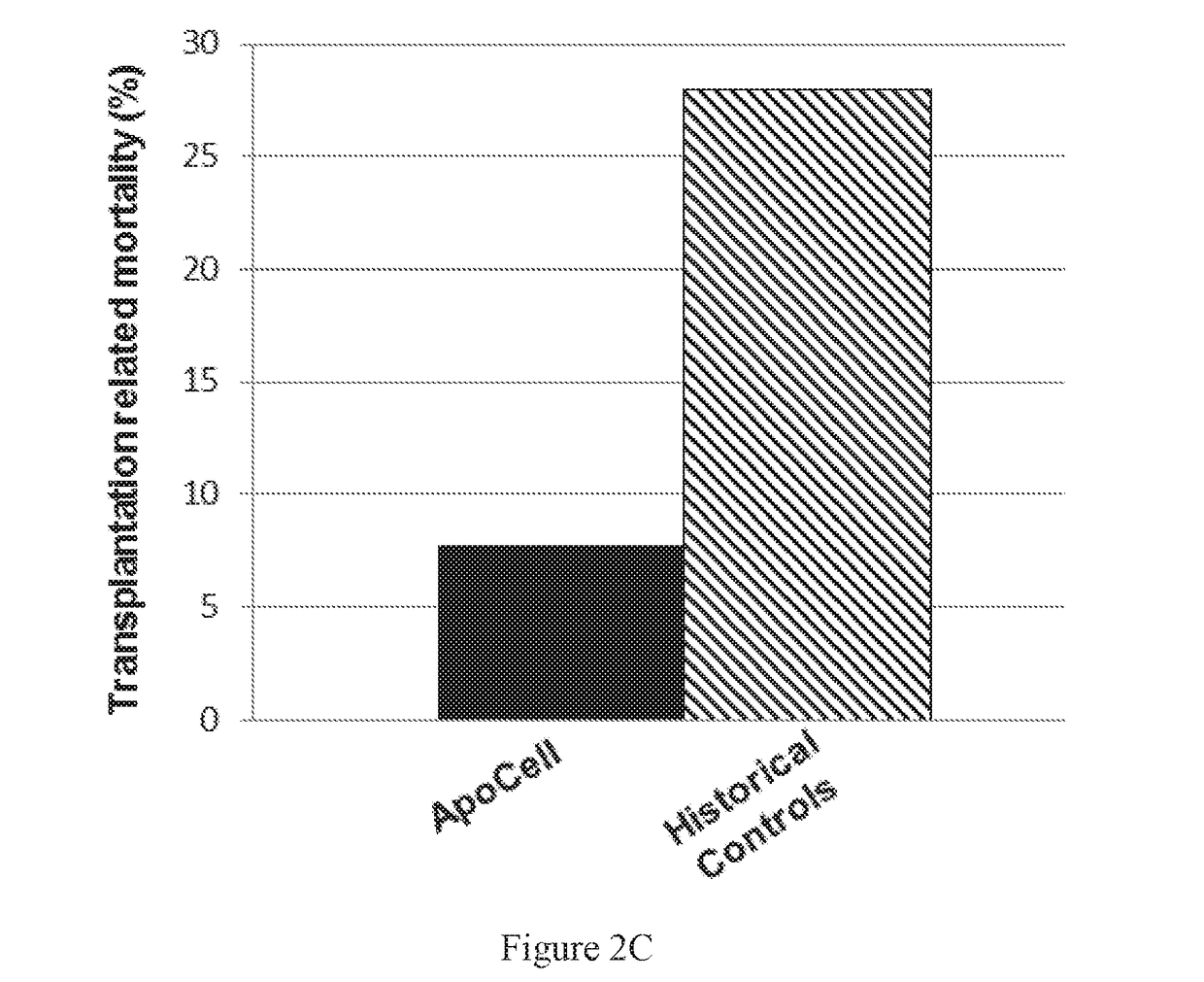
73 results about "Intestinal GVHD" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
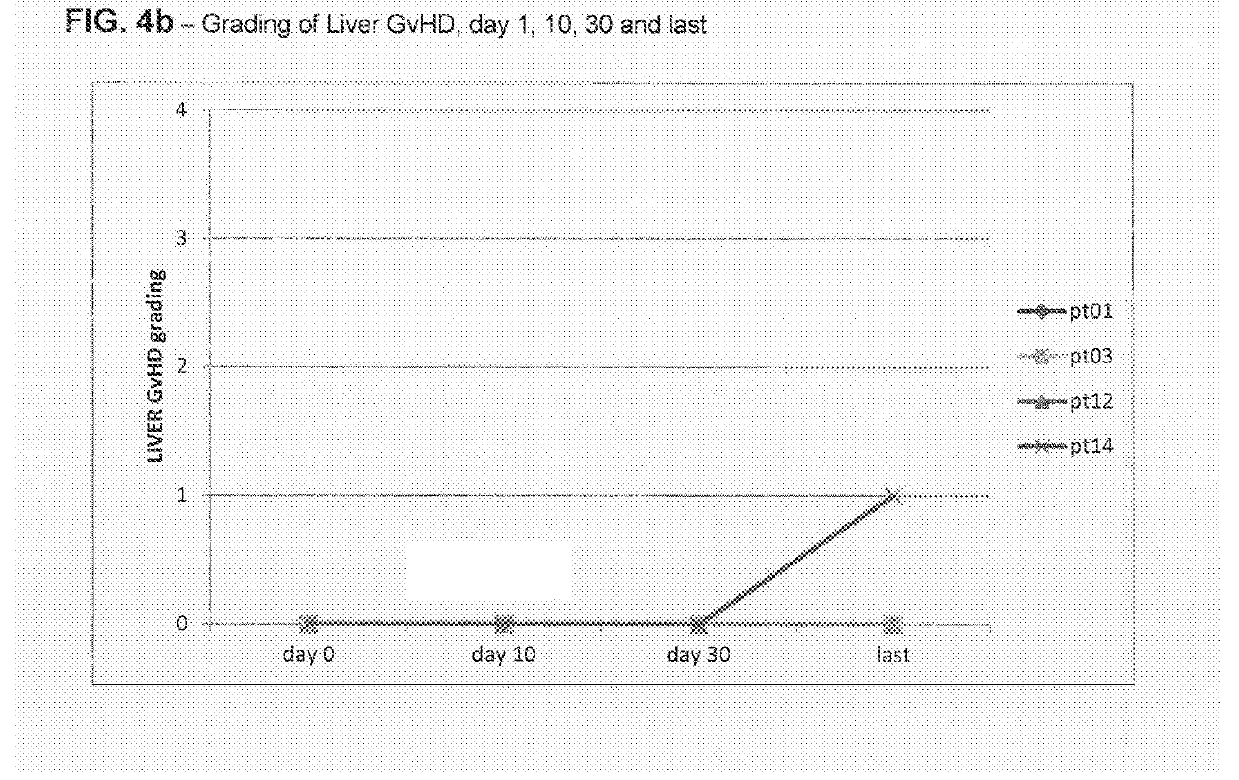
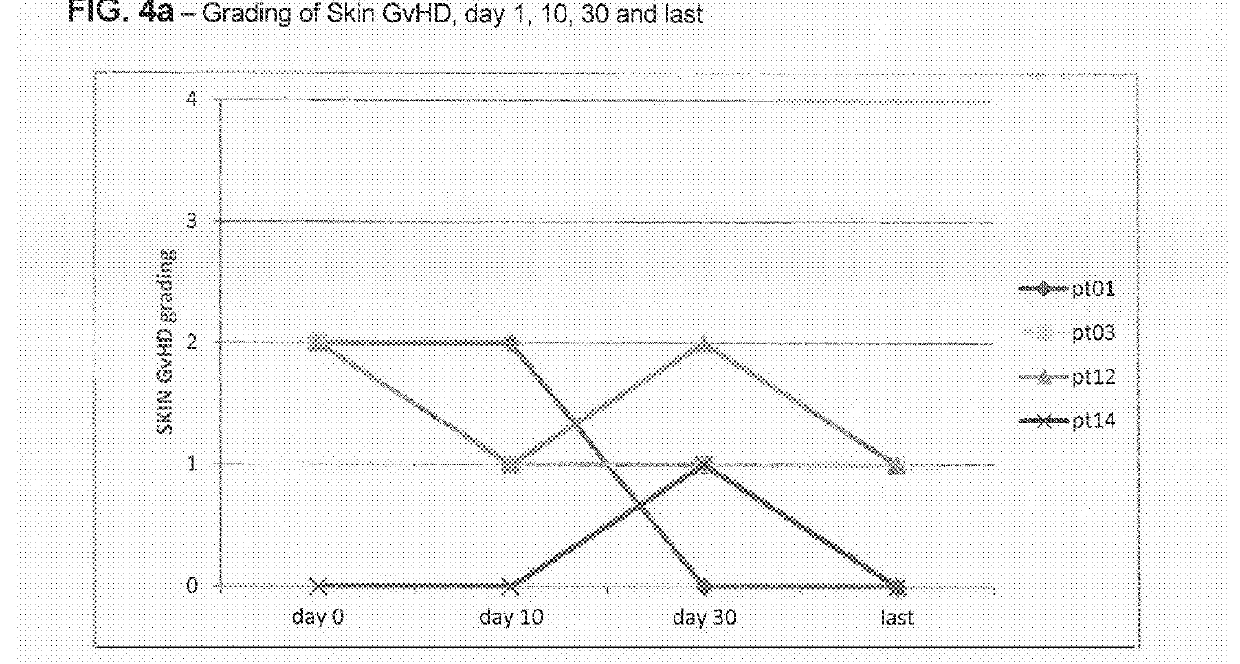
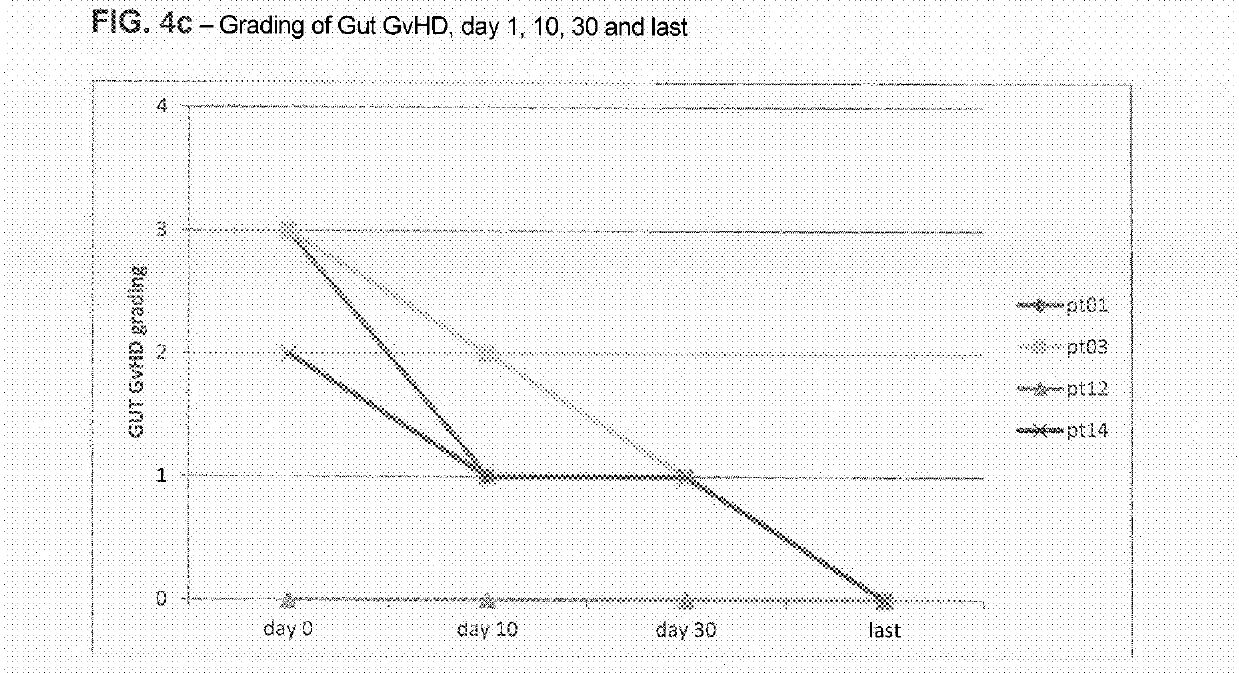
Liver GvHD is measured by the bilirubin level in acute patients. Skin GvHD results in a diffuse red maculopapular rash, sometimes in a lacy pattern. Mucosal damage to the vagina can result in severe pain and scarring, and appears in both acute and chronic GvHD.
