Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Lymphoproliferative disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lymphoproliferative disorders (LPDs) refer to several conditions in which lymphocytes are produced in excessive quantities. They typically occur in people who have a compromised immune system. They are sometimes equated with "immunoproliferative disorders", but technically lymphoproliferative disorders are a subset of immunoproliferative disorders, along with hypergammaglobulinemia and paraproteinemias.
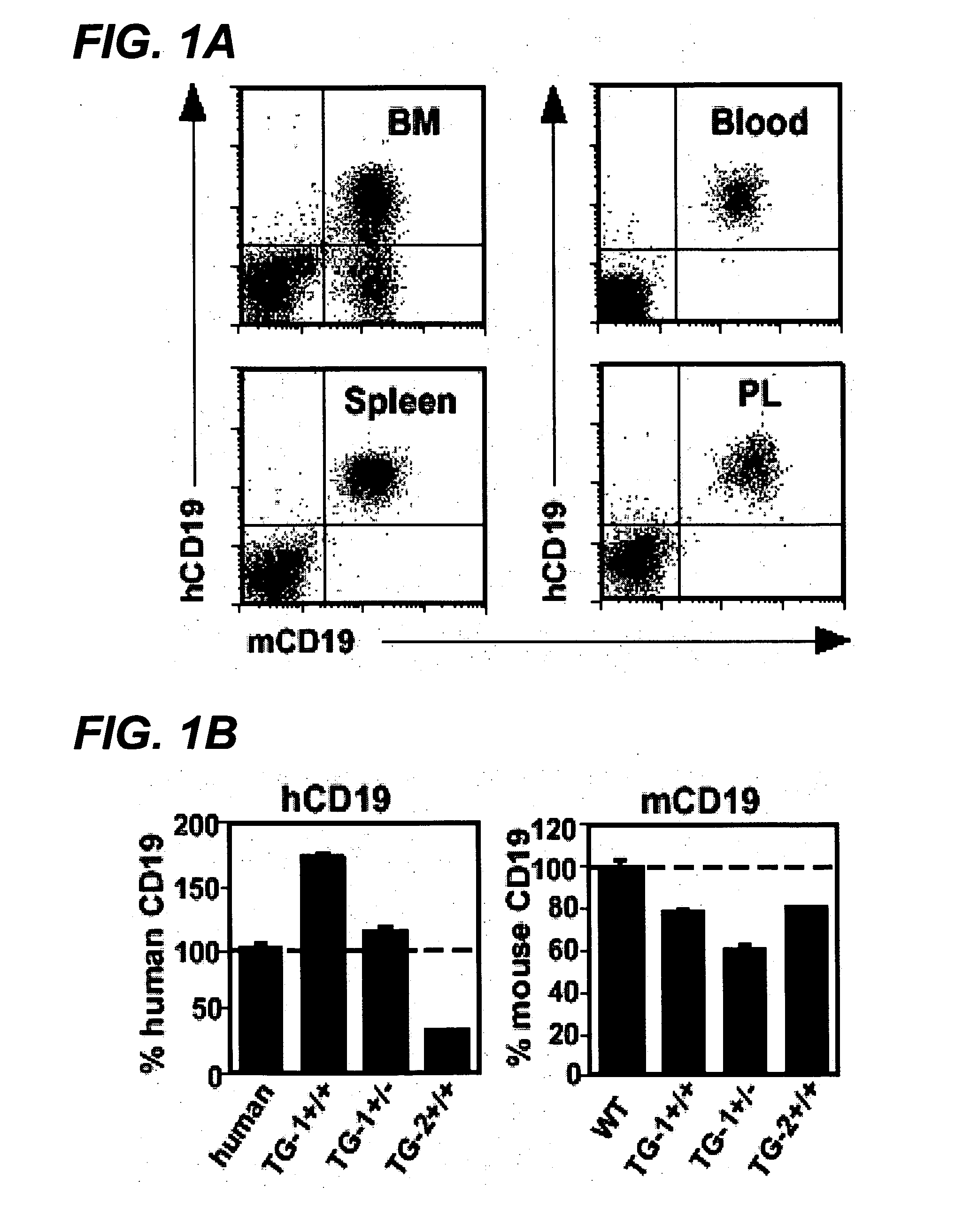
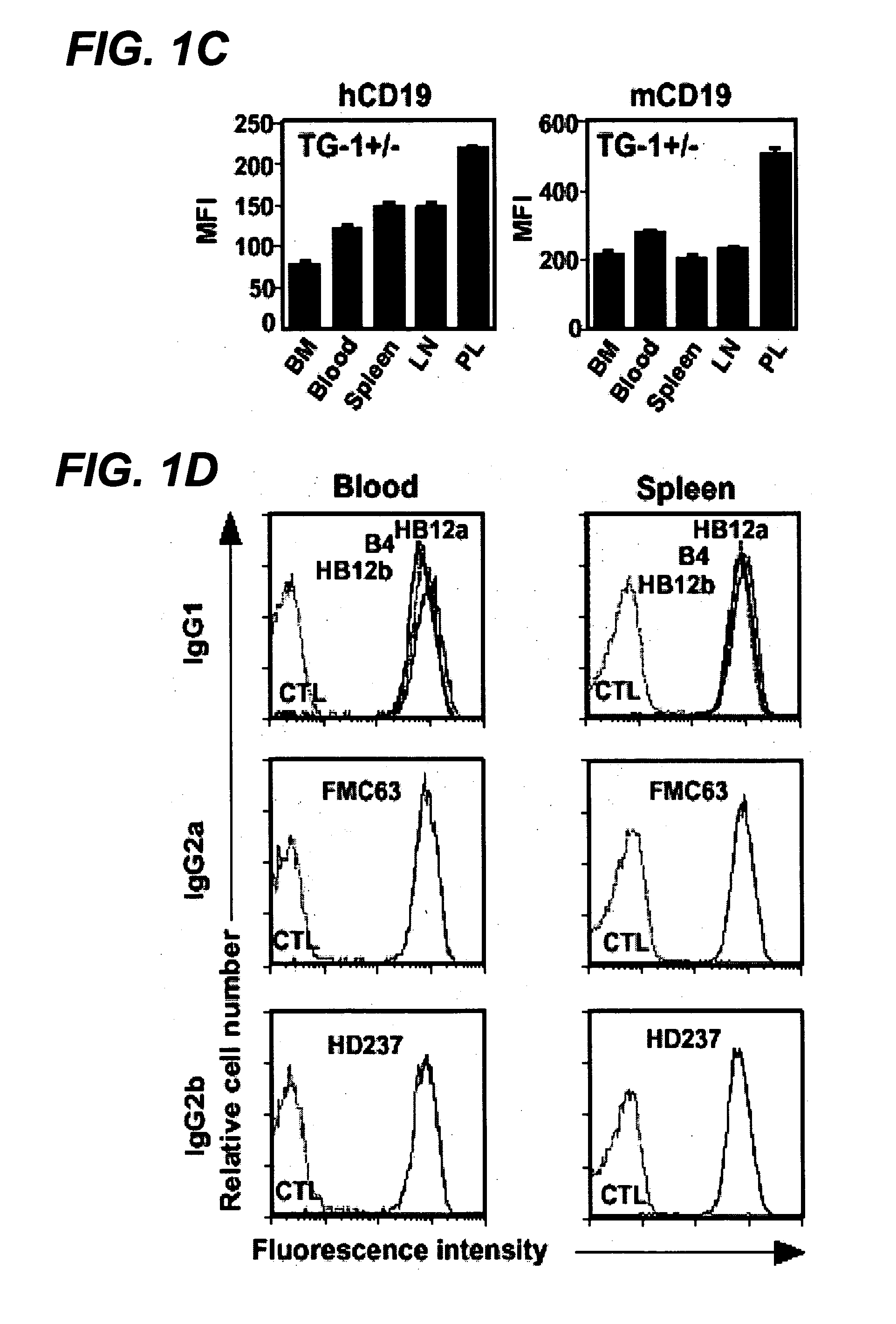
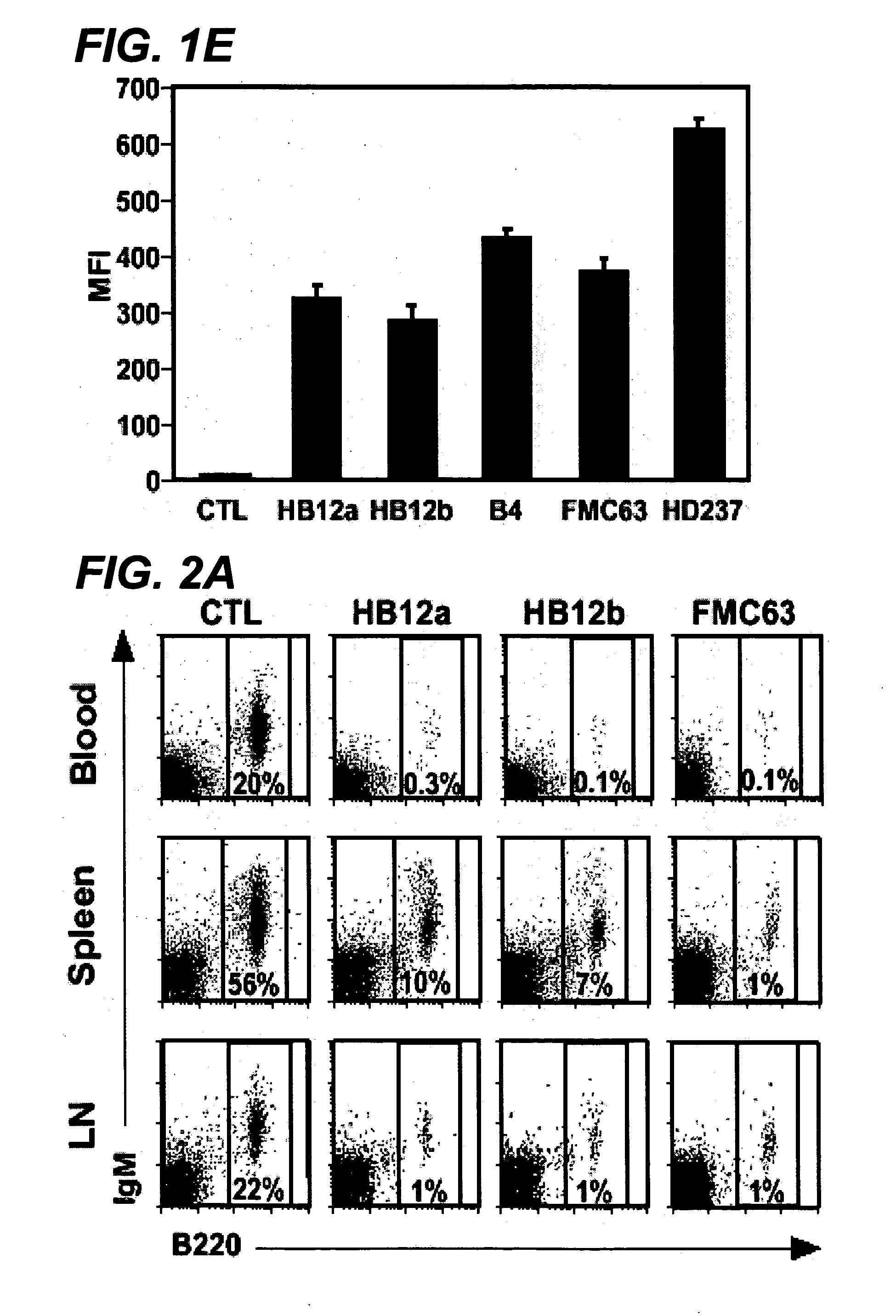
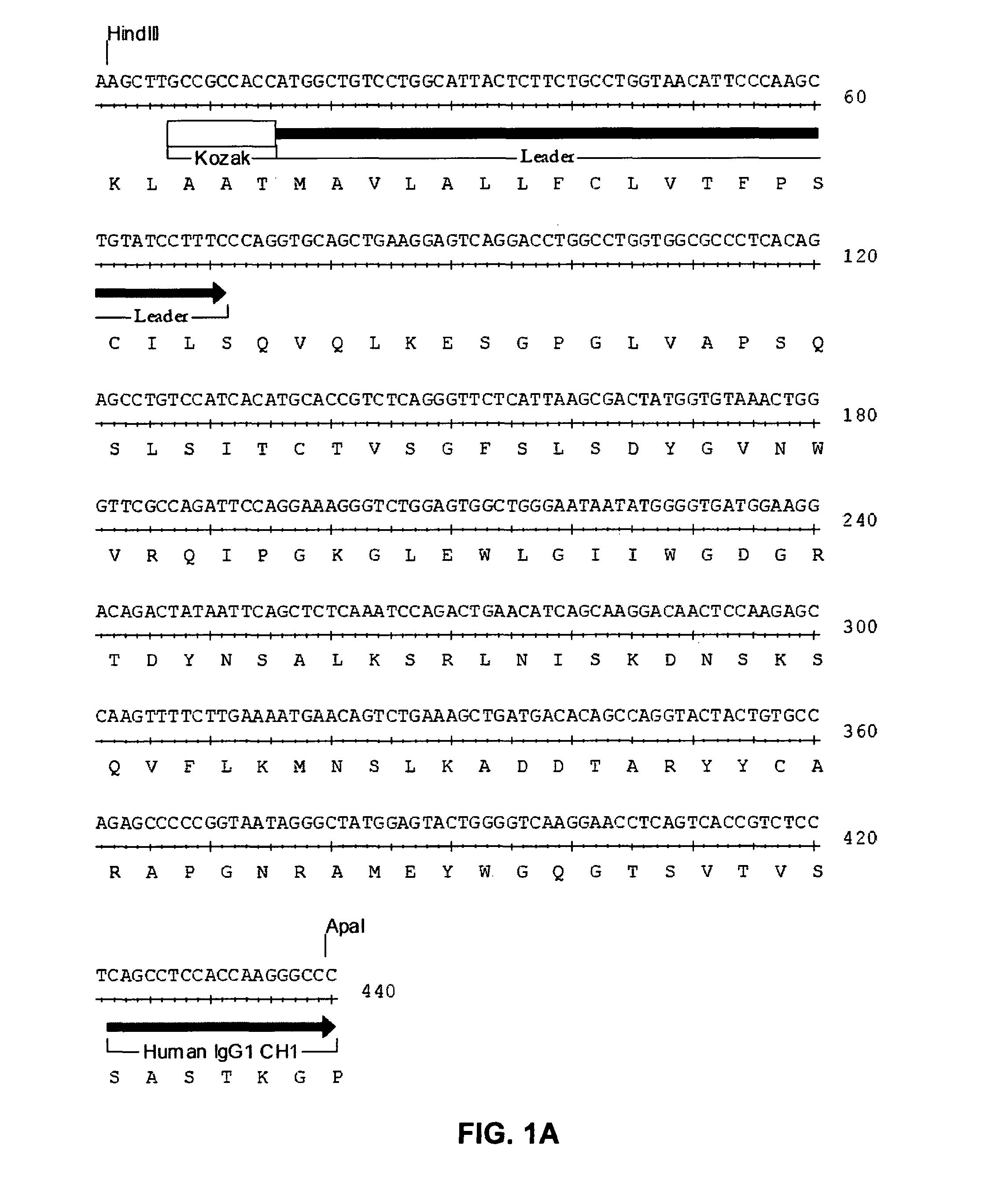
Anti-CD19 antibody therapy for transplantation
InactiveUS20060280738A1Efficient productionEfficiently depletedBiocidePhosphorous compound active ingredientsAntigenDisease
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD 19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
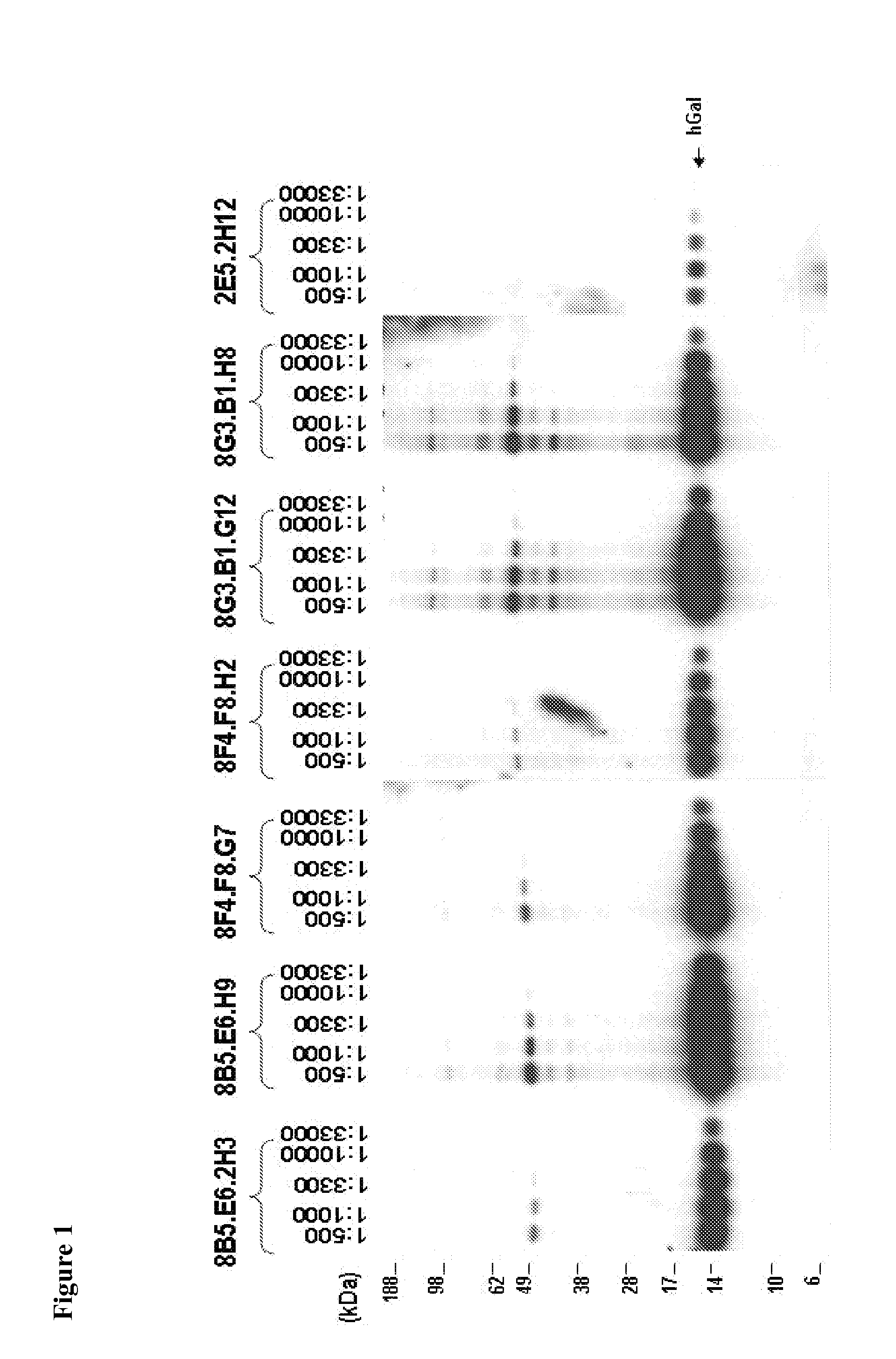
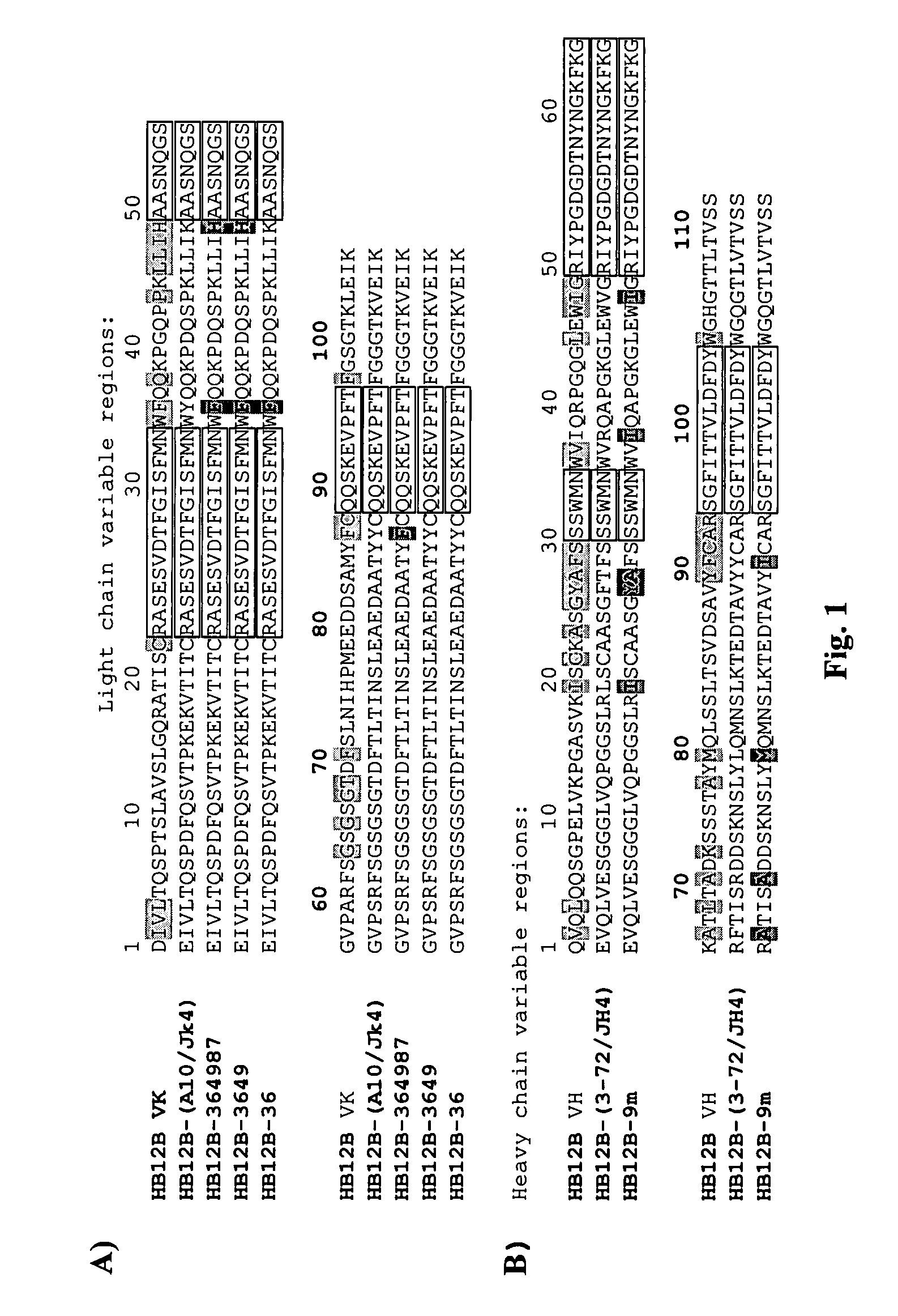
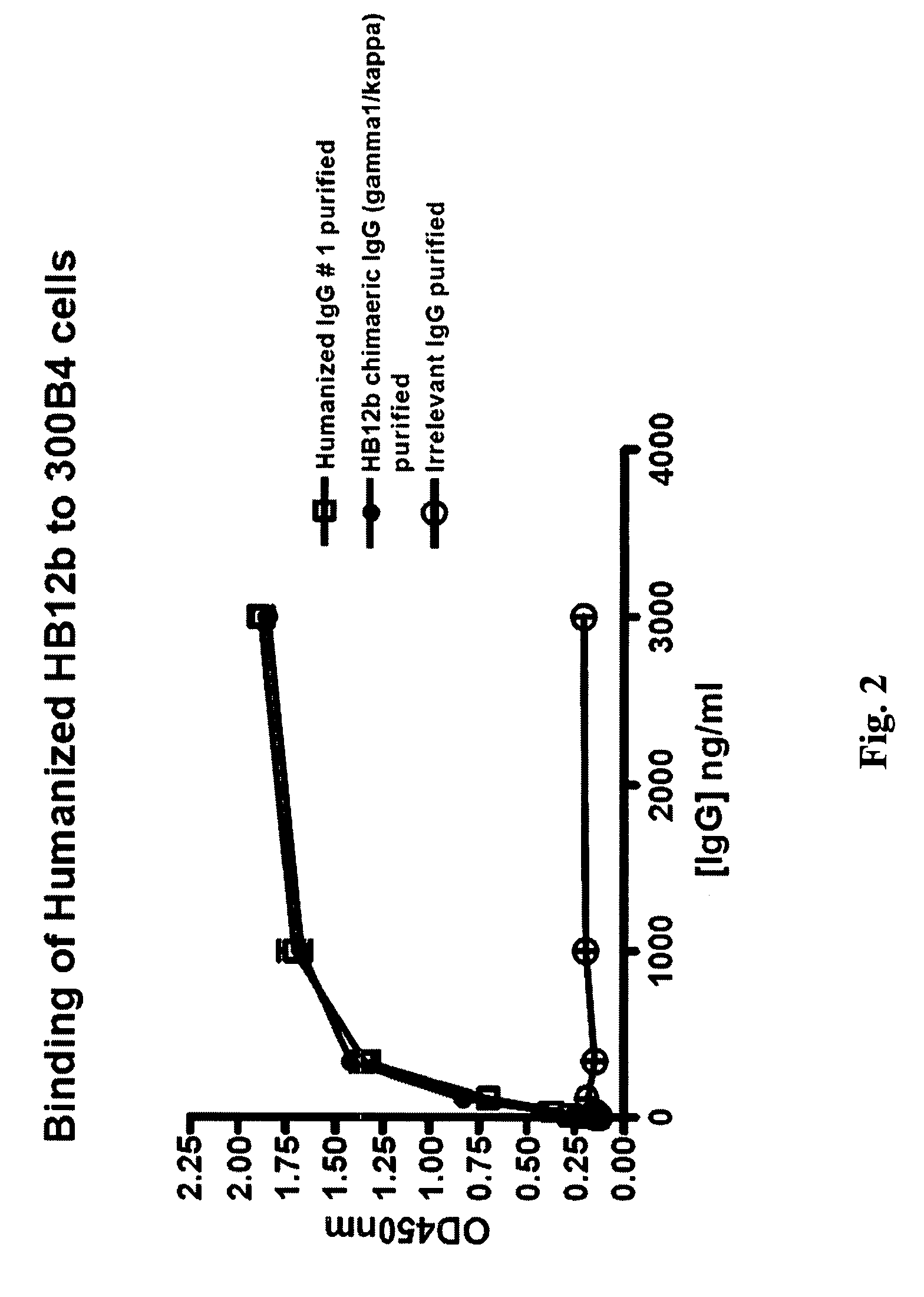