Method of judging leukemia, pre-leukemia or aleukemic malignant blood disease and diagnostic therefor
InactiveUS7479371B2Biological material analysisImmunoglobulins against growth factorsGackstroemiaPreleukemia
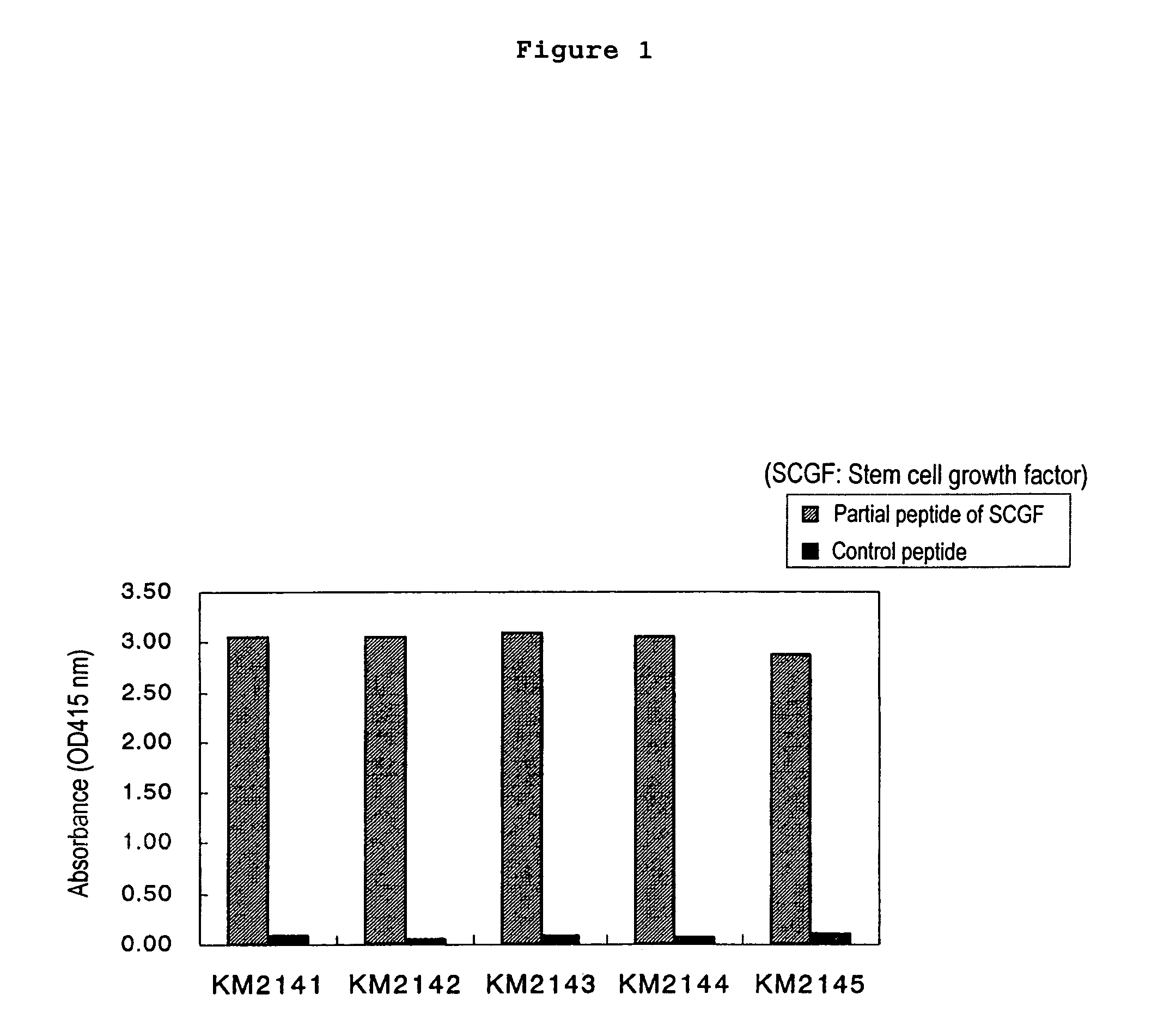
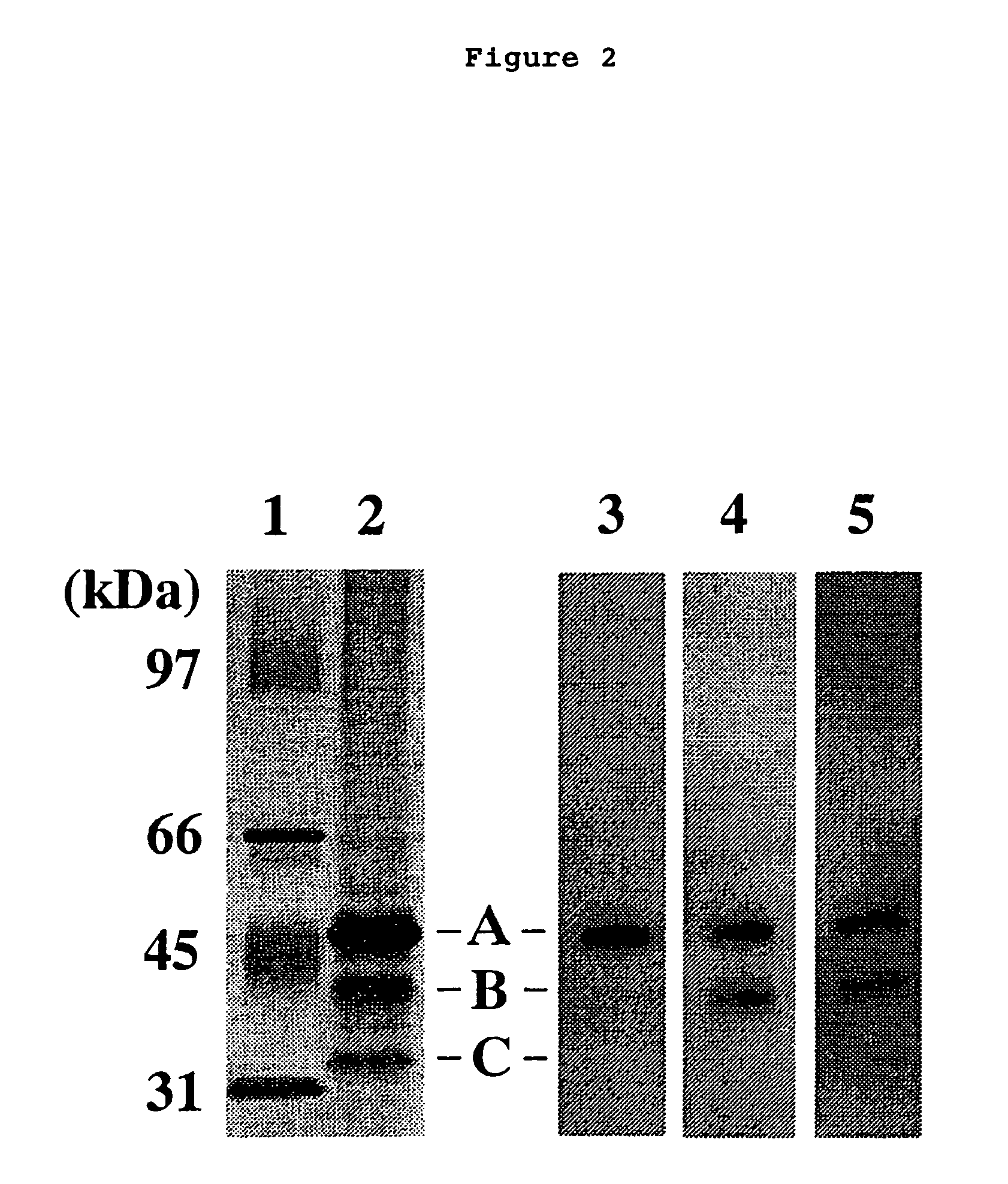
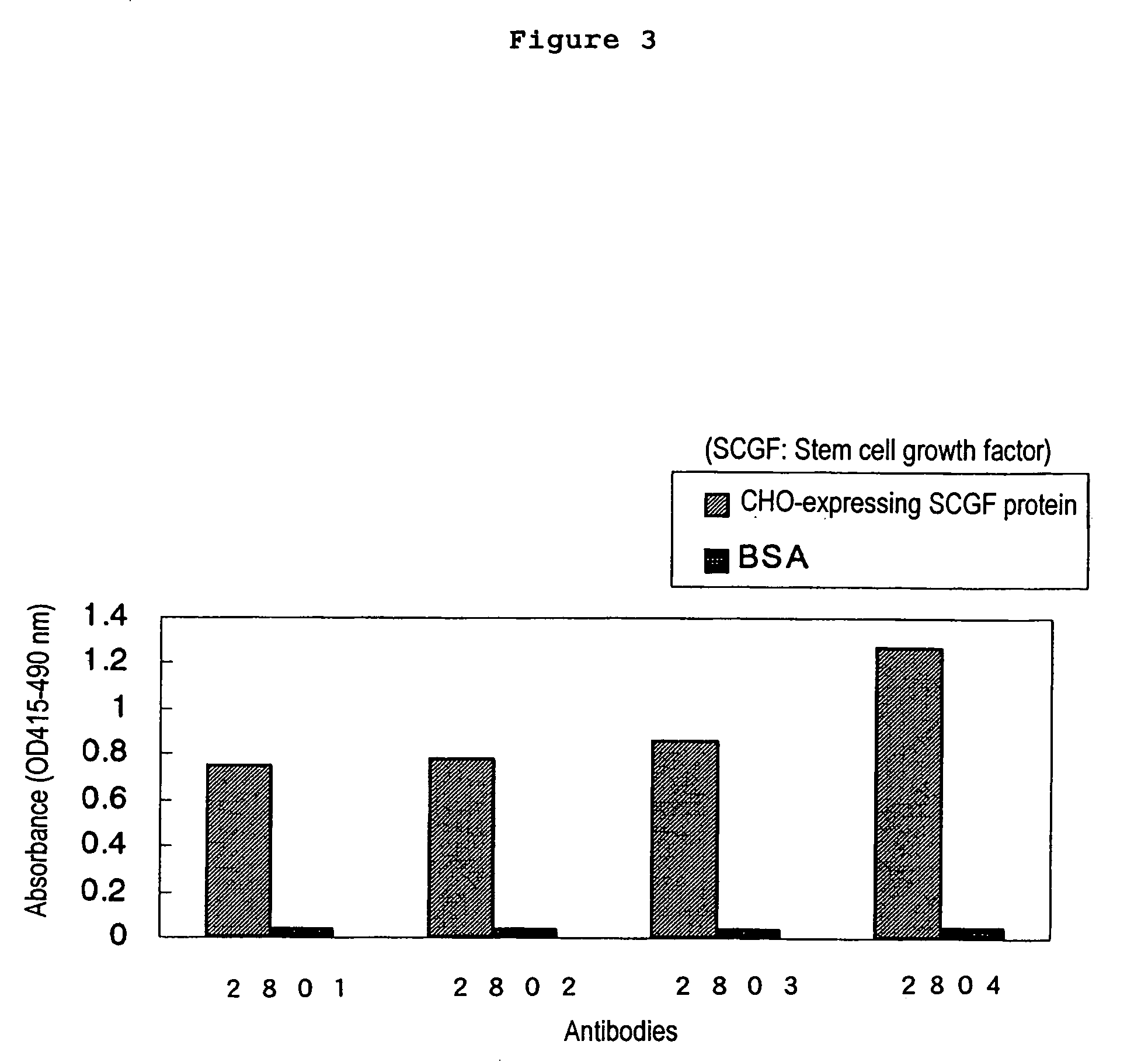
The present invention relates to a method for diagnosing leukemia, pre-leukemia or aleukemic malignant blood diseases, a method of discriminating leukemia from pre-leukemia or aleukemic malignant blood diseases, a method of discriminating aplastic anemia from myelodysplastic syndrome, a method of diagnosing delayed engraftment of the hematopoietic stem cells after transplantation of the hematopoietic stem cells, and a method of diagnosing the graft versus host disease, each of said methods comprising quantifying stem cell growth factor (SCGF). The present invention also makes it possible to provide an agent for diagnosing leukemia, pre-leukemia or aleukemic malignant blood diseases and an agent for diagnosing delayed engraftment of the hematopoietic stem cells after transplantation of the hematopoietic stem cells or an agent for diagnosing graft versus host disease (GVHD), each containing as an active ingredient an antibody reacting with stem cell growth factor (SCGF).
Owner:TOKAI UNIV +2
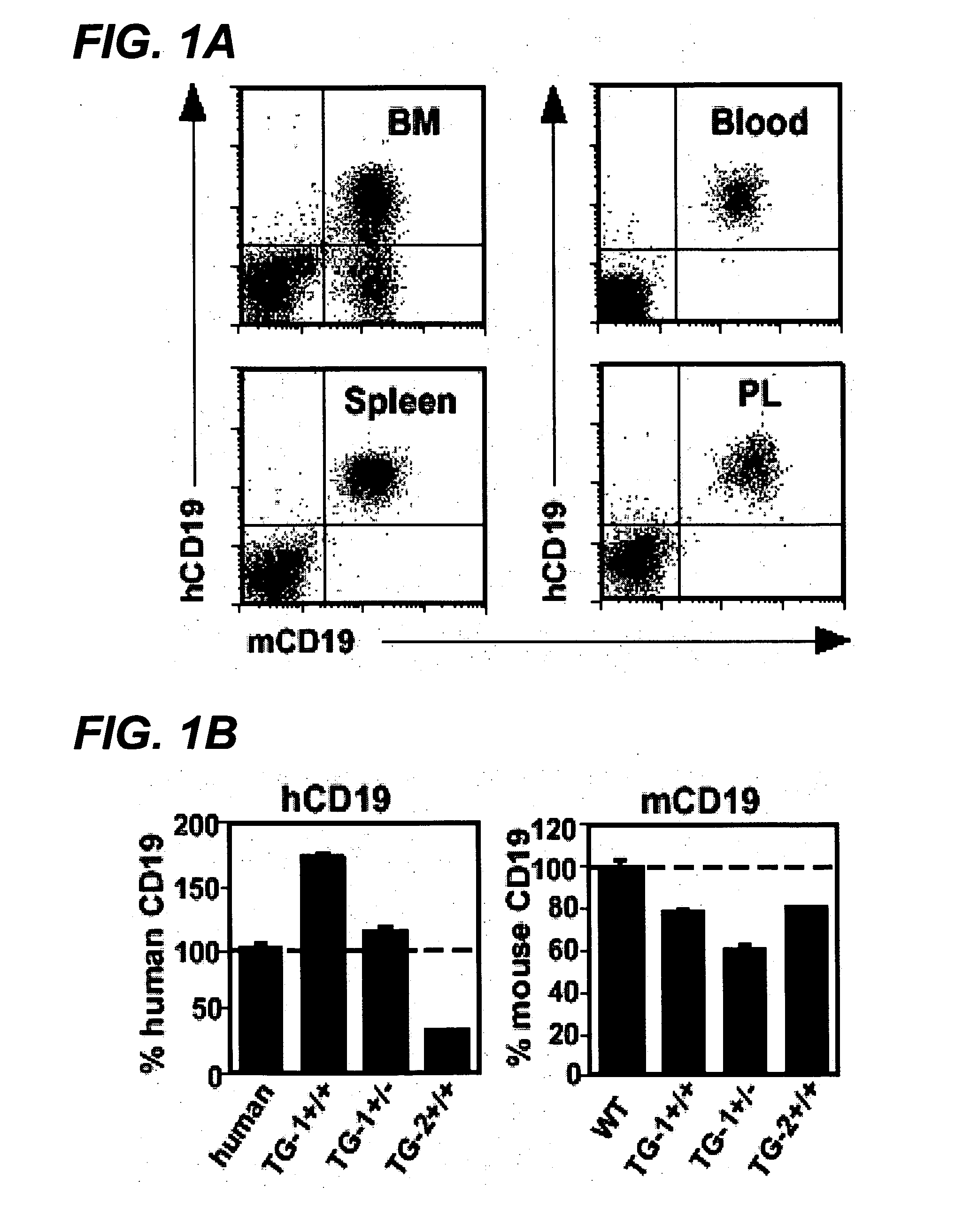
Anti-CD19 antibody therapy for transplantation
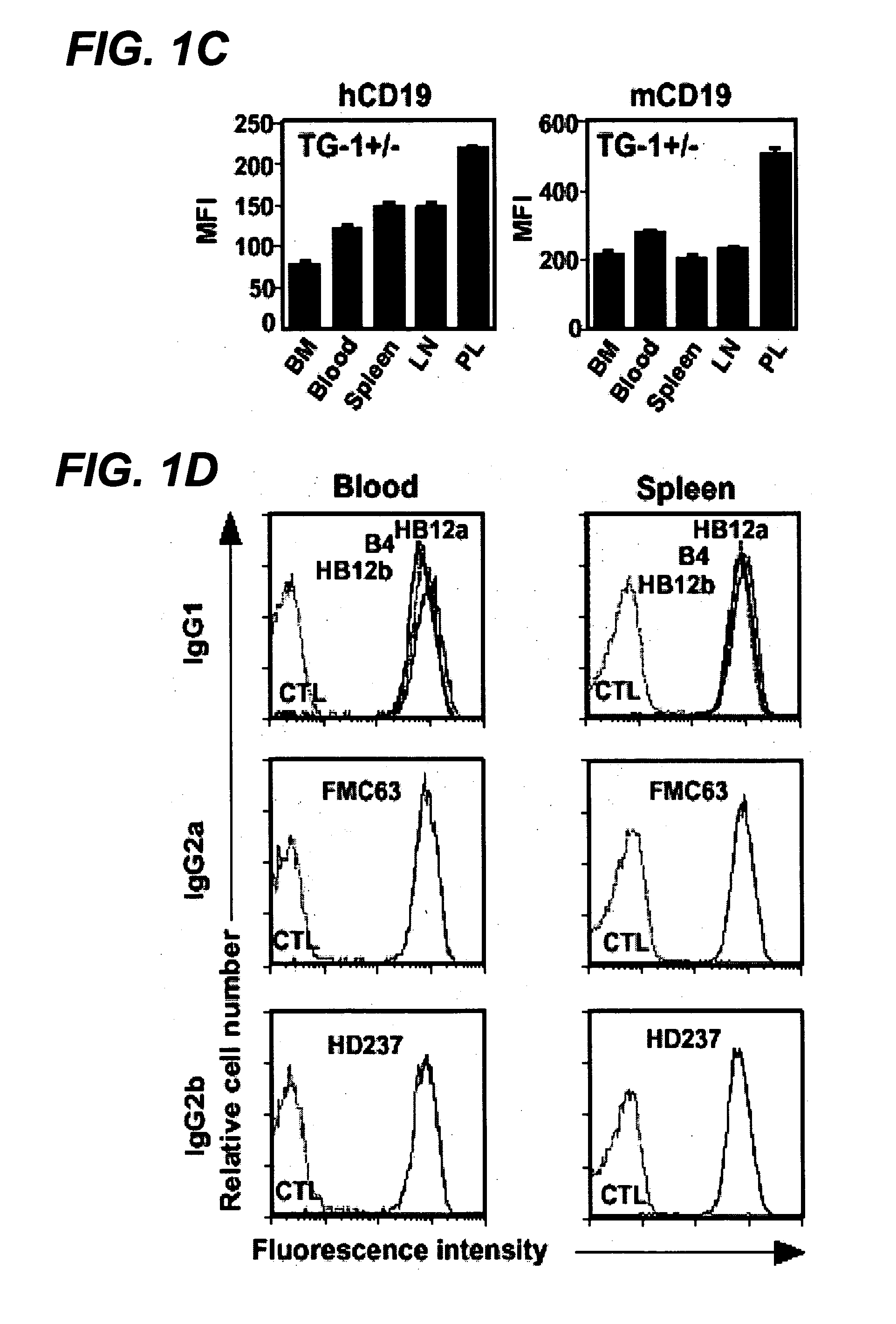
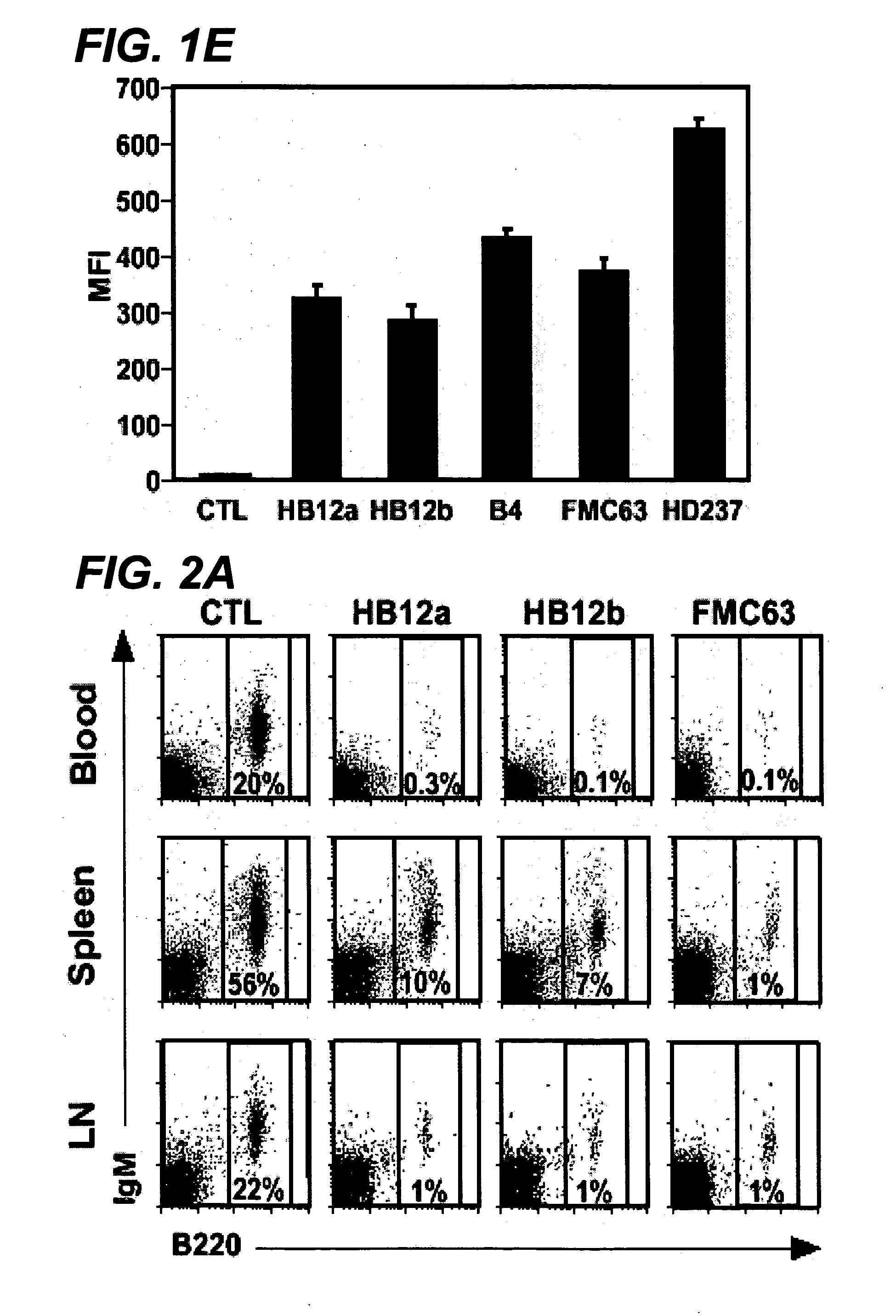
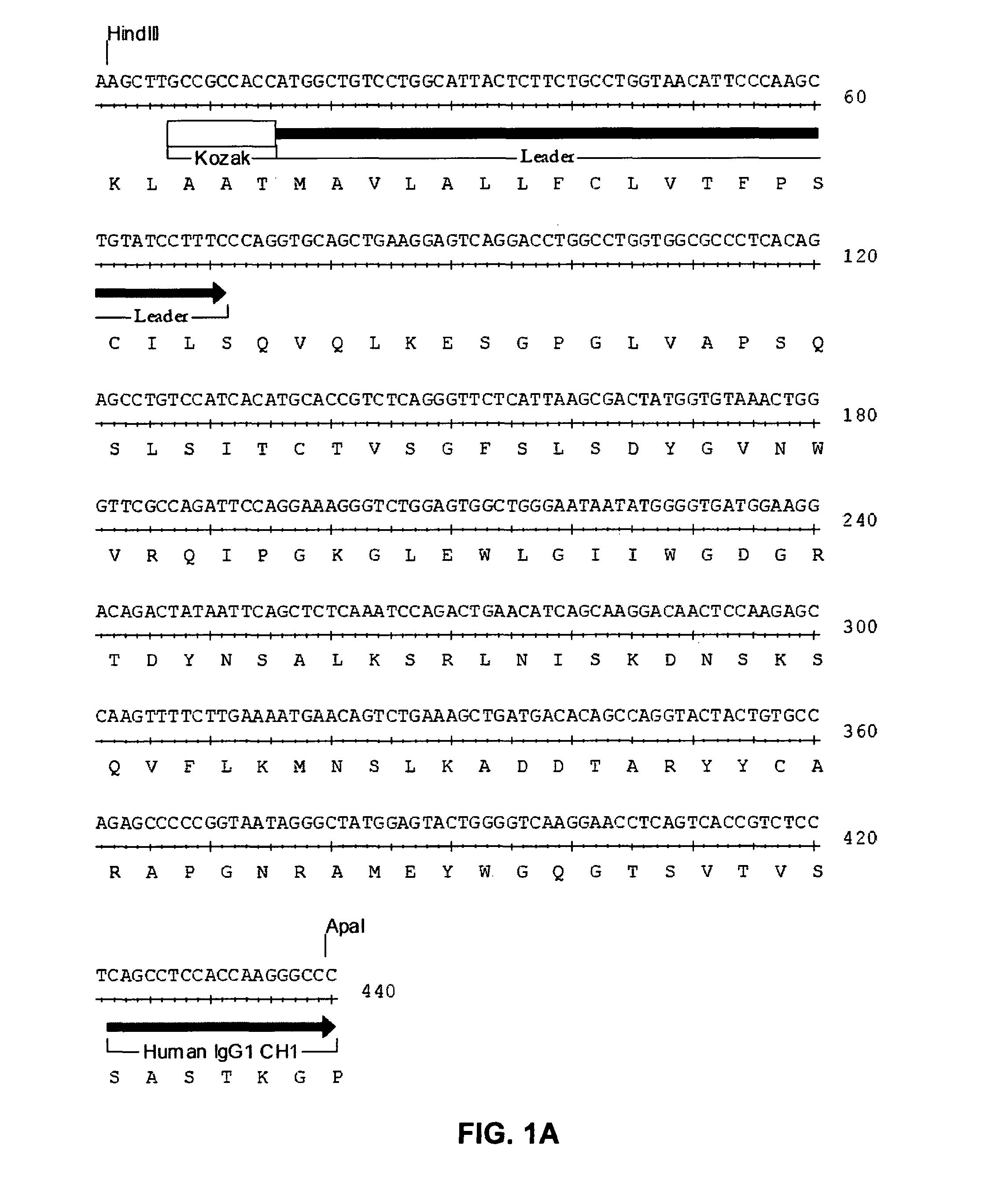
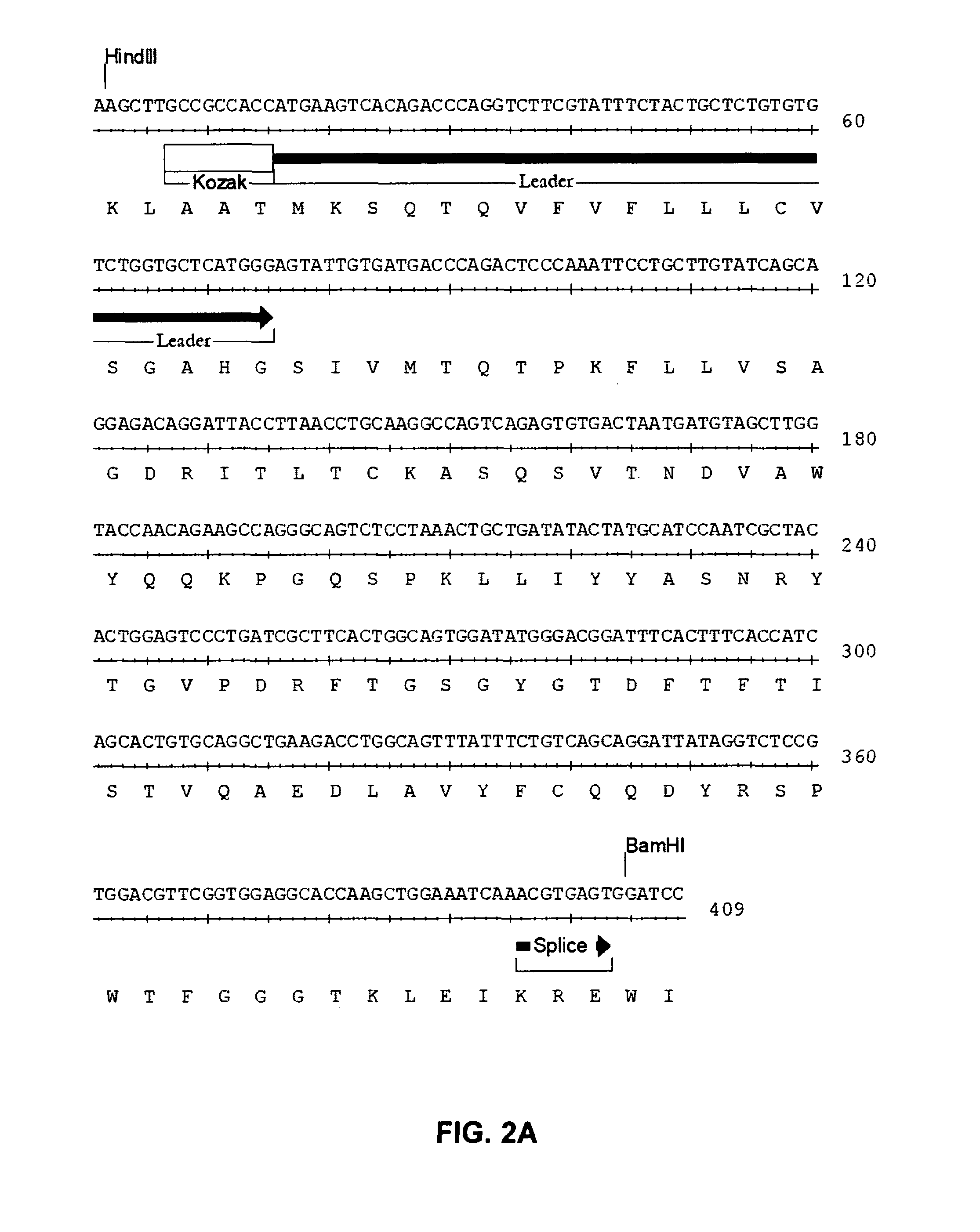