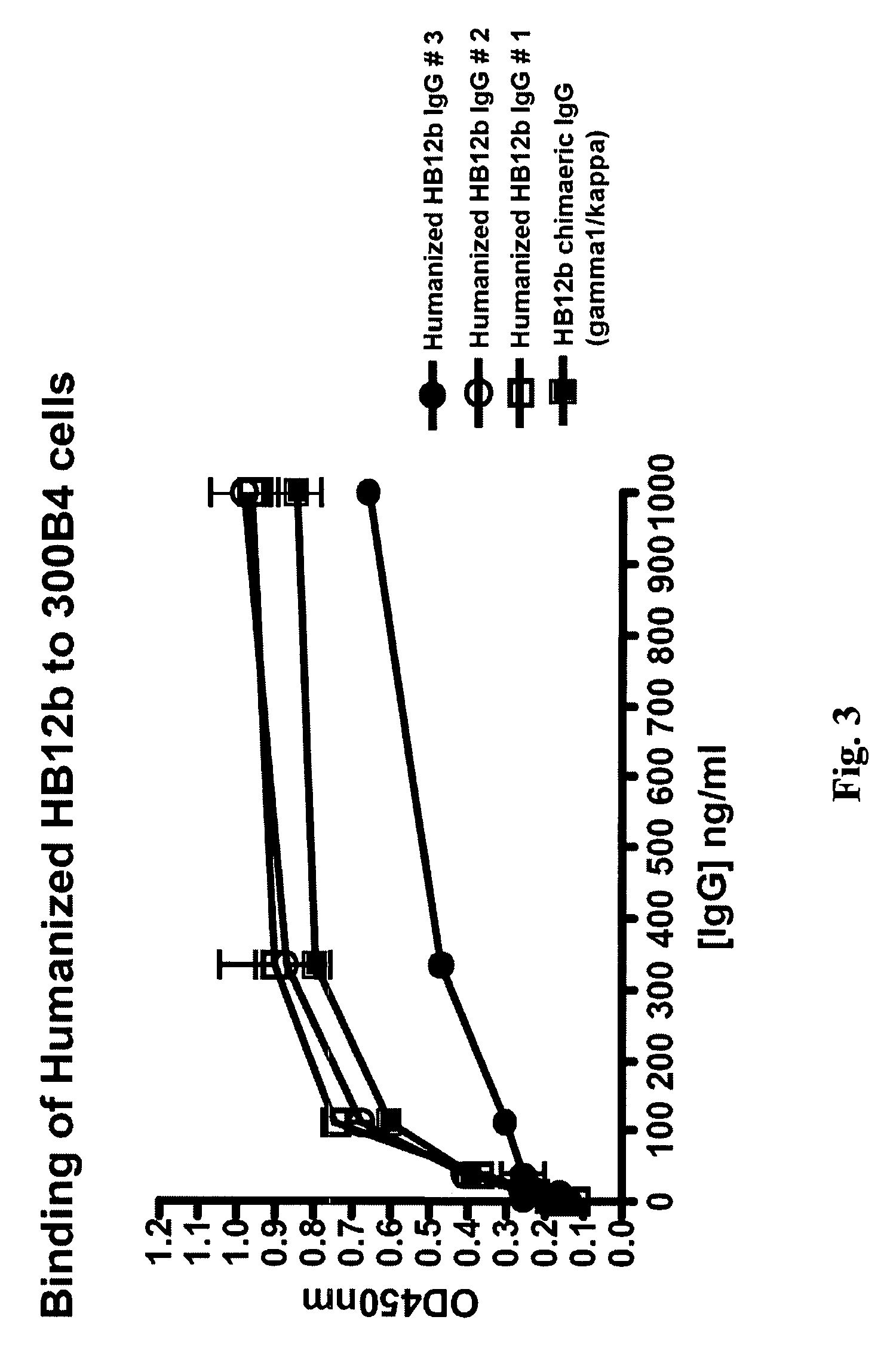
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
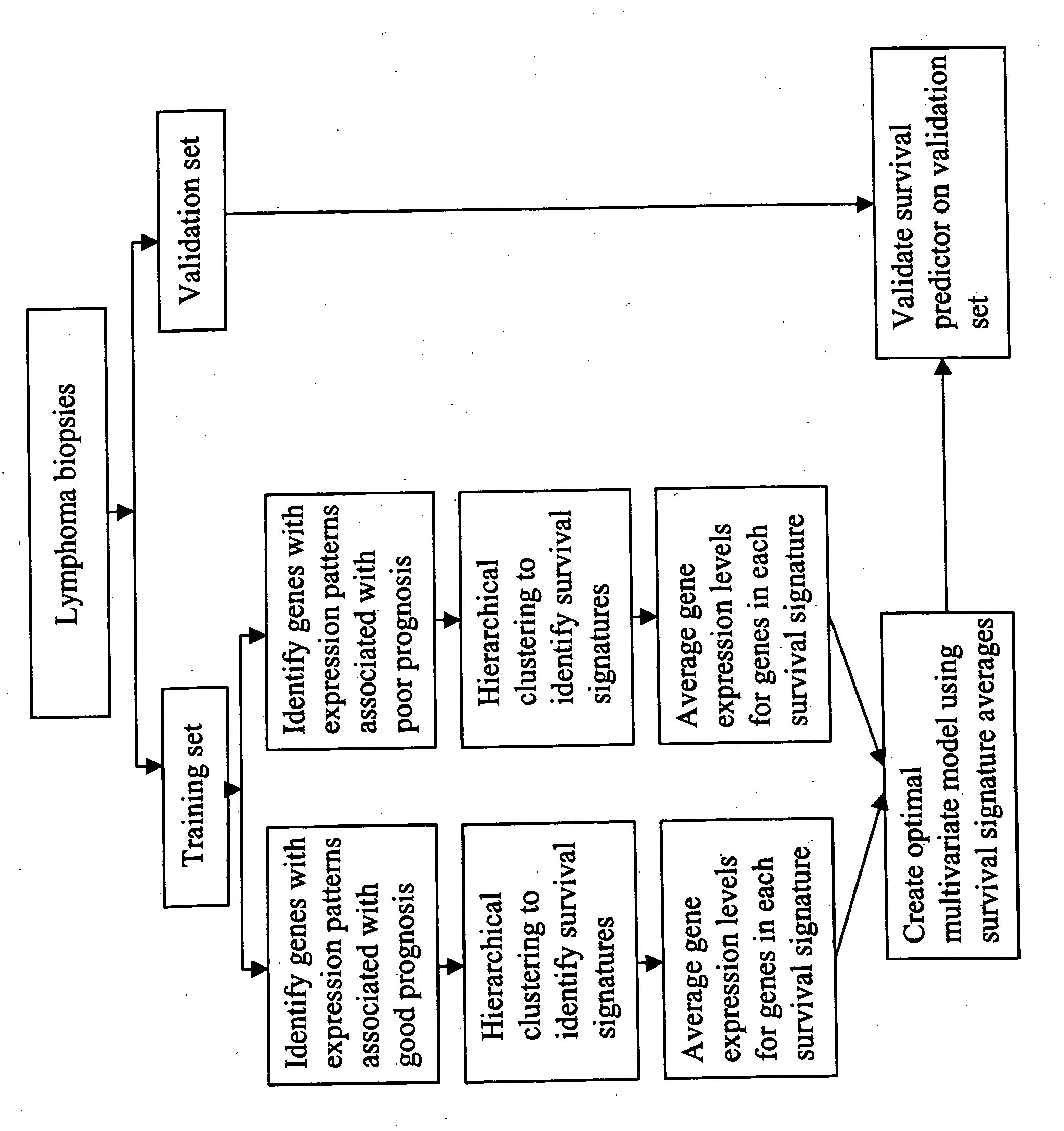
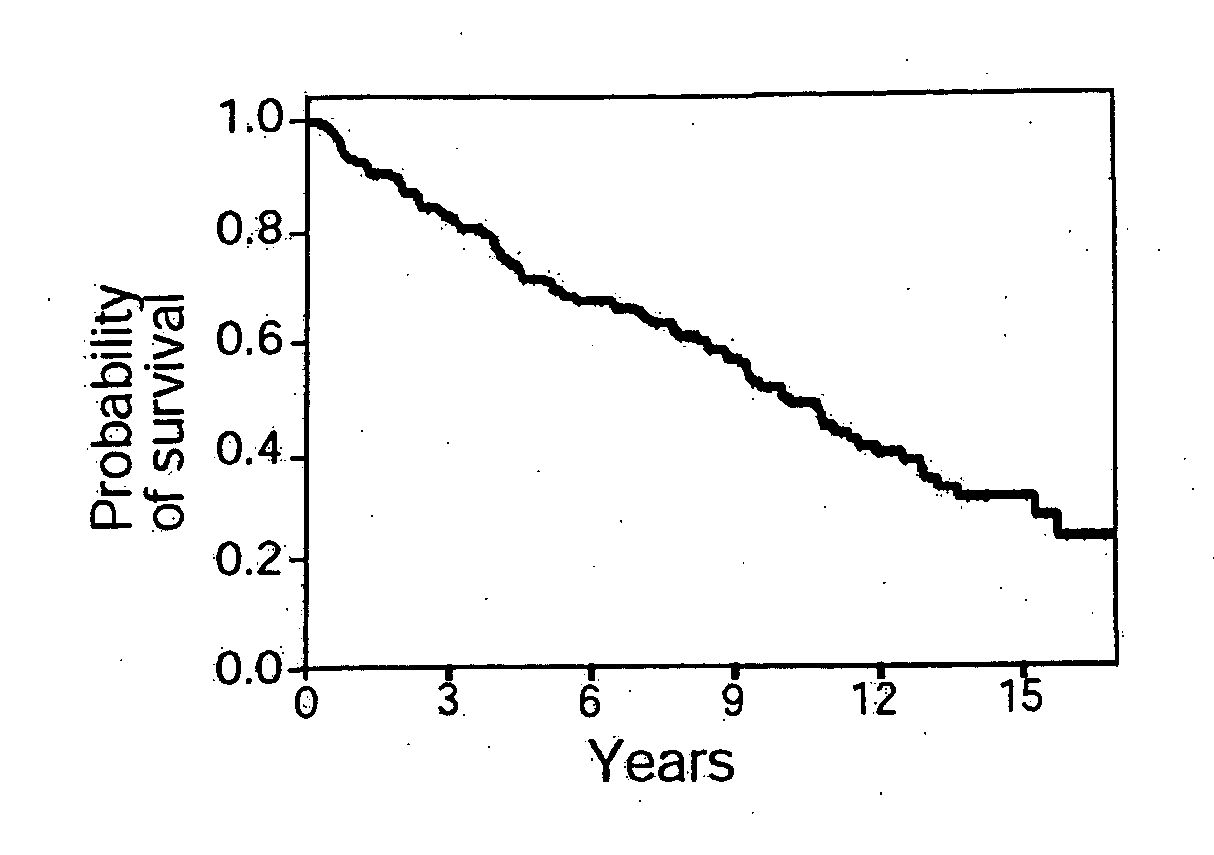
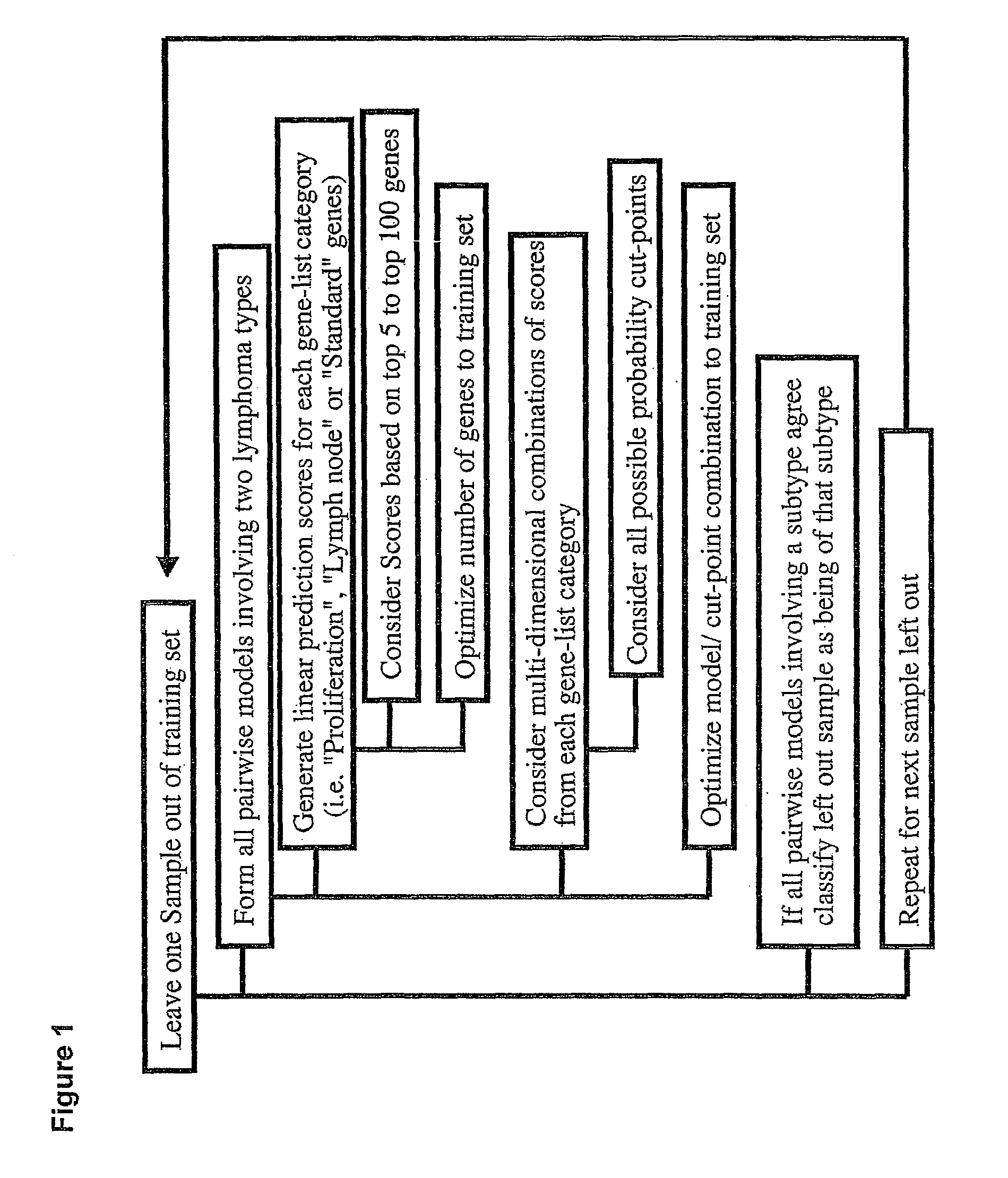
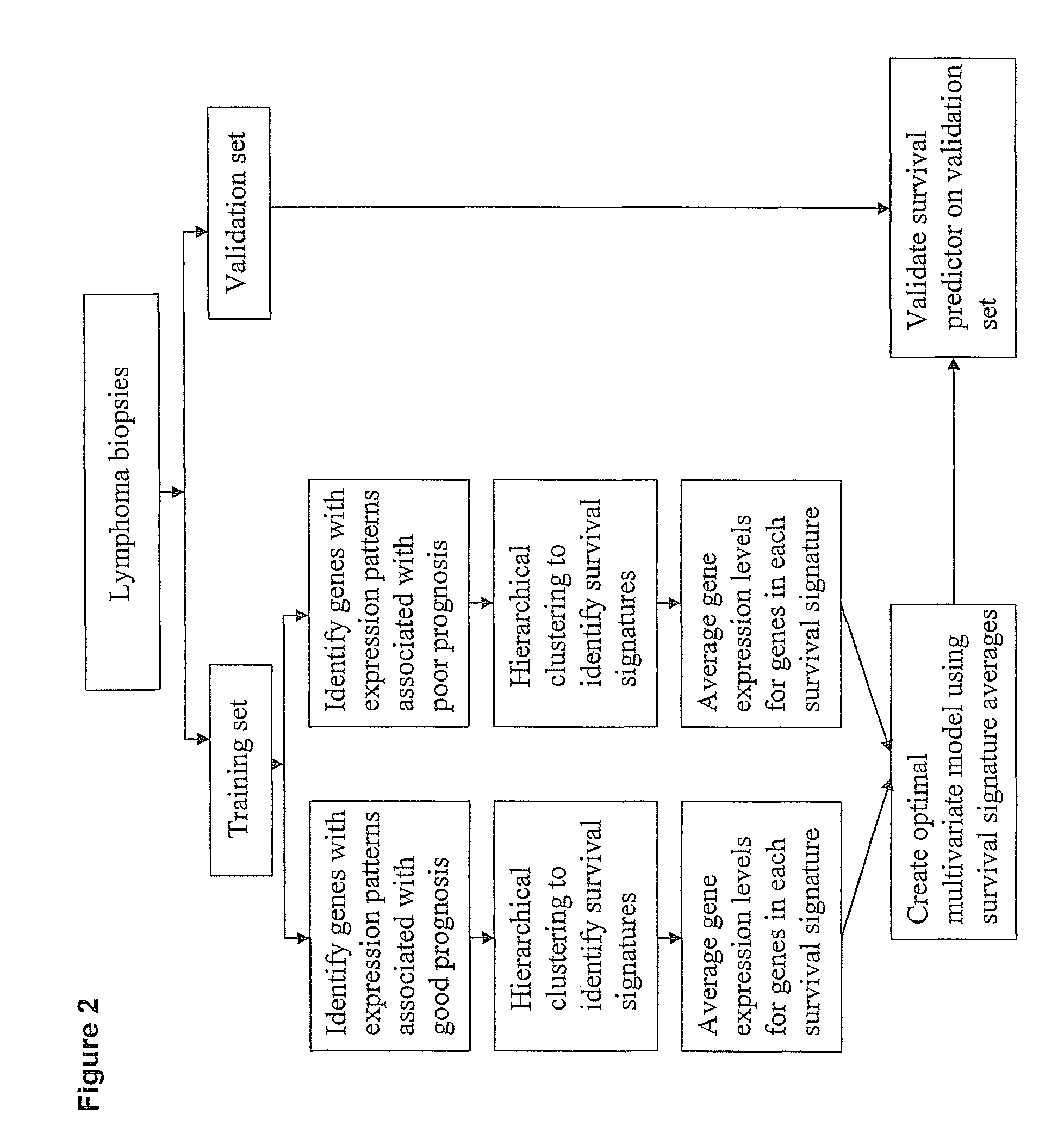
Methods for identifying, diagnosing, and predicting survival of lymphomas