InactiveUS20060280738A1Efficient productionEfficiently depletedBiocidePhosphorous compound active ingredientsAntigenDisease
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD 19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
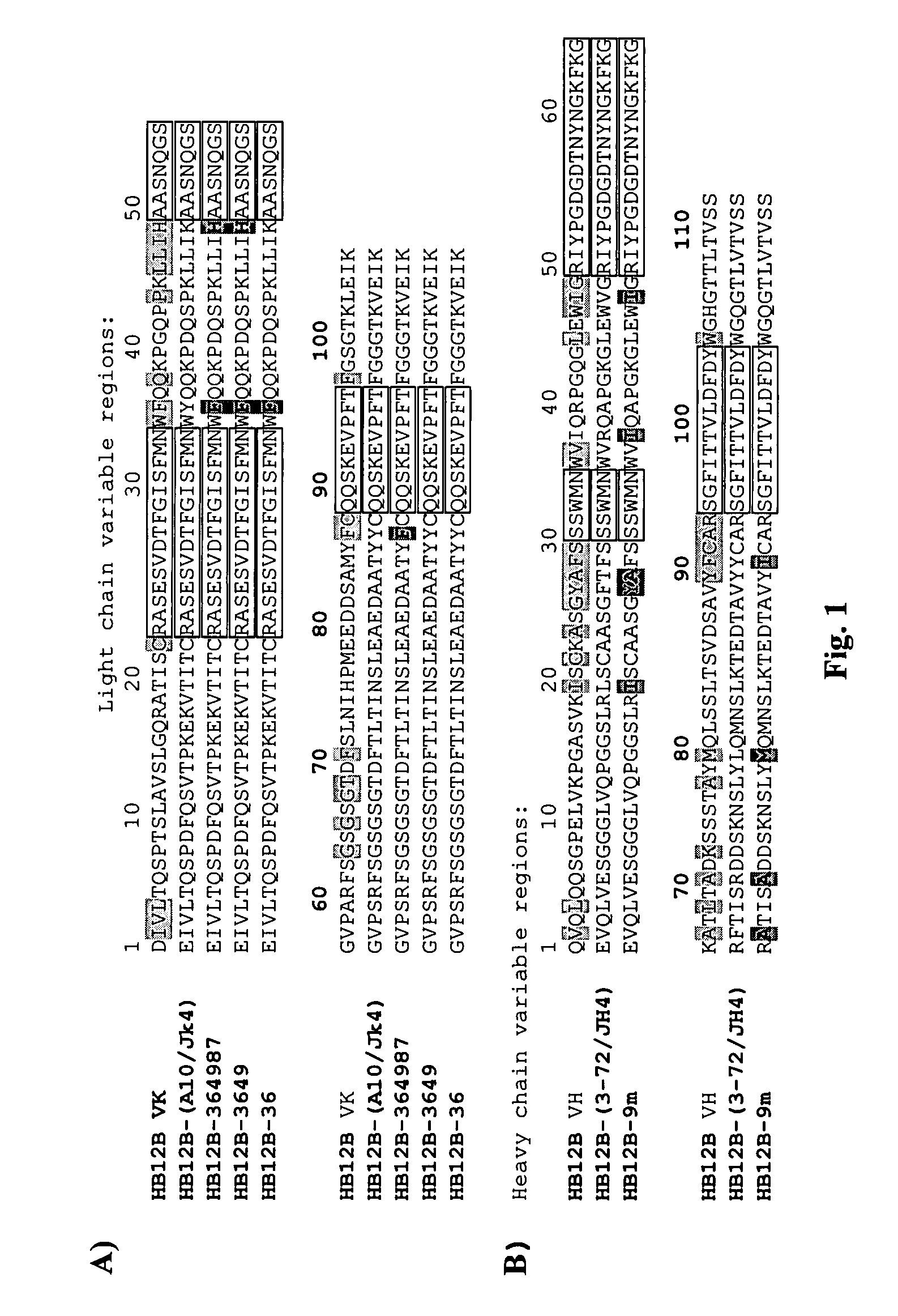
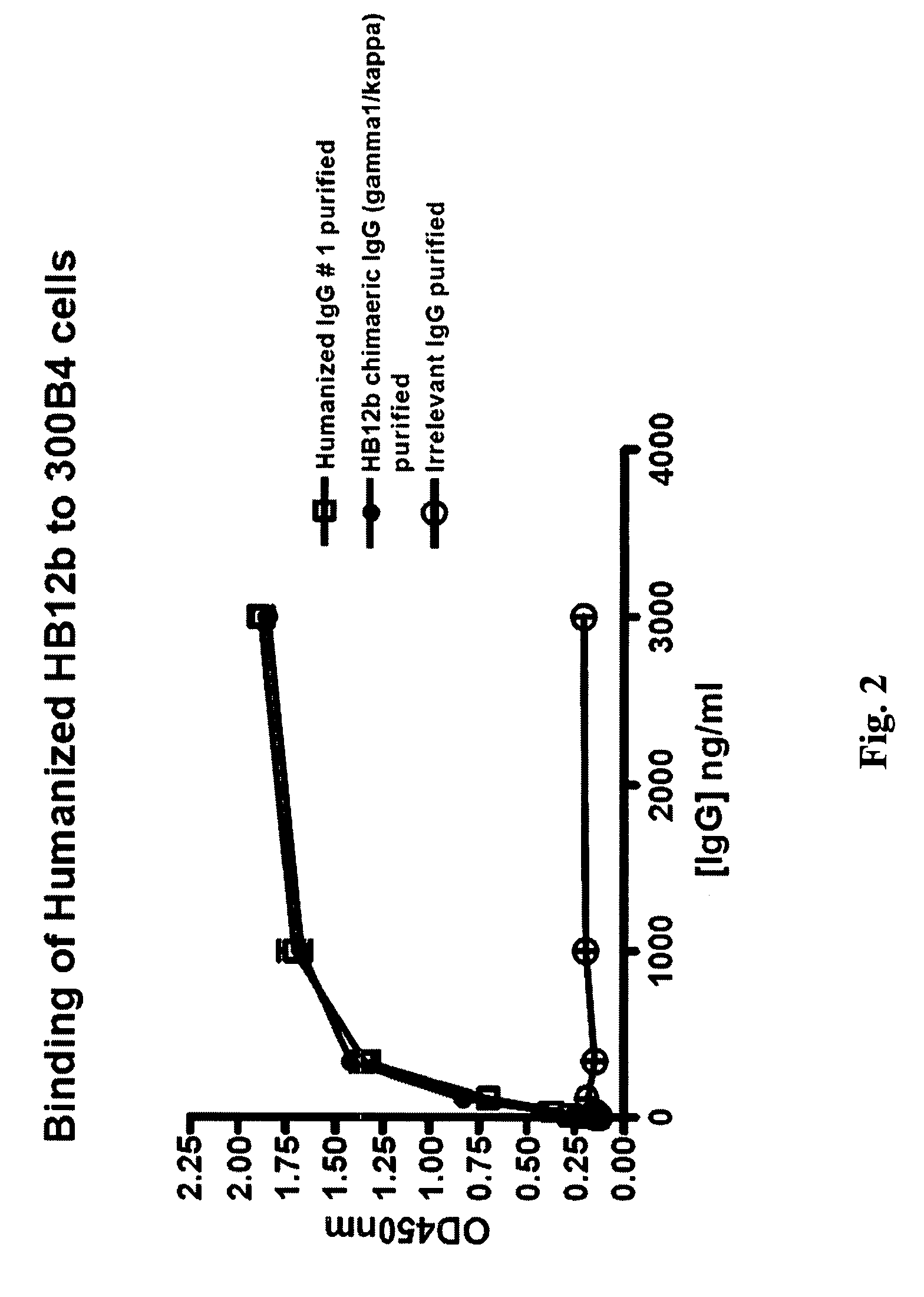
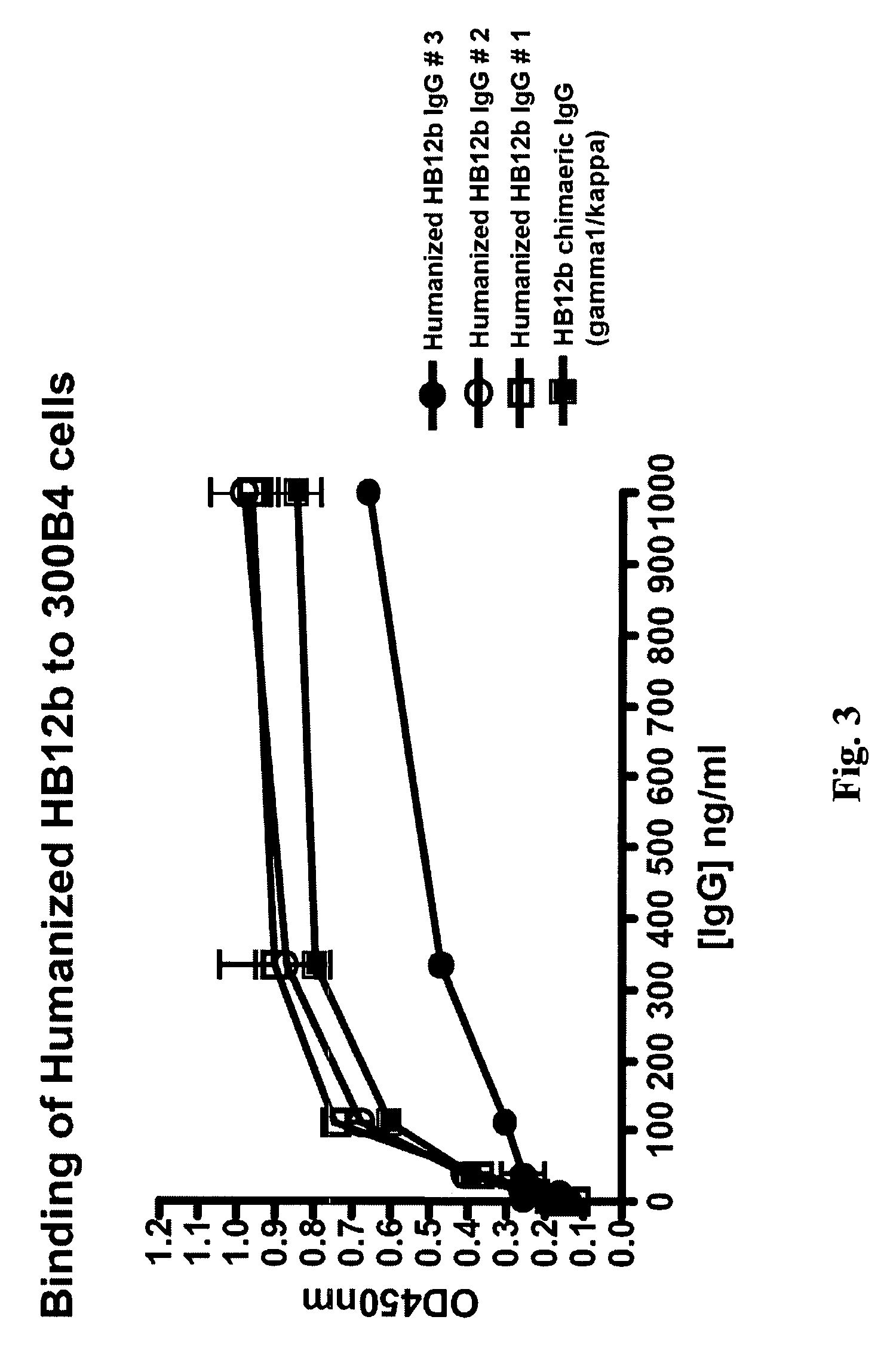
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Therapeutic agents for graft-versus-host disease comprising interleukin 6 receptor inhibitor as active ingredient
ActiveUS20100255007A1Organic active ingredientsAntibody ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention provides a novel therapeutic agent for graft-versus-host disease (GVHD).A therapeutic agent for graft-versus-host disease (GVHD), which comprises an interleukin 6 (IL-6) receptor inhibitor as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
Method for suppressing the development of graft-versus-host-disease by administering interleukin 6 receptor antibodies
ActiveUS8529895B2Organic active ingredientsAntibody ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention provides a novel therapeutic agent for graft-versus-host disease (GVHD).A therapeutic agent for graft-versus-host disease (GVHD), which comprises an interleukin 6 (IL-6) receptor inhibitor as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
Methods for treatment of acute lymphocytic leukemia
InactiveUS7026330B2Improved prognosisAvoid seizuresBiocideAntibody ingredientsChronic lymphocytic leukemiaAcute lymphocytic leukemia
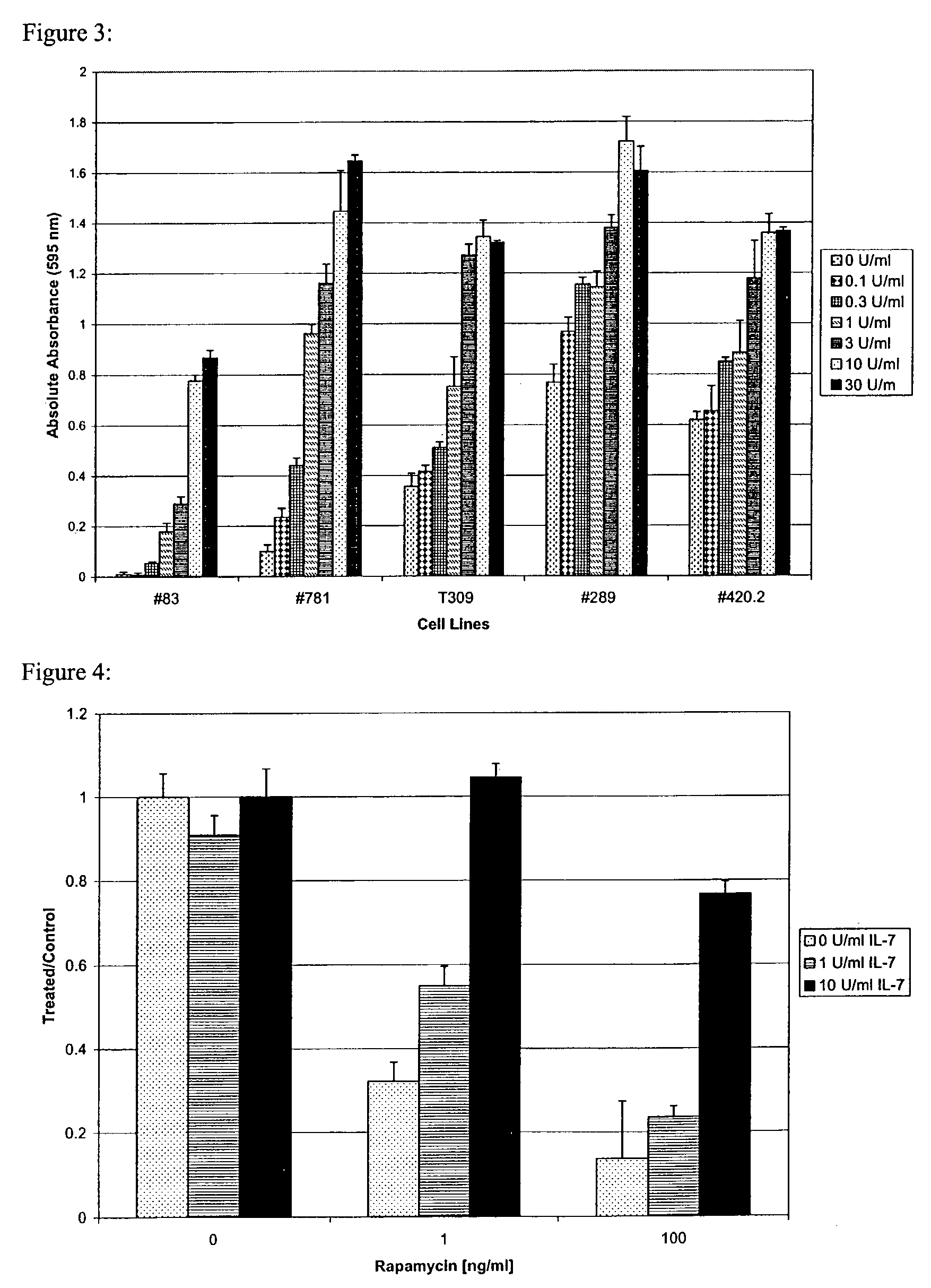
Methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof are provided. Also provided are methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof in combination with an IL-7 inhibitor. Finally methods for preventing GVHD in ALL patients following a bone marrow transplant are disclosed.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Humanized anti-CD19 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
ActiveUS8323653B2Alleviation and mitigation and decreaseSenses disorderNervous disorderAntigenApoptosis
Owner:VIELA BIO INC
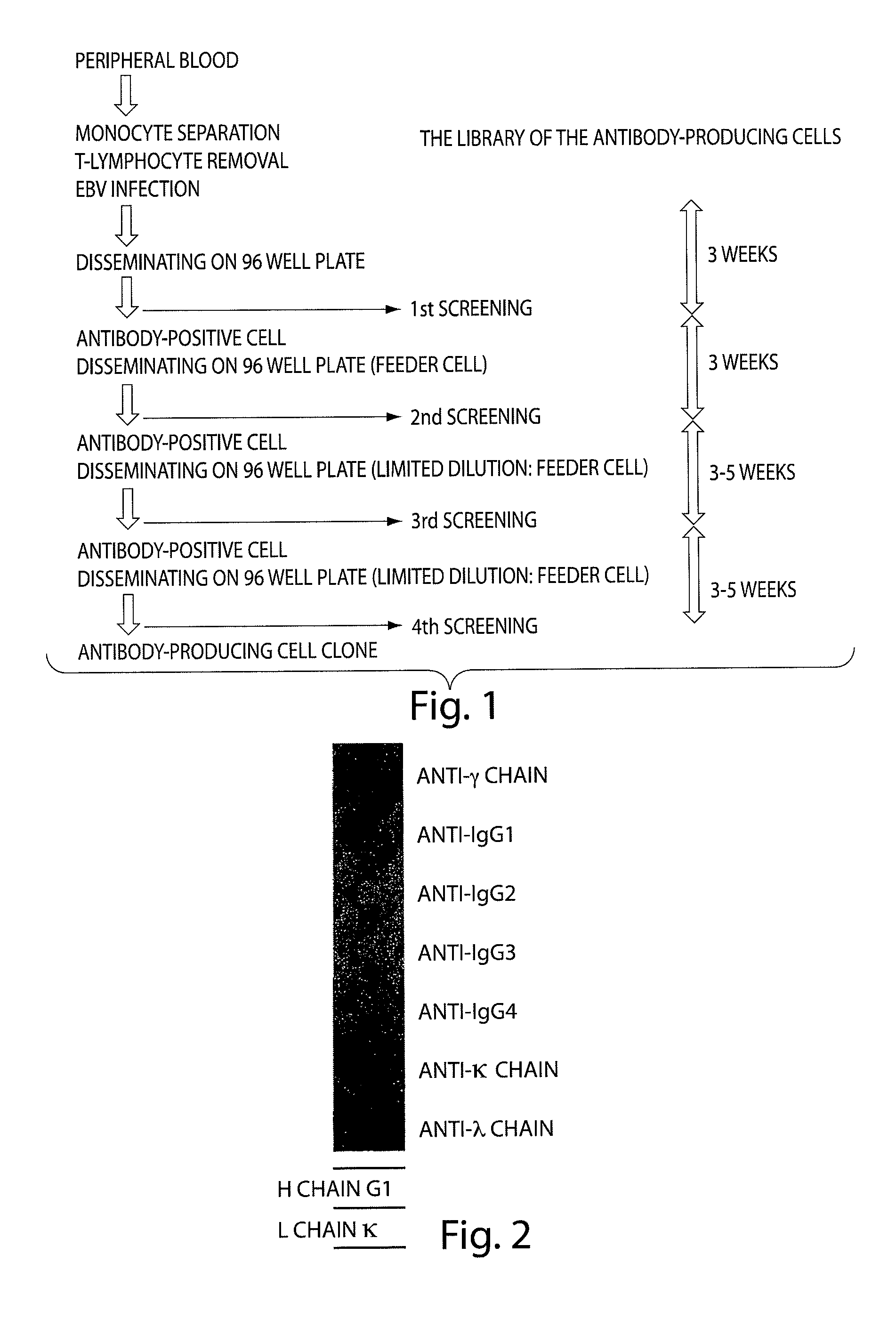
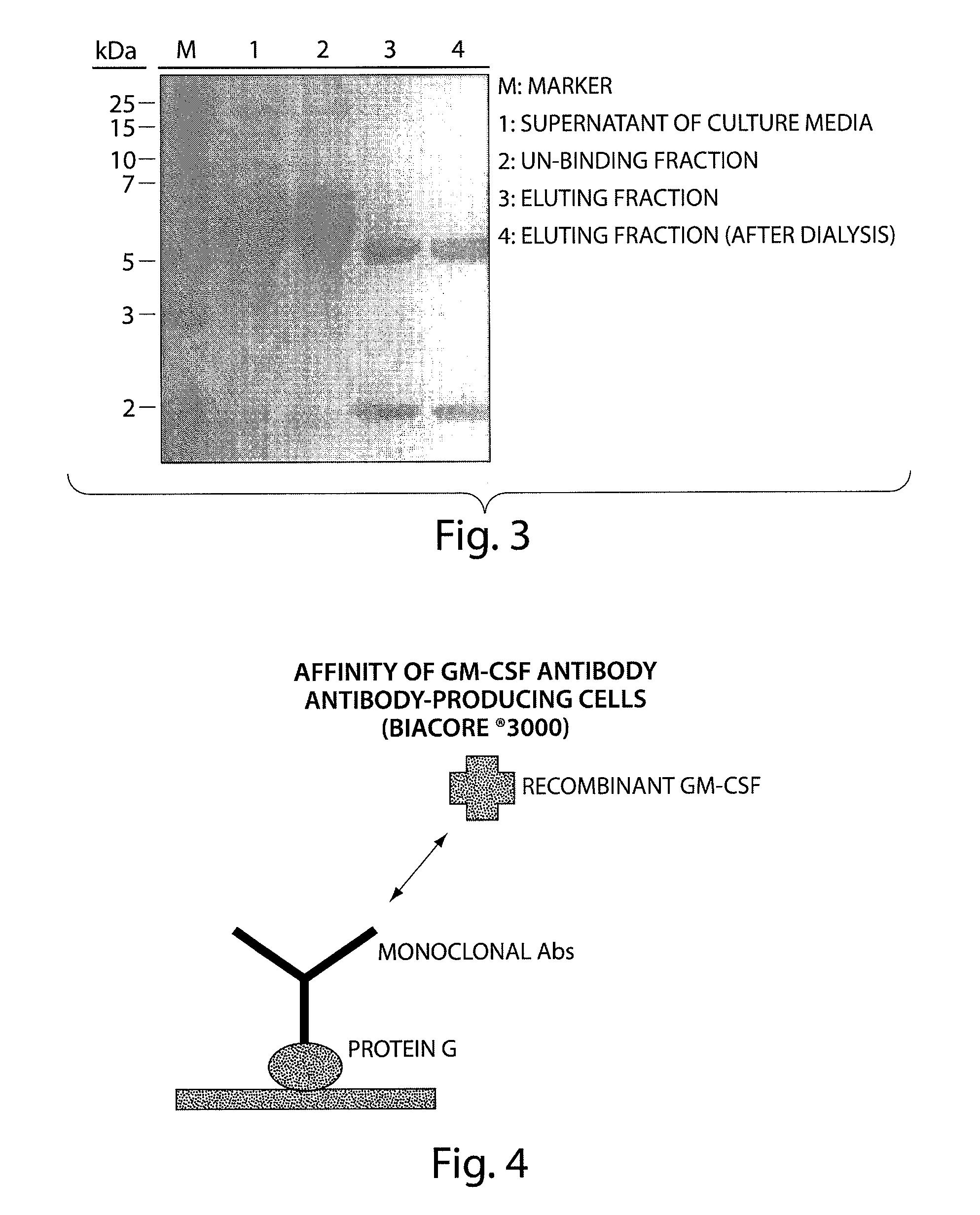
Human monoclonal antibody binding to hGM-CSF and its antigen binding portion
ActiveUS7935795B2High affinityPrevent proliferationSenses disorderAntipyreticComplementarity determining regionAutoimmune disease
The present invention provides a human monoclonal antibody, and antigen-binding portions thereof, capable of binding to human granulocyte-macrophage colony stimulating factor (hGM-CSF) and neutralizing the bioactivity of the hGM-CSF, wherein the anti-hGM-CSF monoclonal antibody has a light chain (L chain) including an amino acid sequence SEQ ID NO:1 and a heavy chain (H chain) including an amino acid sequence SEQ ID NO: 2. Also provided are human monoclonal anti-hGM-CSF antibodies, and antigen-binding portions thereof, characterized by complementarity determining regions (CDRs) or H chain and L chain variable regions related to SEQ ID NO:1 and SEQ ID NO:2. Antibodies, and antigen-binding portions thereof, of the invention are useful in the treatment of diseases associated with overproduction of hGM-CSF, including allergic disease, graft rejection and graft-versus-host disease (GVHD), and autoimmune diseases.