ActiveUS20050164231A1Sugar derivativesMicrobiological testing/measurementLymphoid tissue hyperplasiaLymphocyte disorder
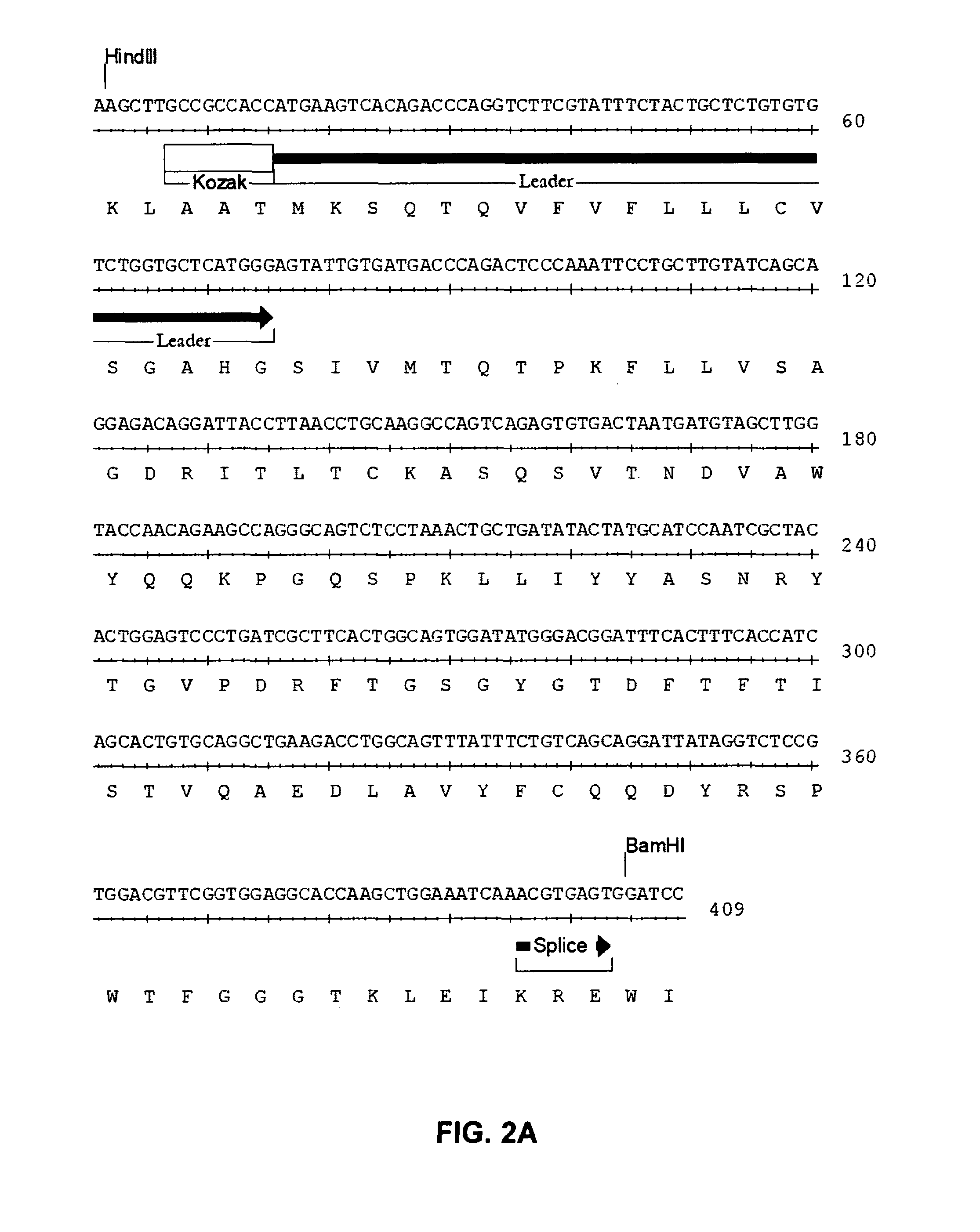
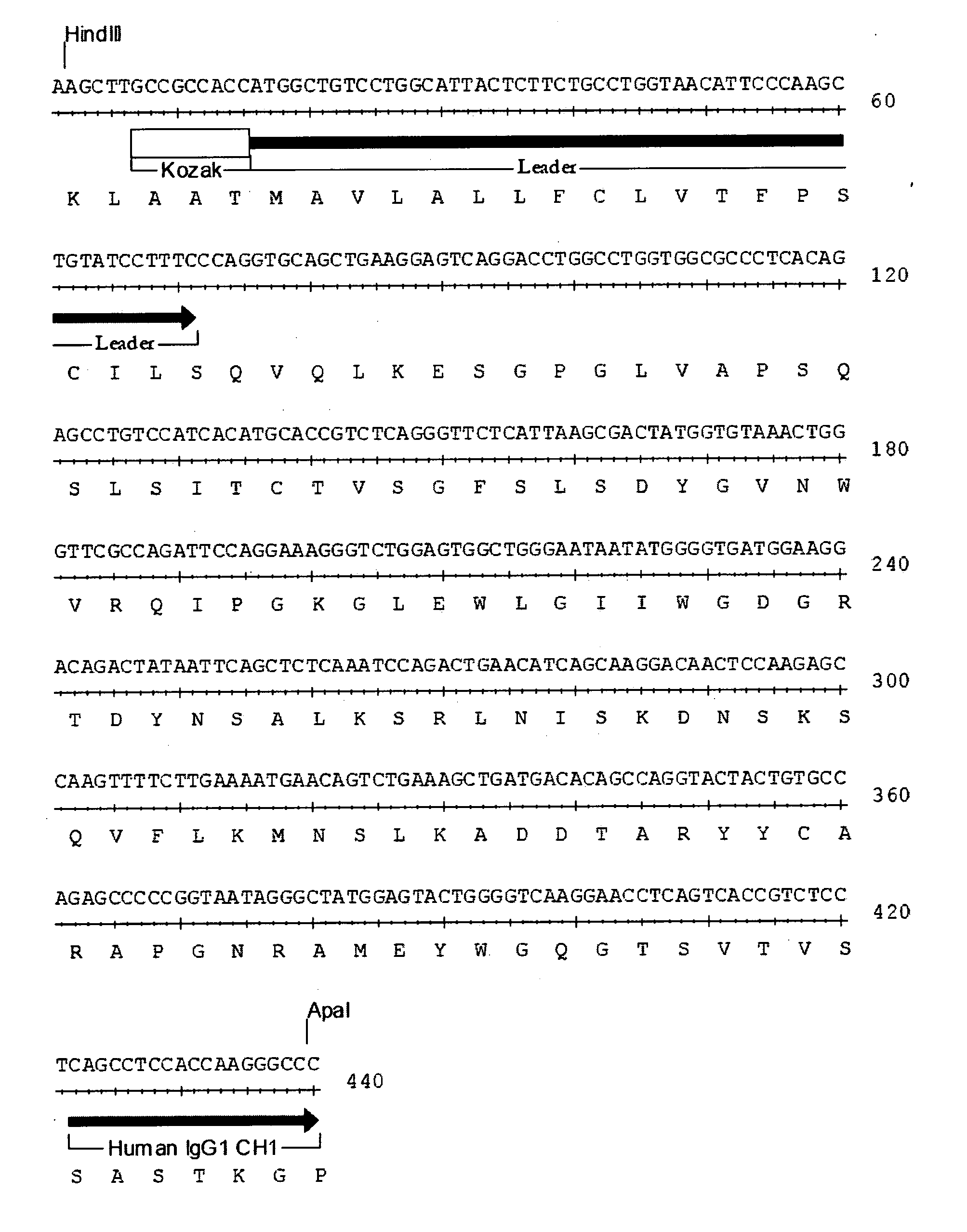
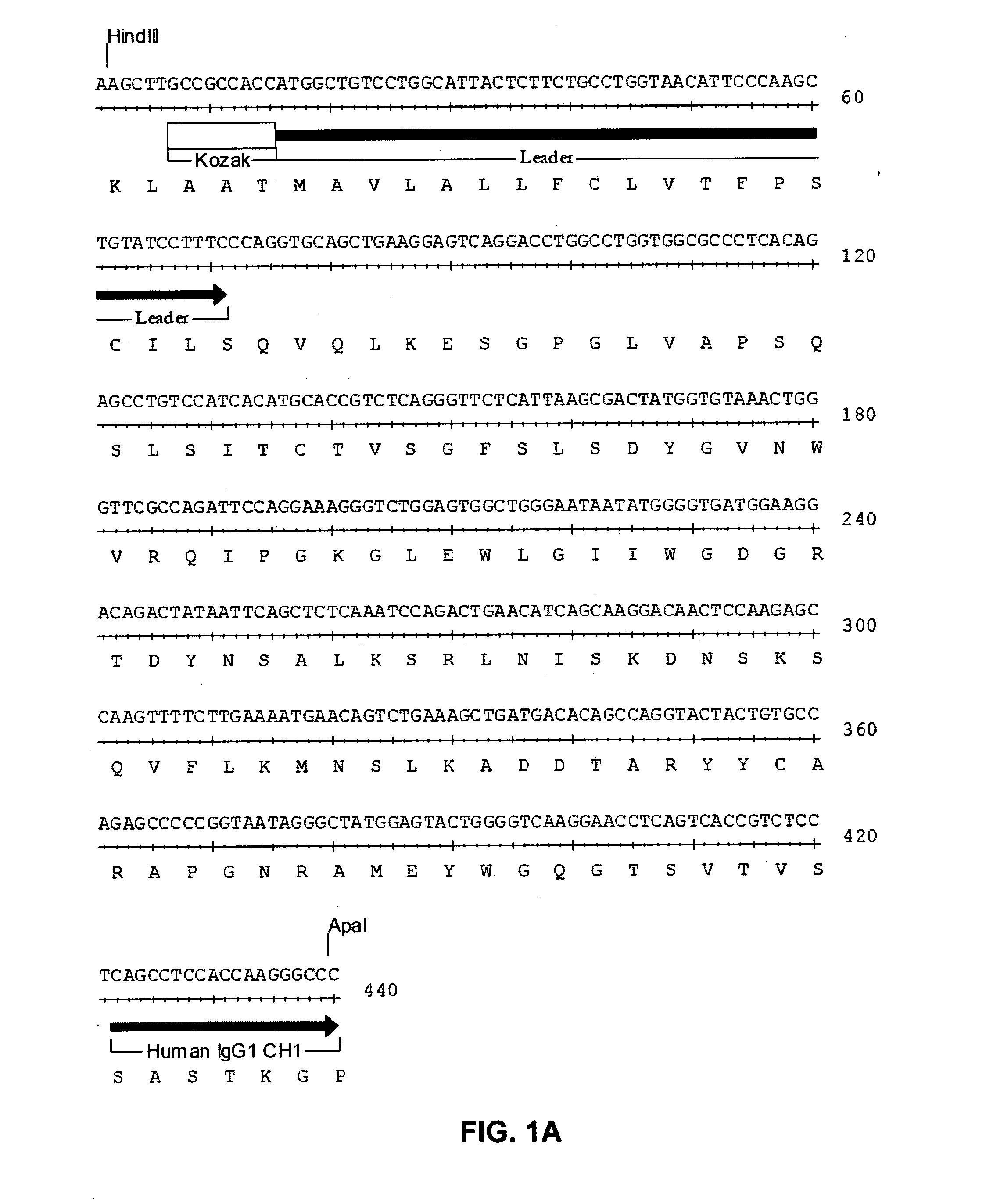
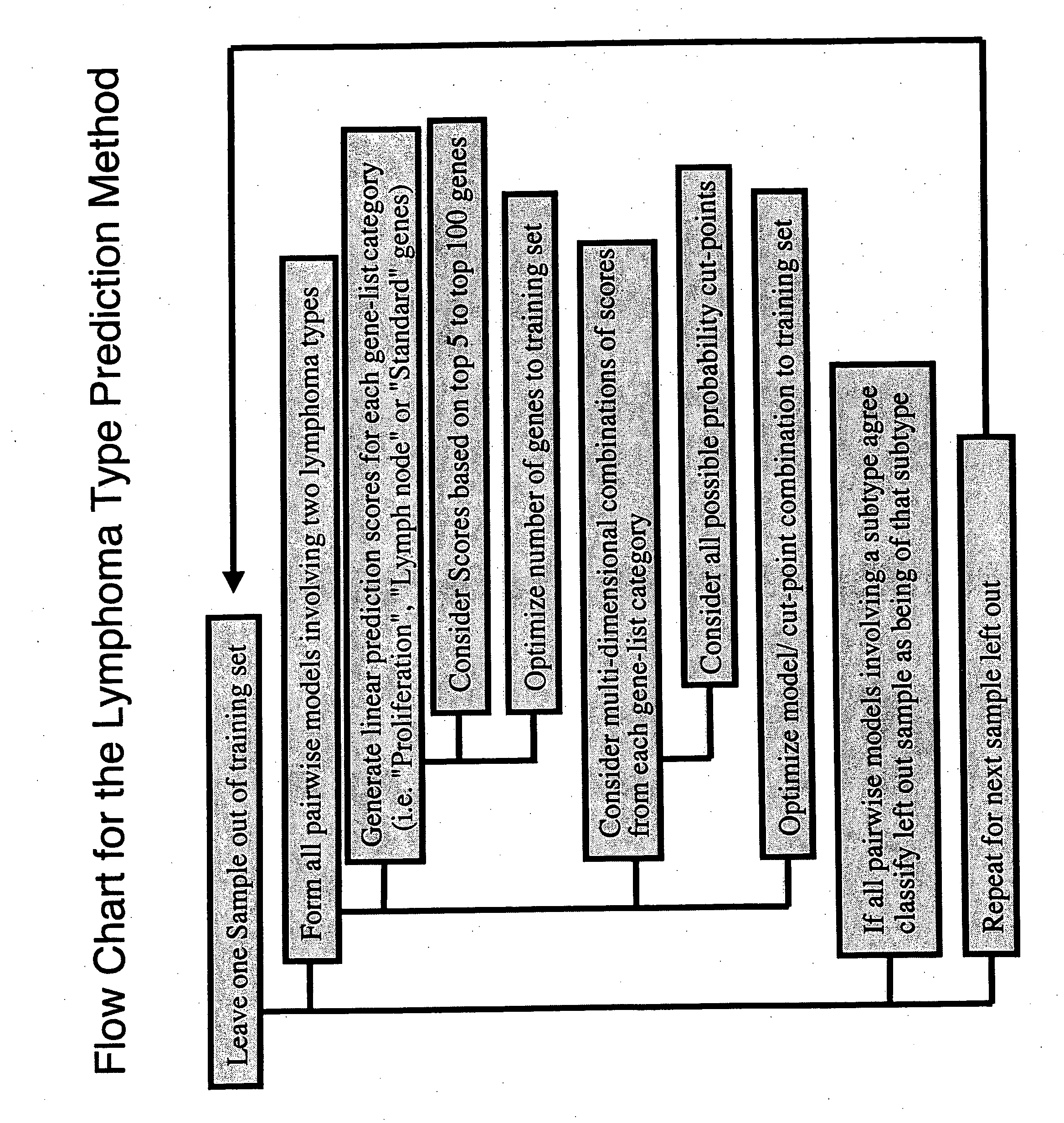
Gene expression data provides a basis for more accurate identification and diagnosis of lymphoproliferative disorders. In addition, gene expression data can be used to develop more accurate predictors of survival. The present invention discloses methods for identifying, diagnosing, and predicting survival in a lymphoma or lymphoproliferative disorder on the basis of gene expression patterns. The invention discloses a novel microarray, the Lymph Dx microarray, for obtaining gene expression data from a lymphoma sample. The invention also discloses a variety of methods for utilizing lymphoma gene expression data to determine the identity of a particular lymphoma and to predict survival in a subject diagnosed with a particular lymphoma. This information will be useful in developing the therapeutic approach to be used with a particular subject.