Owner:EVEC
Therapeutic apoptotic cell preparations, method for producing same and uses thereof
ActiveUS20150275175A1Reduce morbidityReduced incidence of hepatotoxicityBiocideOrganic active ingredientsInflammatory Bowel DiseasesGraft versus host disease induction
The present application provides pharmaceutical compositions comprising a population of mononuclear-enriched cells in an early-apoptotic state, methods for the production of said compositions and uses thereof in the treatment of diseases characterized by pathological immune responses. The pharmaceutical compositions may be used in treatment of conditions such as, but not limited to, graft versus host disease (GVHD) and autoimmune diseases including but not limited to inflammatory bowel disease, gout and arthritis.
Owner:ENLIVEX THERAPEUTICS R&D LTD
Function Inhibitor of Effector Cell
InactiveUS20070270429A1Inhibit functioningBiocideOrganic chemistryAutoimmune conditionIslet cell transplantation
The present invention relates to a function inhibitor of an effector cell comprising a CCR5 antagonist. Since the function inhibitor of an effector cell comprising a CCR5 antagonist inhibits the function of effector cells which play an important role in formation of diseases and the like, it is effective for the prevention and / or treatment of, for example, a transplant rejection (e.g., rejection of a solid organ graft, rejection of islet cell transplantation in diabetes mellitus, graft-versus-host disease (GVHD), etc.), an autoimmune disease (e.g., arthritis, rheumatoid arthritis, multiple sclerosis, ulcerative colitis, etc.), an allergic disease (e.g., asthma, etc.), an ischemic disease (e.g., ischemia-reperfusion injury, etc.), cancer or a metastatic disease or the like.
Owner:ONO PHARMA CO LTD
Anti-cd19 antibody therapy for transplantation
InactiveUS20090246195A1Reduce or deplete circulating B cellsReduce or deplete circulating immunoglobulin (Ig)Dead animal preservationAntibody ingredientsAntigenTherapeutic antibody
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
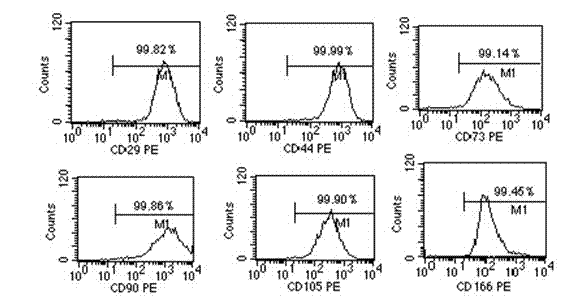
CD106-positive cells, and identification and preparation method and application thereof
ActiveCN102539736APrevent rejectionSuppress immune responseSkeletal/connective tissue cellsEmbryonic cellsAdipogenesisAutoimmune disease
The invention discloses an identification and preparation method and application CD106-positive mesenchymal stem cells. The method comprises the following steps: identifying and isolating CD106-positive cells from mononuclear cells isolated from bone marrow, placenta or other tissues through an immunological method, carrying out adherent culture, and collecting adherent CD106-positive cells; or carrying out adherent culture of mononuclear cells isolated from bone marrow, placenta or other tissues, collecting adherent mesenchymal cells, and isolating the CD106-positive mesenchymal stem cells when the count of the obtained cells is large enough. The CD106-positive cells are mesenchymal stem cells with adherent growth, express mesenchymal stem cell-related immunological phenotype, and have osteogenesis, adipogenesis and other differentiation potencies. Compared with CD106-negative mesenchymal stem cells, the CD106-positive cells have higher immunosuppression capacity, can better inhibit proliferation of IFN-gamma (interferon-gamma) secretion of lymphocytes, and can be used for treating graft versus host disease (GVHD) and autoimmune diseases.
Owner:北京汉氏干细胞科技有限公司
Freeze-drying composition of posaconazole prodrug and preparation method and application of freeze-drying composition of posaconazole prodrug
InactiveCN105287403AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliverySolubilityCase fatality rate
The invention relates to a freeze-drying composition of a posaconazole prodrug and a preparation method and application of the freeze-drying composition of the posaconazole prodrug. The freeze-drying composition has the advantages that the freeze-drying composition is high in water solubility, and safety of the freeze-drying composition is guaranteed due to the fact that cyclodextrins auxiliary materials need not to be added during the preparation of the freeze-drying composition; the freeze-drying composition is suitable for being used for treating various amphotericin-intolerant or refractory adult invasive fungal infections; the freeze-drying composition is used as a preventive drug for high-risk patients, the freeze-drying composition is applicable to patients above 13 years old and with impaired immunity and especially applicable to patients who have graft versus host disease (GVHD) after hematopoietic stem cell transplant, patients with leukemia and patients with long-term leukopenia due to chemotherapy; compared with control drugs such as fluconazole and itraconazole, the freeze-drying composition can effectively prevent invasive aspergillosis and can lower the mortality related to the invasive fungal infections.
Owner:HC SYNTHETIC PHARMA CO LTD
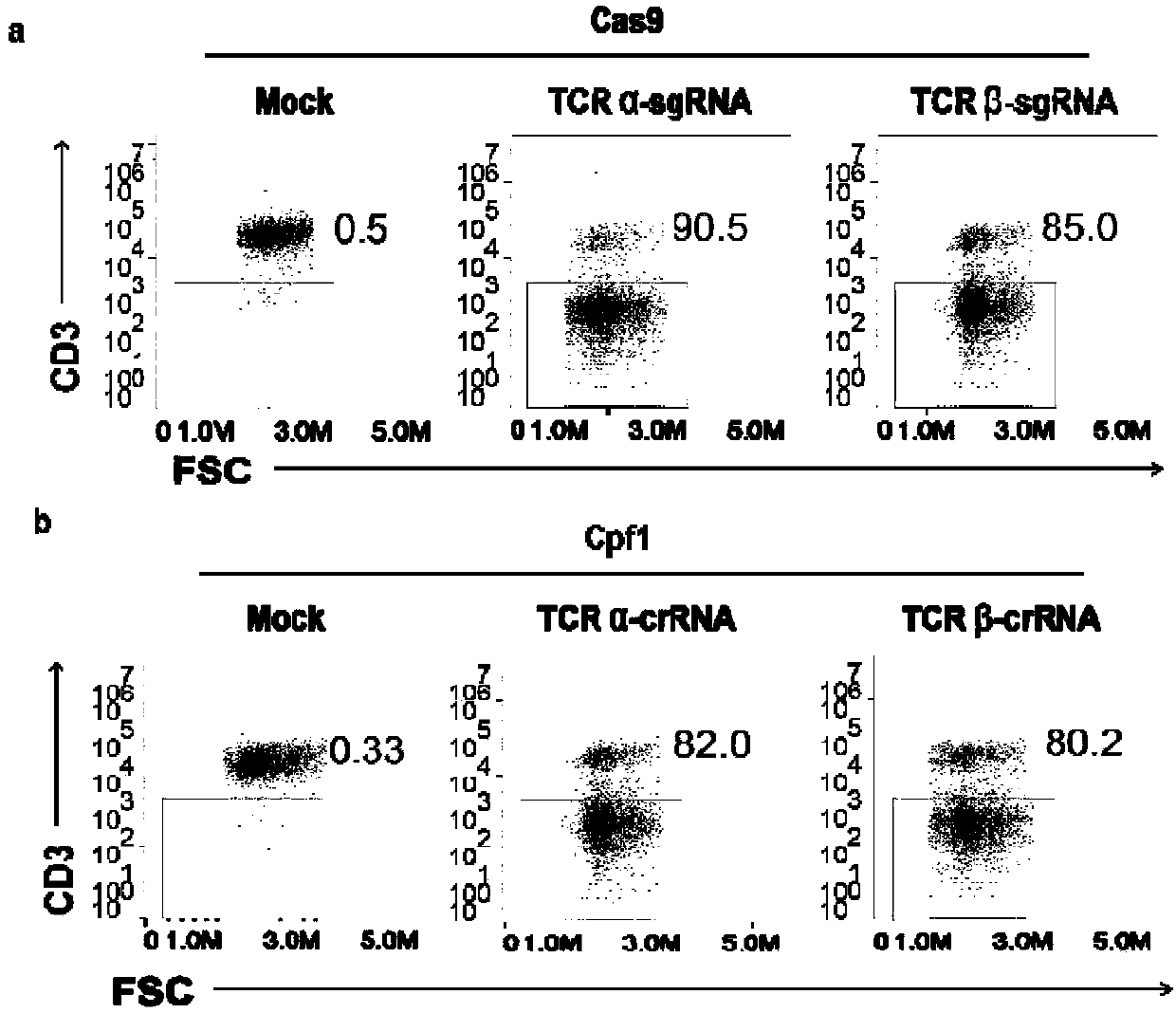
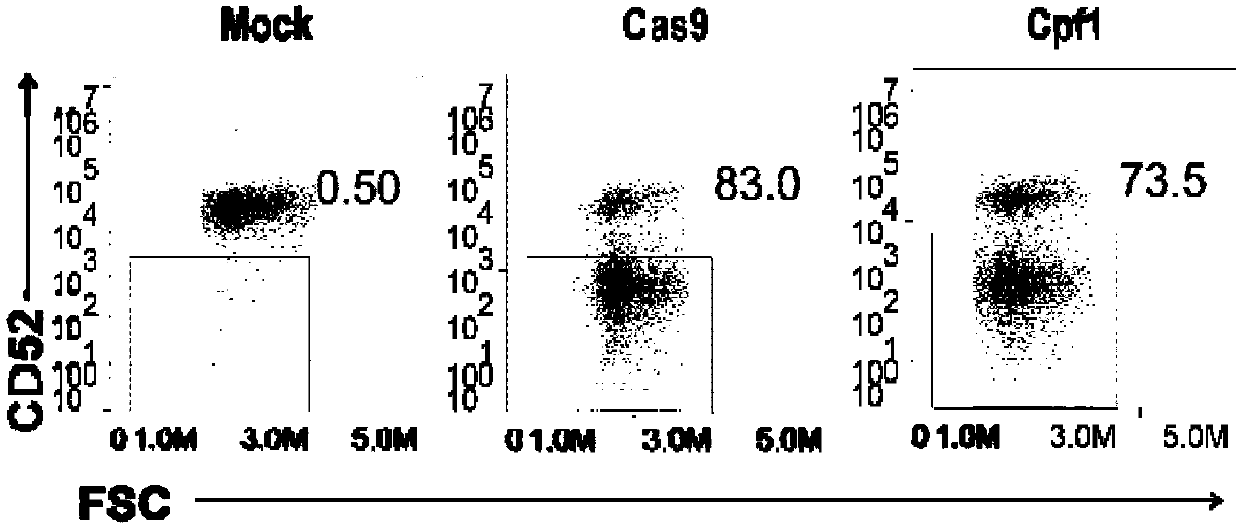
General type CART/TCRT cells with antibody drug resistance as well as construction method of general type CART/TCRT cells
ActiveCN107828730APreserve targetingAvoid exclusionImmunoglobulin superfamilyMammal material medical ingredientsAntigenAntigen receptors
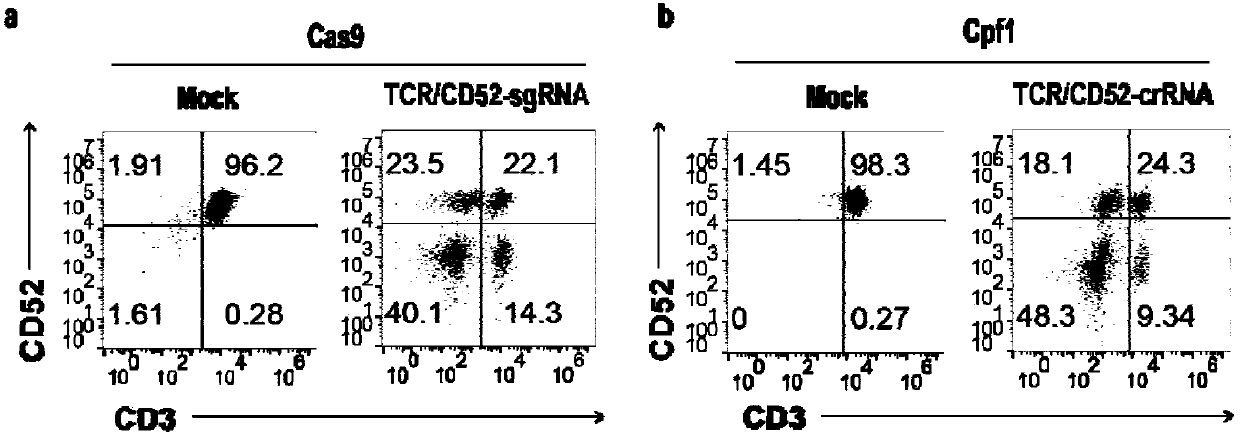
The invention belongs to the fields of genetic engineering and synthetic biology, and in particular relates to general type CART / TCRT cells with antibody drug resistance as well as a construction method of the general type CART / TCRT cells. The general type CART / TCRT cells are allogeneic T cells having chimeric antigen receptors and T cell receptors, and alpha and beta chains and CD52 molecules ofthe T cell receptors are knocked out; a technology for knocking out the alpha and beta chains and the CD52 molecules of the T cell receptors is a CRISPR technology; the general type CART / TCRT cells not only retain the targeting of tumor specific antigen, but also eliminate the problems of GvHD and rejection, and weaken the sensitivity to an antibody drug Alemtuzumab; the general type CART / TCRT cells can be used as a general type simple, low-cost and high-activity CART / TCRT cell preparation.
Owner:NANJING BIOHENG BIOTECH CO LTD
Steroid sparing agents and methods of using same
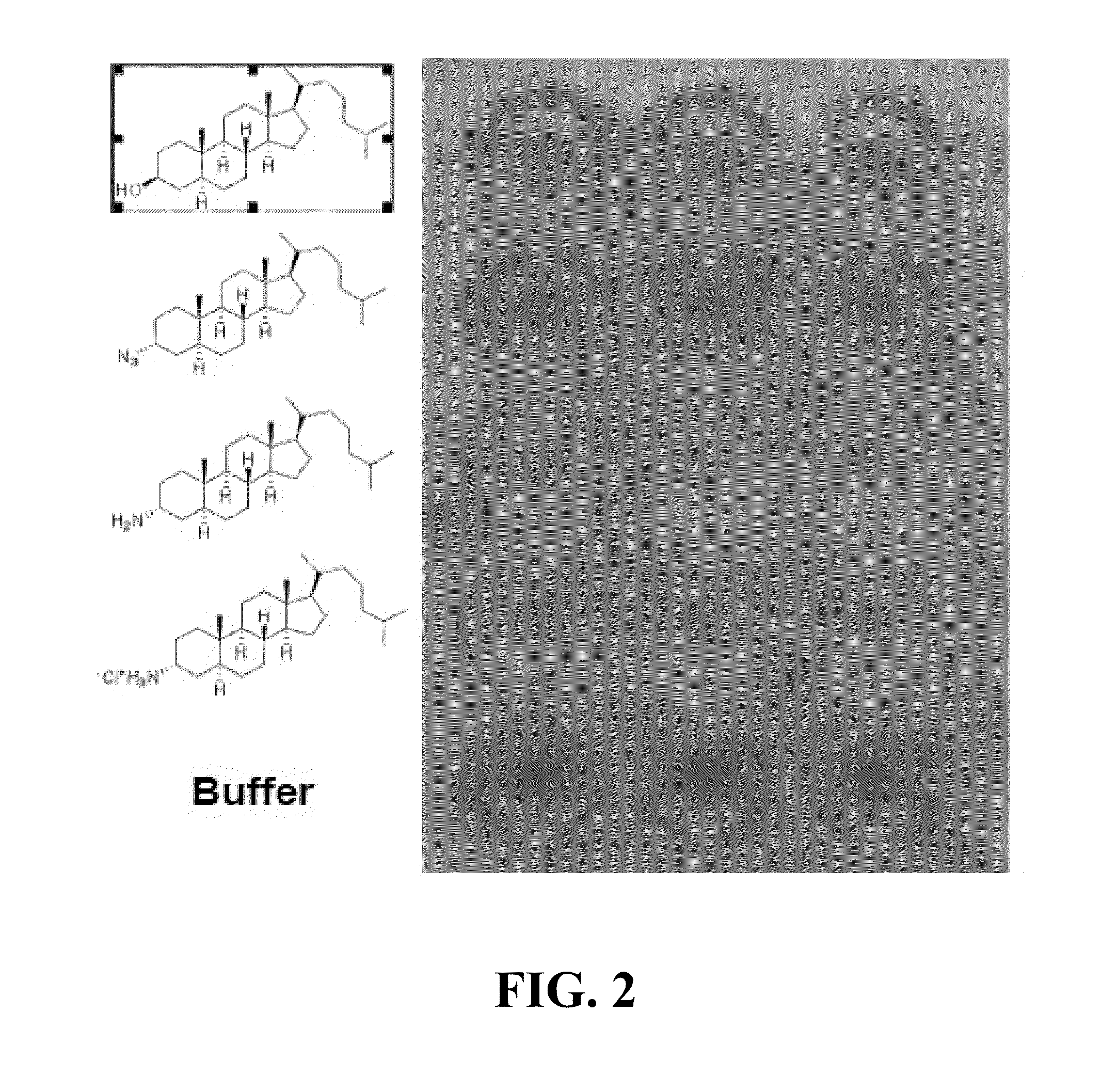
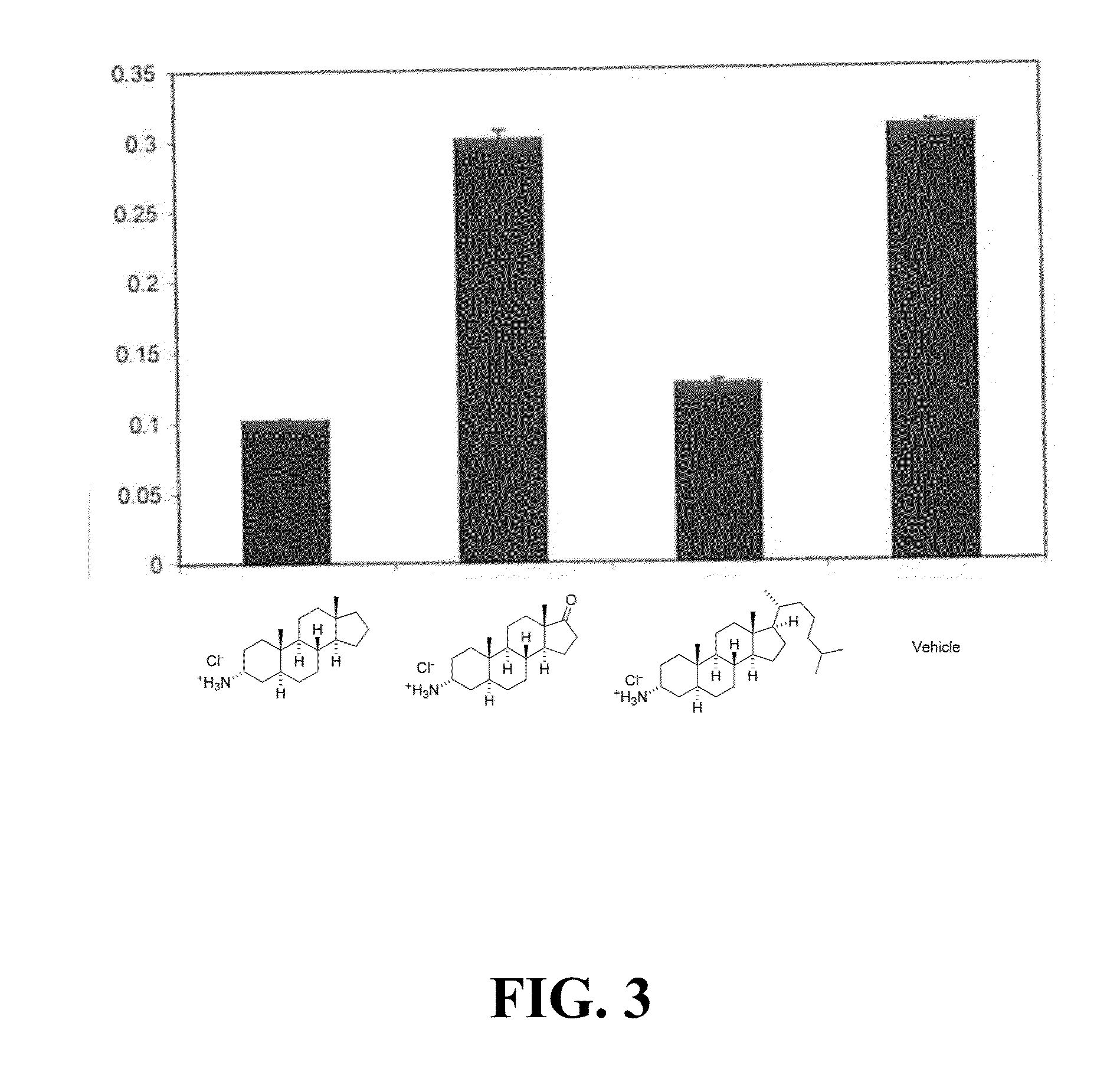
InactiveUS20050260193A1Effective treatmentInhibition is effectiveNervous disorderAntipyreticCombination therapyIntestinal GVHD
This invention relates generally to methods of treating inflammatory bowel diseases (IBD), asthma, Crohn's Disease (CD), multiple sclerosis (MS), rheumatoid arthritis (RA), graft versus host disease (GVHD), host versus graft disease, and various spondyloarthropathies, comprising administering a steroid-sparing effective amount of an immunoglobulin or small molecule composition to a patient in need thereof. The invention also relates generally to combination therapies for the treatment of these conditions.
Owner:BIOGEN IDEC MA INC
Therapeutic apoptotic cell preparations, method for producing same and uses thereof
ActiveUS10077426B2Reduce morbidityReduced incidence of hepatotoxicityOrganic active ingredientsMammal material medical ingredientsAutoimmune conditionInflammatory Bowel Diseases
The present application provides pharmaceutical compositions comprising a population of mononuclear-enriched cells in an early-apoptotic state, methods for the production of said compositions and uses thereof in the treatment of diseases characterized by pathological immune responses. The pharmaceutical compositions may be used in treatment of conditions such as, but not limited to, graft versus host disease (GVHD) and autoimmune diseases including but not limited to inflammatory bowel disease, gout and arthritis.
Owner:ENLIVEX THERAPEUTICS R&D LTD
Anti-CD26 antibodies and uses thereof
ActiveUS9376498B2Engraftment can be facilitatedImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAplastic bone marrowIntestinal GVHD
The present invention pertains to novel antibodies capable of binding to CD26, as well as to their use as a medicament. Moreover, the present invention provides antibodies for use in treating and / or preventing Graft-versus-Host Disease (GvHD), for use in treating Aplastic Anemia and / or for use in promoting engraftment after haematopoietic stem cell transplantation. Furthermore, the present invention provides pharmaceutical compositions comprising at least one antibody of the present invention, as well as provides a kit of parts.
Owner:ADIENNE SA
Umbilical cord mesenchymal stem cell injection liquid, preparation method and application thereof
InactiveCN106237313AGreat differentiation potentialImprove proliferative abilityOrganic active ingredientsPeptide/protein ingredientsVitamin CHuman albumin
The invention relates to the technical field of stem cells and especially relates to an umbilical cord mesenchymal stem cell injection liquid, a preparation method and an application thereof. The injection liquid comprises umbilical cord mesenchymal stem cells, human albumin, low molecular heparin calcium, vitamin C, panax japonicus majoris saponin and a solvent. The injection liquid is prepared from the umbilical cord mesenchymal stem cells, so that the materials are convenient to obtain and the method does not violate ethical issue. The injection liquid can effectively prolong preservative time of the cells and the preserved stem cells have high activity, so that the injection liquid is convenient to use. After refusion, the injection can promote differentiation and maturation of the mesenchymal stem cell to liver cells, thus reducing incidence rate of GVHD.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
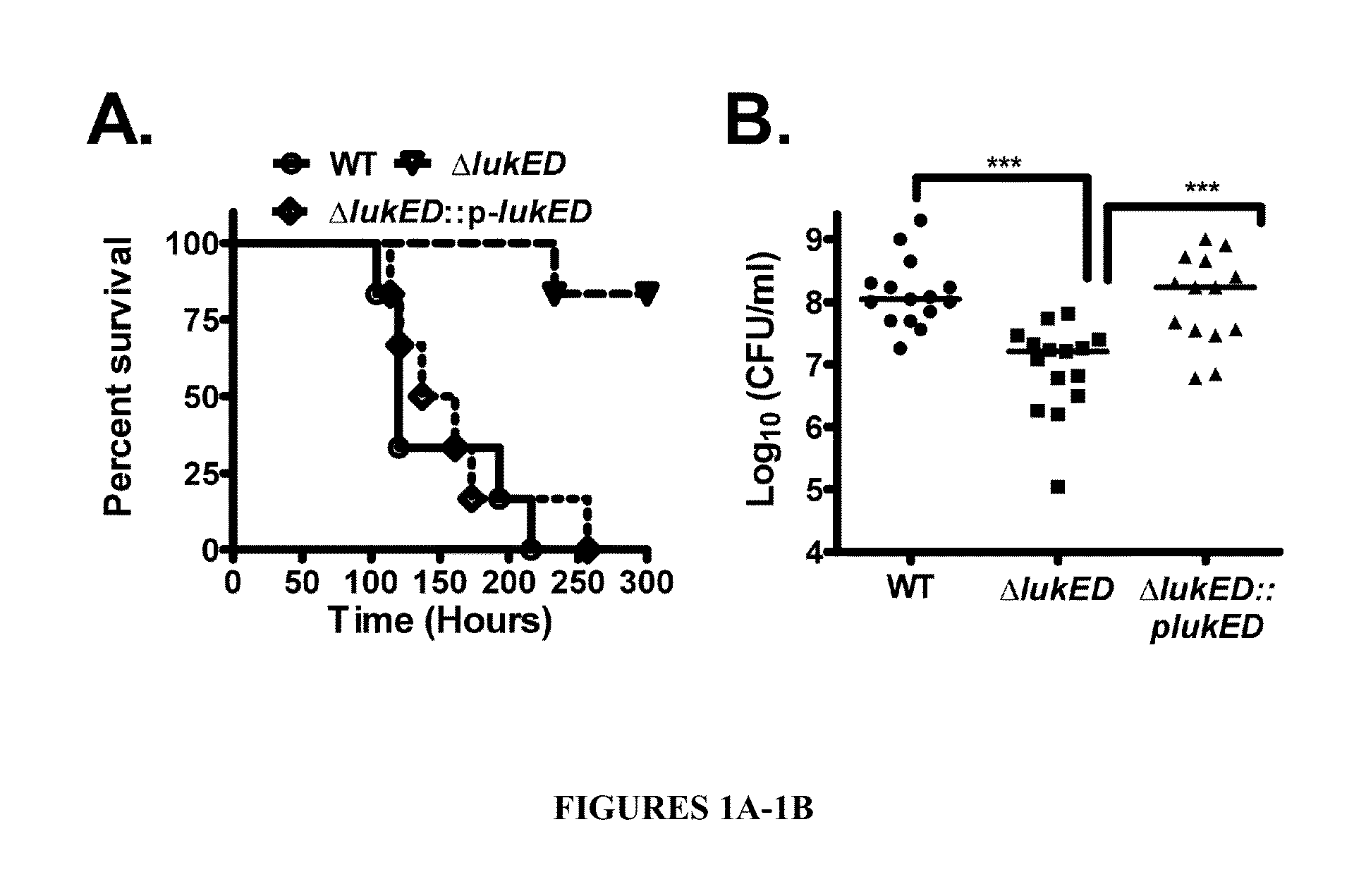
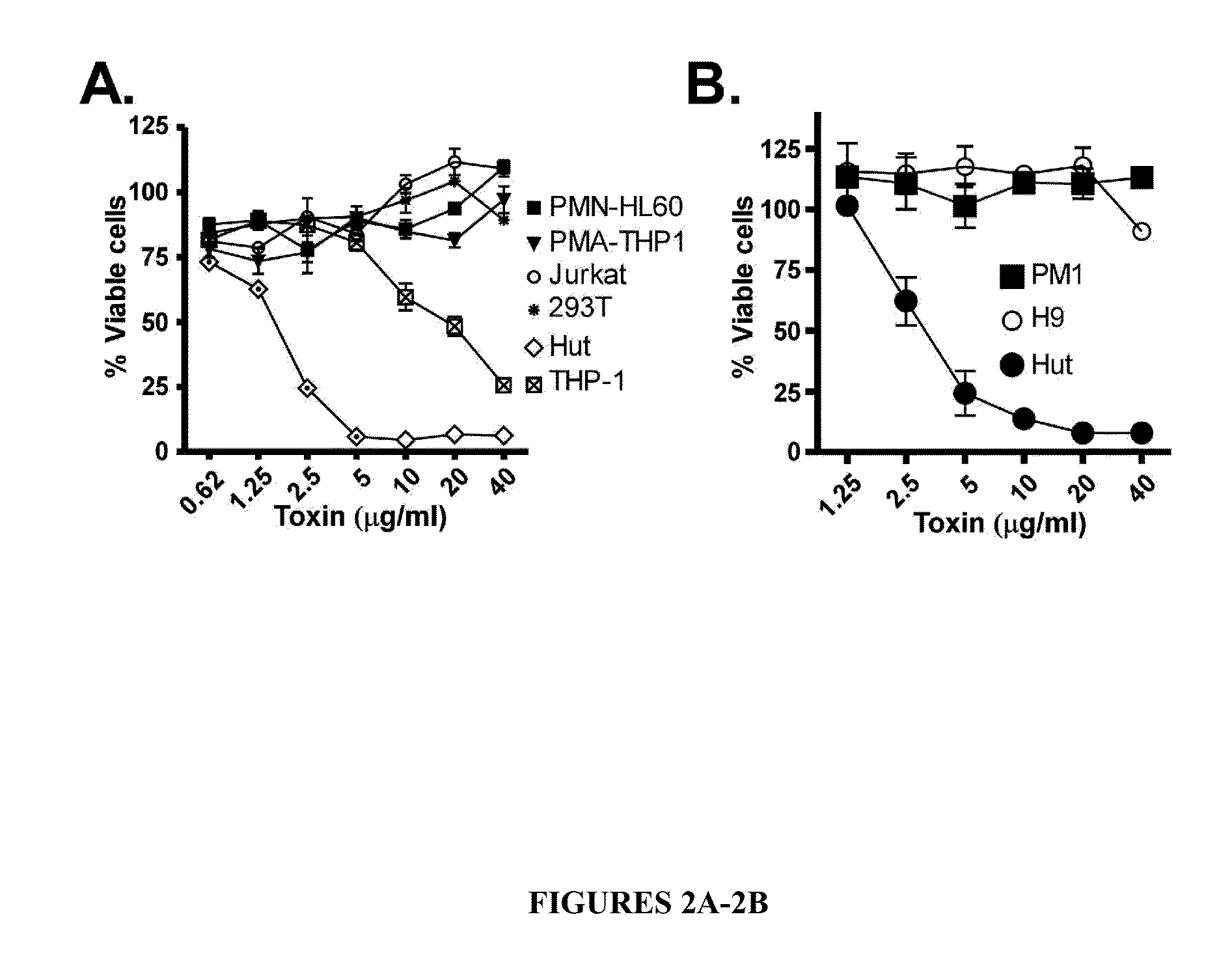
Leukotoxin e/d as a new Anti-inflammatory agent and microbicide
ActiveUS20130039885A1Superior therapeutic strategyAvoid spreadingBiocideNervous disorderStaphylococcus cohniiWhite blood cell
The present invention relates to methods for preventing or treating Human Immunodeficiency Virus (HIV) infection, inflammatory conditions, and graft-versus-host-disease (GVHD) in a subject. Therapeutic compositions of the present invention comprise Leukocidin E (LukE) and / or D proteins or polypeptides. The invention further relates to methods of treating Staphylococcus aureus infection by administering a composition comprising a CCR5 antagonist or any molecule that blocks LukE / D interaction with CCR5+ cells in an amount effective to treat the S. aureus infection in the subject.
Owner:NEW YORK UNIV
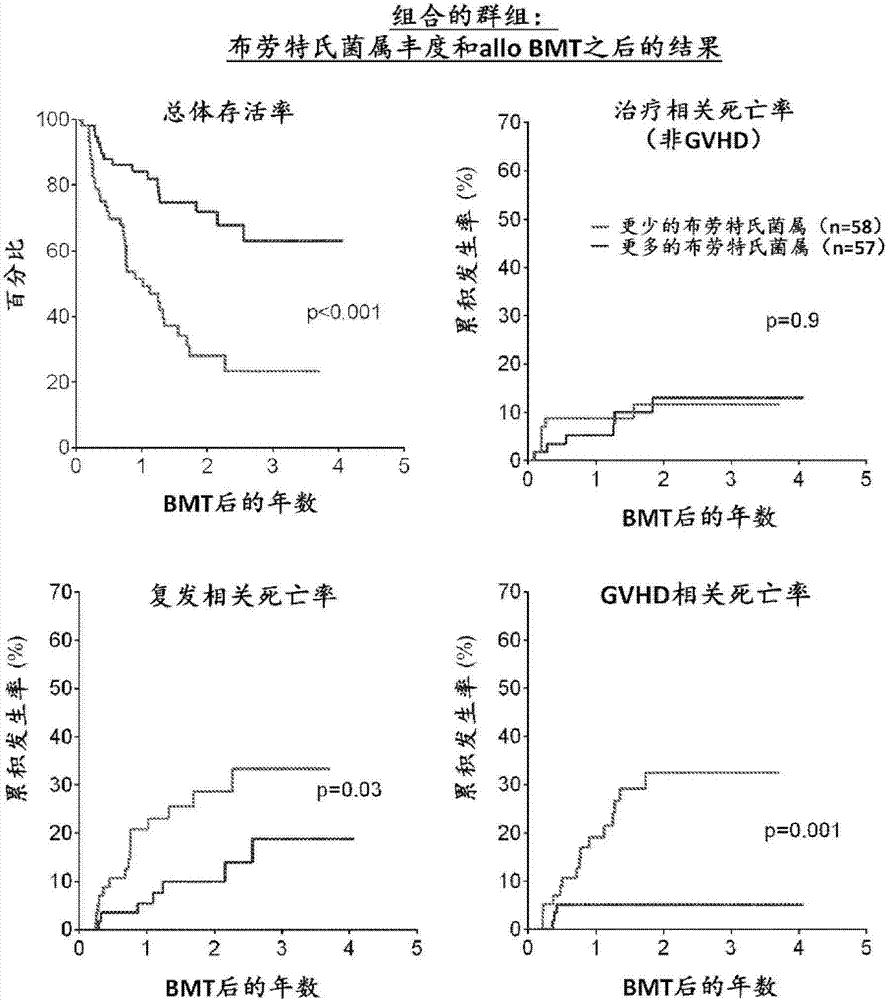
Intestinal microbiota and gvhd
PendingCN107249609AReduce abundanceRisk reduction or eliminationBacteria material medical ingredientsMicrobiological testing/measurementTreatment choicesSymbiotic bacteria
The present disclosure describes compositions and methods for increasing the abundance of commensal bacteria belonging to the order Clostridiales, including Blautia, Ruminococcus, Clostridium, Eubacterium, Holdemania and Dorea species, that are as-sociated with reduced lethal GVHD and improved overall survival following bone mar-row or hematopoietic stem cell transplant. The present disclosure, therefore, provides methods for reducing the likelihood, incidence or severity of GVHD by (1) avoiding the loss of endogenous beneficial species through antibiotic selection; (2) by administering a therapeutically effective amount of a composition comprising one or more Clostridiales associated with reduced GVHD to individuals who may lack or have lost those strains from their intestinal microbiota. Additionally, support for endogenous or reestablished Clostridiales related to reduced GVHD as a treatment option for reducing GVHD can also be provided in the form of nutritional supplementation, for example, sugars fermented by some species of Clostridiales with GVHD reducing activity.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Ship inhibitors and uses thereof
ActiveUS20130102577A1Additional componentEngraftment can be facilitatedOrganic active ingredientsBiocideLymphatic SpreadGackstroemia
The present invention relates to SHIP inhibitor compounds and methods for using these compounds. In particular, the present invention discloses the following methods: (i) a method of treating graft versus host disease in a subject; (ii) a method of inhibiting a SHIP1 protein in a cell; (iii) a method of selectively inhibiting a SHIP1 protein in a cell; (iv) a method for treating or preventing graft-versus-host disease (GVHD) in a recipient of an organ or tissue transplant; (v) a method of modulating SHIP activity in a cell expressing SHIP1 or SHIP2; (vi) a method of ex vivo or in vitro treatment of transplants; (vii) a method of inhibiting tumor growth and metastasis in a subject; (viii) a method of treating a hematologic malignancy in a subject; (ix) a method of inducing apoptosis of multiple myeloma cells; (x) a method of treating multiple myeloma in a subject; (xi) a method of inhibiting the proliferation of a human breast cancer cell; and (xii) a method of treating breast cancer in a subject.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
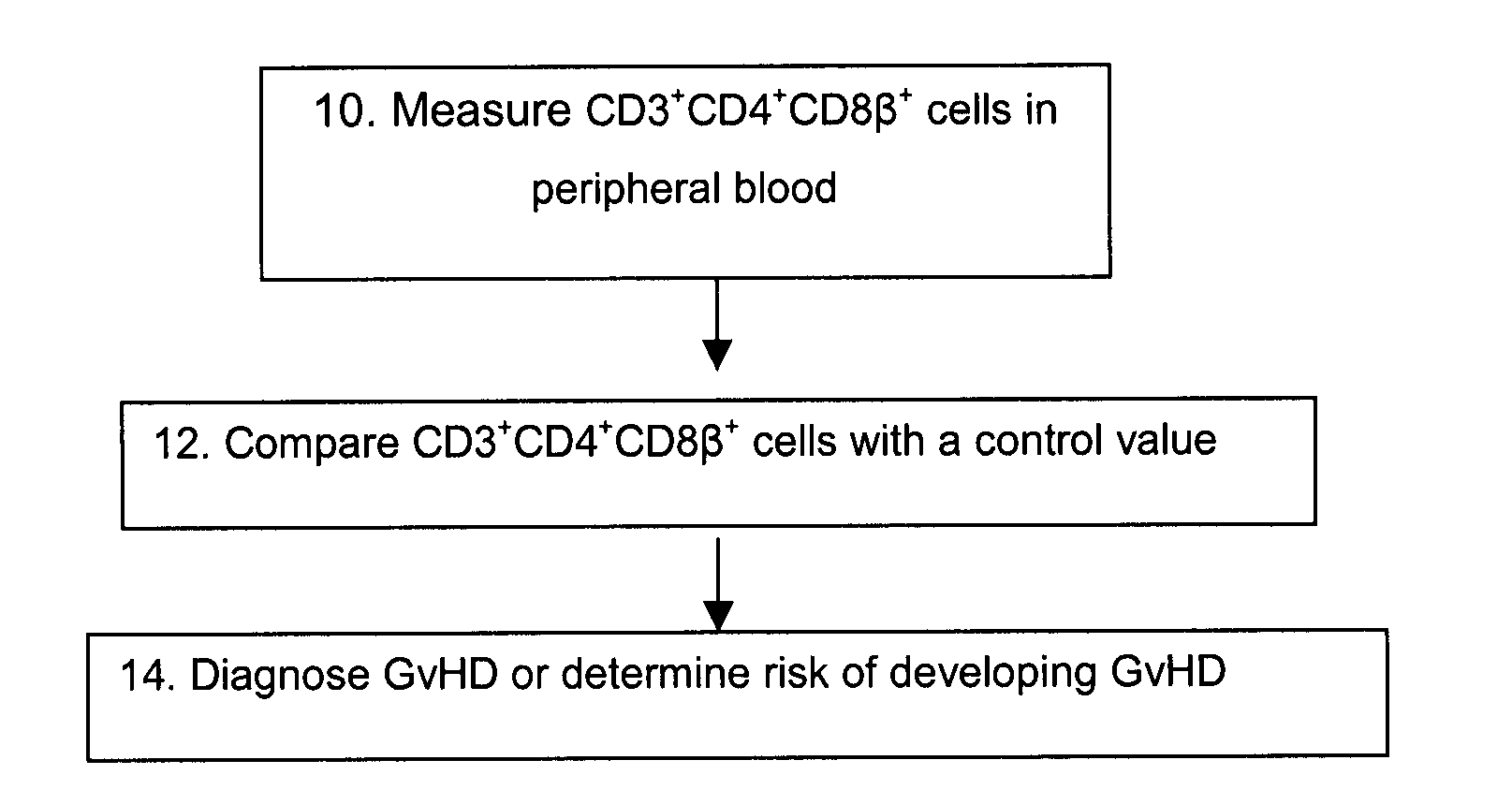
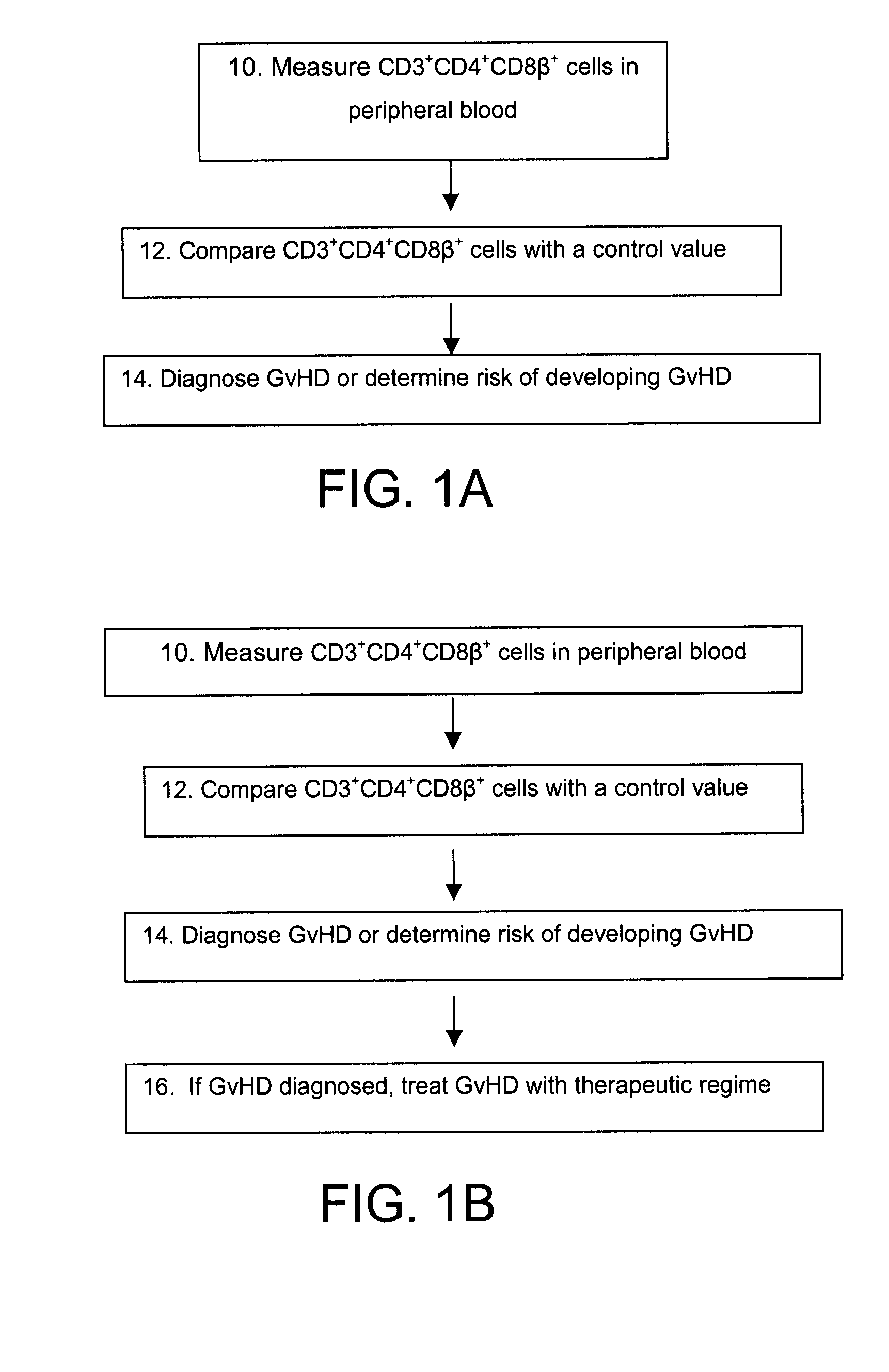
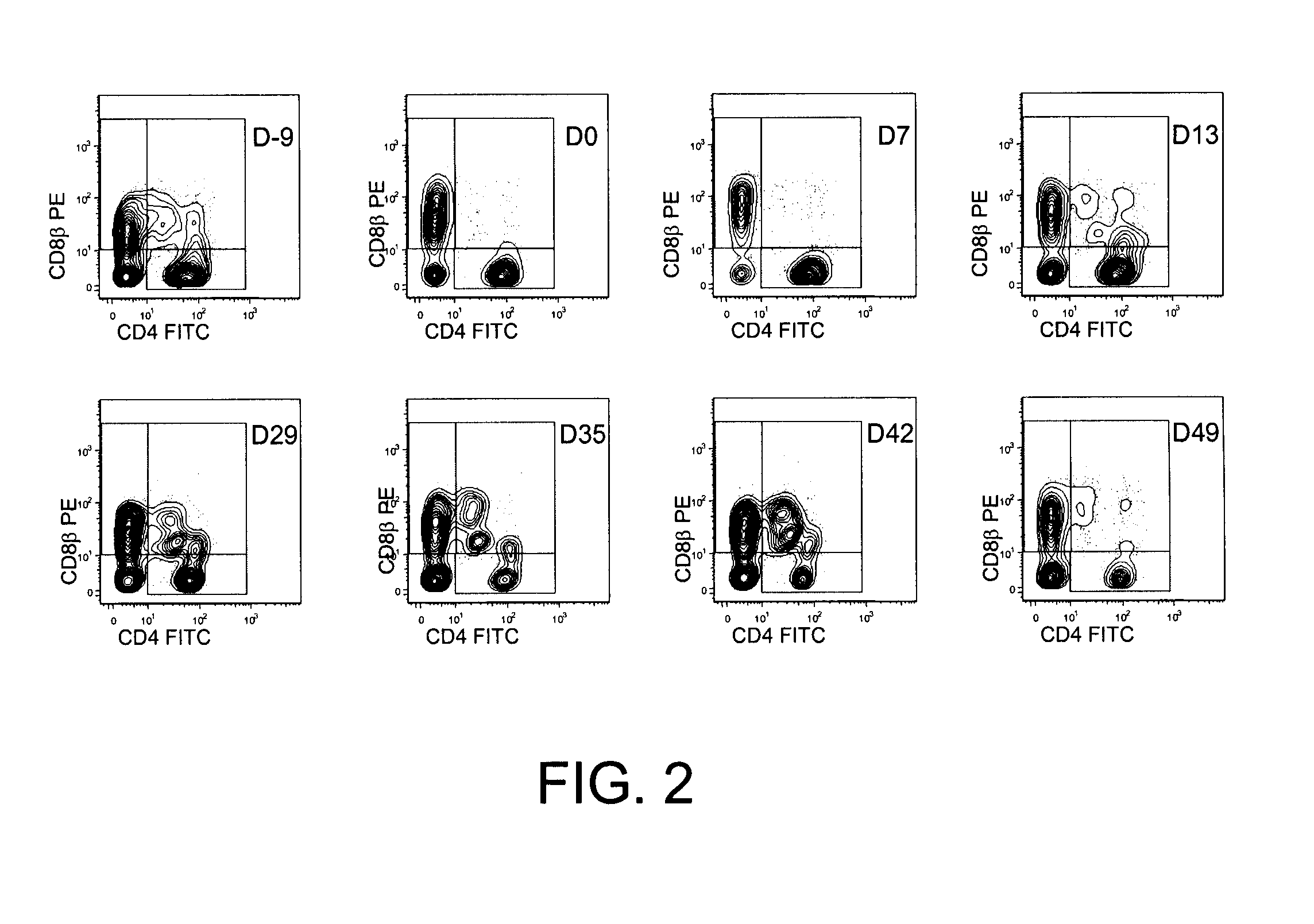
Methods for the Diagnosis and Prognosis of Graft Versus Host Disease By Measurement of Peripheral Cd3+Cd4+Cd8Beta+ Cells
InactiveUS20090011456A1Increase relative volatilityMicrobiological testing/measurementBiological material analysisCD8Intestinal GVHD
Acute graft versus host disease (GvHD) is one of the most significant clinical problems in allogeneic blood and marrow transplantation. Currently, there is no unequivocal diagnostic test for GvHD until the disease is well developed and can be recognized histologically. The invention provides a blood based test for diagnosis and / or prognosis of GvHD, allowing assessment of risk for developing GvHD prior to appearance of clinical symptoms. Using flow cytometry, peripheral blood mononuclear cells are assessed for an increase in proportion and fluctuation of CD3+CD4+CD8β+ cells. An increase in presence or proportion, or high fluctuation in this cell population prior to onset of clinically recognized indicators of GvHD is predictive of later development of GvHD.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
Method for extracting original mesenchyma and hematopoiesis trunks/ancestral cell from caesarean birth placenta for treating medulla scathe
InactiveCN101161249AFor long-term storageSkeletal disorderMammal material medical ingredientsProgenitorTissue repair
The present invention is a method for clinic hematopoietic stem cells transplantation by extracting original mesenchymal and hematopoietic stem / progenitor cells from health post-caesarean birth placenta tissue. Each placenta can extract 3.8 + / - 0.7 X 10<9> cells having nucleus, wherein original mesenchymal and hematopoietic stem / progenitor cells are 1.8 + / - 0.2 X10<7>, cell viability is more than 95 percent, microorganism contamination rate is less than 6 percent. The method uses counter monoclonal antibody method to eliminate 90 to 95 percent contaminated mature cell having nucleus single cell in mother blood, reducing immunological rejection reaction (GVHD) after transplanting. The finished product of the method can be used for clinic treating hematopoietic stem cells transplantation due to marrow injury, can be used for applying to placenta tissue supplying person or other patient having various hematopoietic stem cells transplantation indications, and can also combine with various cell growth factors in clinic to develop cell repair and gene treatment. The present invention supplies another cell source for hematopoietic stem cells transplantation, promotes the development of cells transplantation and tissue repairing, also supplies new research direction for early tissue cell differentiation.
Owner:上海厚东生物科技有限公司
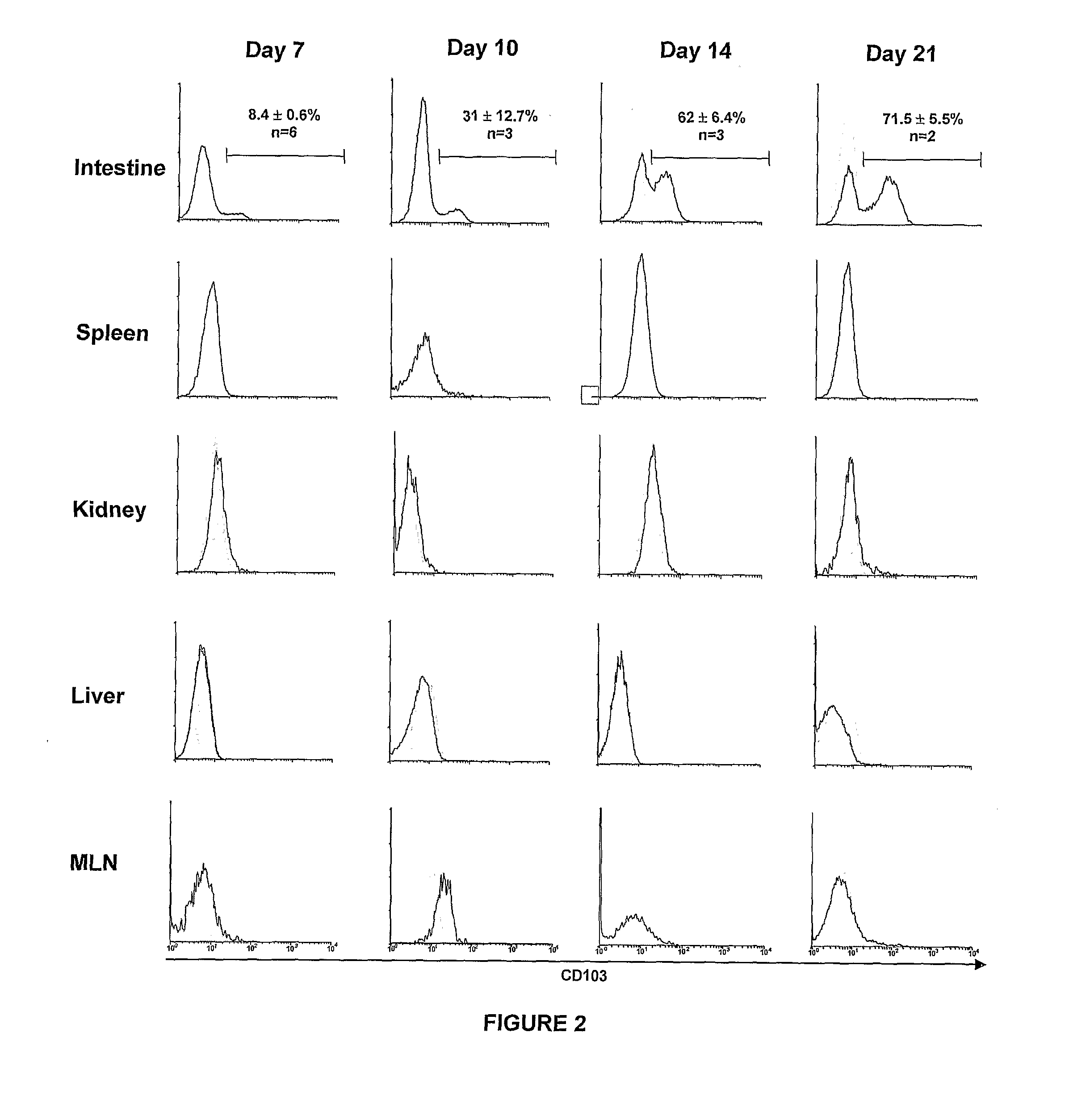
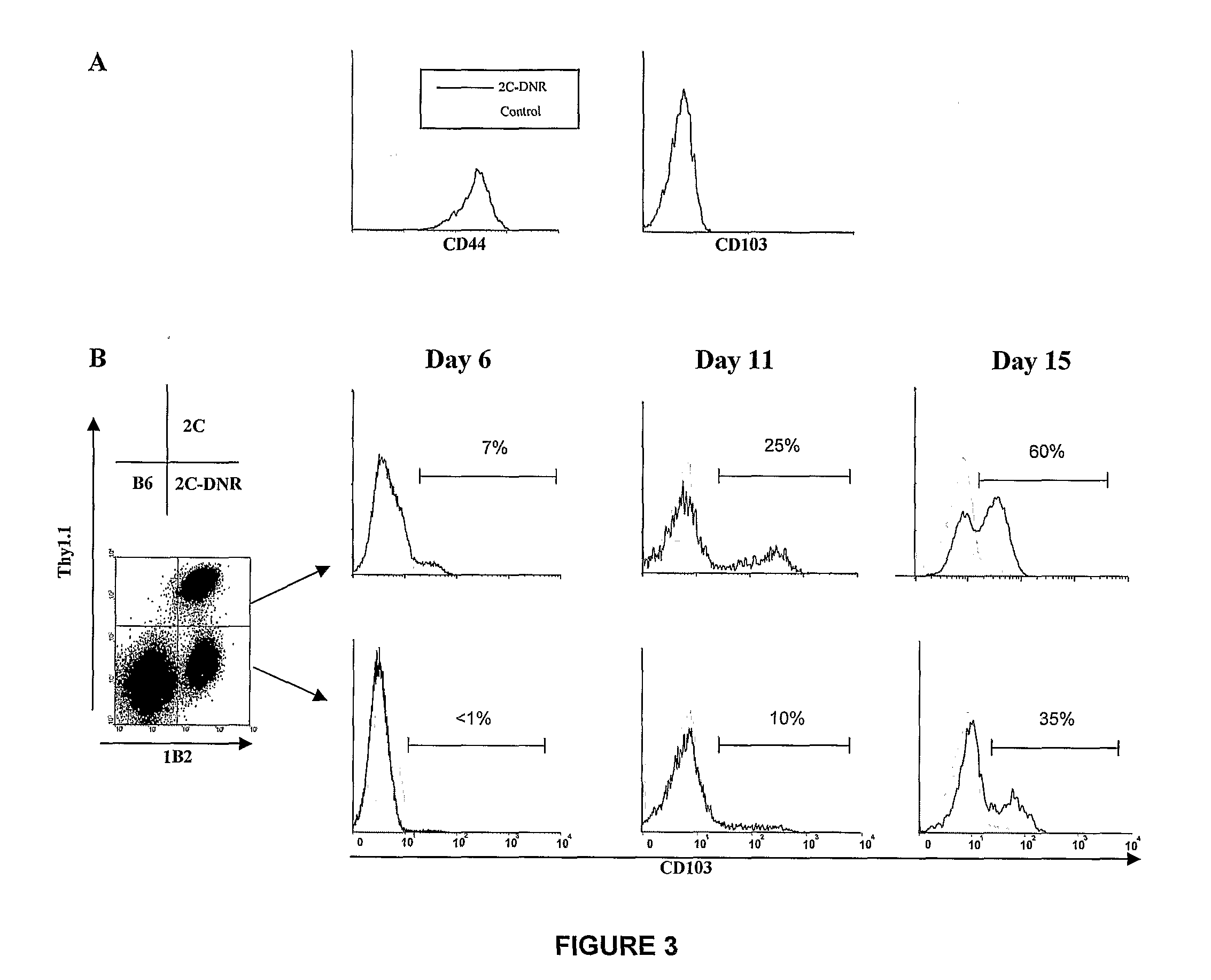
Methods of Treating Graft Versus Host Disease
InactiveUS20080171047A1Reduced activityAntibody ingredientsPharmaceutical non-active ingredientsGraft versus host disease inductionSurgery
The present invention relates to methods for treating or preventing graft versus host disease (GVHD) in a host subject, with the methods comprising treating either the graft or the host subject or both to reduce the interaction of graft-origin, CD103-expressing cells with cells in the host. The present invention also relates to methods of screening a graft to determine the likelihood of the graft to generate GVHD.
Owner:UNIV OF MARYLAND
Anti CD4 antibodies to prevent in particular graft-versus-host-disease (GvHD)
ActiveUS9745552B2Immunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsMode of actionWhite blood cell
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Use of cytokines and mitogens to inhibit graft versus host disease
InactiveUS7381563B2InhibitionAvoiding and minimizingBiocidePeptide/protein ingredientsFactor iiCytokine
The field of the invention is generally related to pharmaceutical agents useful in treating graft-versus-host disease (GVHD) in patents that have received allogenic bone marrow transplants.
Owner:UNIV OF SOUTHERN CALIFORNIA
Composition and method for treating graft-versus-host disease
InactiveUS7173016B2Minimize ToxicitySuppress T-lymphocyte mediated immune responsesBiocideSugar derivativesPentostatinAdenosine Deaminase Inhibitor
Owner:MAYNE PHARMA USA
Methods modulating immunoregulatory effect of stem cells
The present invention provides methods or kits with inflammatory cytokines to pretreat 1-ISCs to augment their immune modulatory effect, in prevention and treatment of various diseases such as multiple sclerosis, arthritis, lupus, sepsis, hepatitis, cirrhosis, Parkinson's disease, chronic infections, and graft versus-host disease (GvHD). The present invention relates to novel methods for enhancingthe immunosuppressive or the immune stimulatory activities of mesenchymal stem cells (JvfSCs).
Owner:RUTGERS THE STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com