Owner:HEALTH & HUMAN SERVICES NAT INST OF HEALTH GOVERNMENT OF THE UNITED STATES OF AMERICA THE DEPT OF
Methods for diagnosing lymphoma types
ActiveUS7711492B2Sugar derivativesMicrobiological testing/measurementLymphocyte disorderSurvival predictors
Gene expression data provides a basis for more accurate identification and diagnosis of lymphoproliferative disorders. In addition, gene expression data can be used to develop more accurate predictors of survival. The present invention discloses methods for identifying, diagnosing, and predicting survival in a lymphoma or lymphoproliferative disorder on the basis of gene expression patterns. The invention discloses a novel microarray, the Lymph Dx microarray, for obtaining gene expression data from a lymphoma sample. The invention also discloses a variety of methods for utilizing lymphoma gene expression data to determine the identity of a particular lymphoma and to predict survival in a subject diagnosed with a particular lymphoma. This information will be useful in developing the therapeutic approach to be used with a particular subject.
Owner:HEALTH & HUMAN SERVICES NAT INST OF HEALTH GOVERNMENT OF THE UNITED STATES OF AMERICA THE DEPT OF
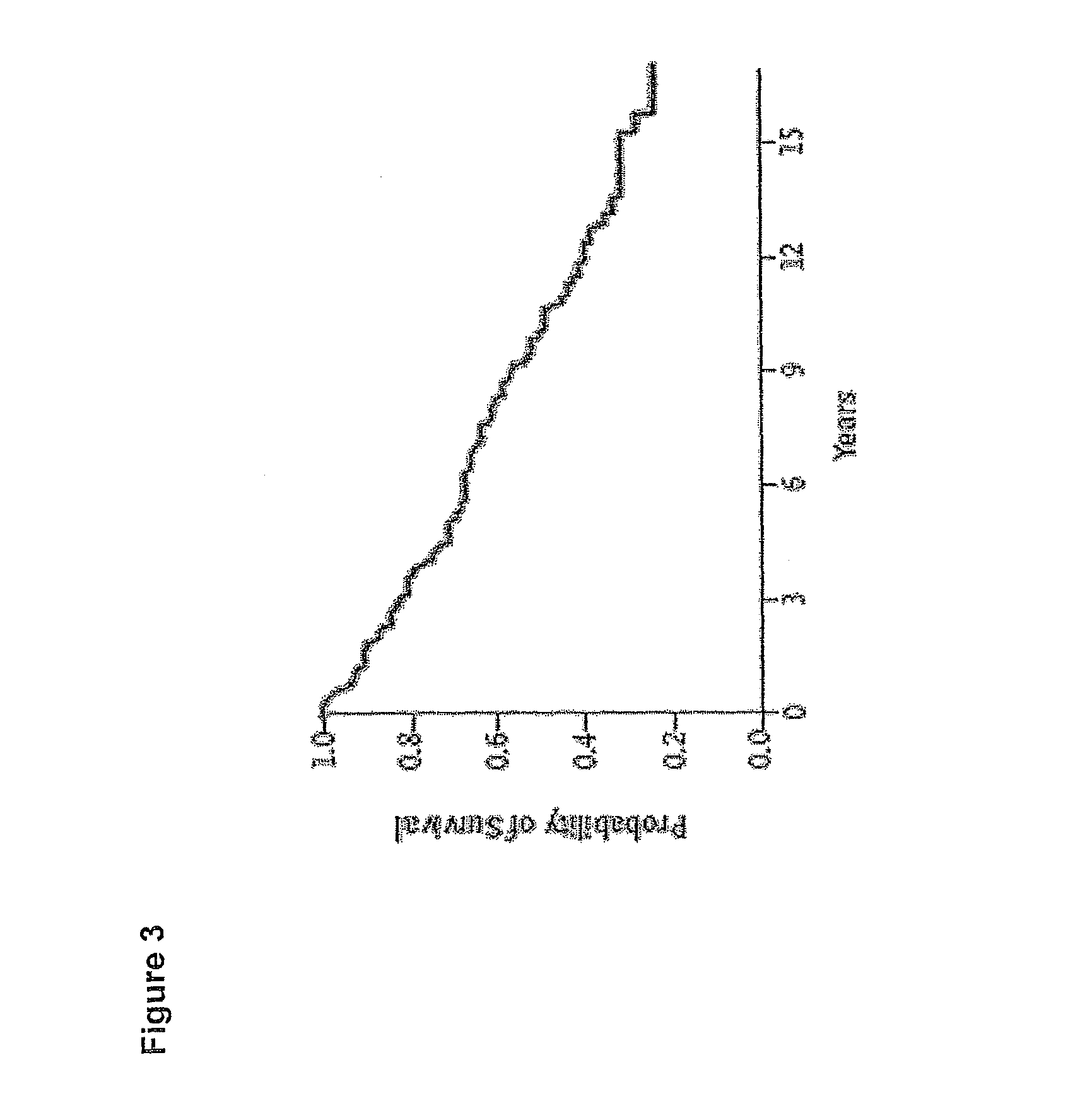
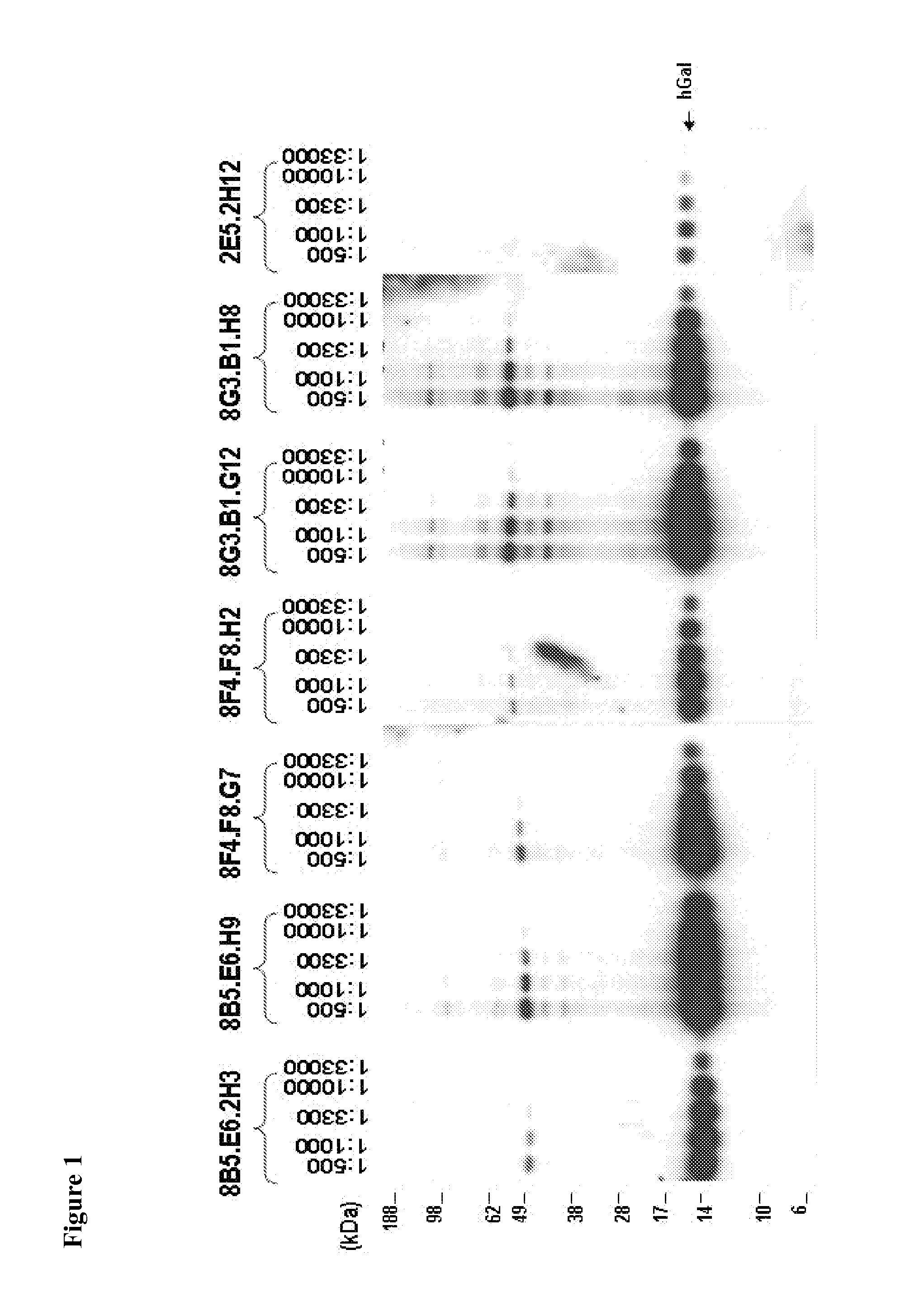
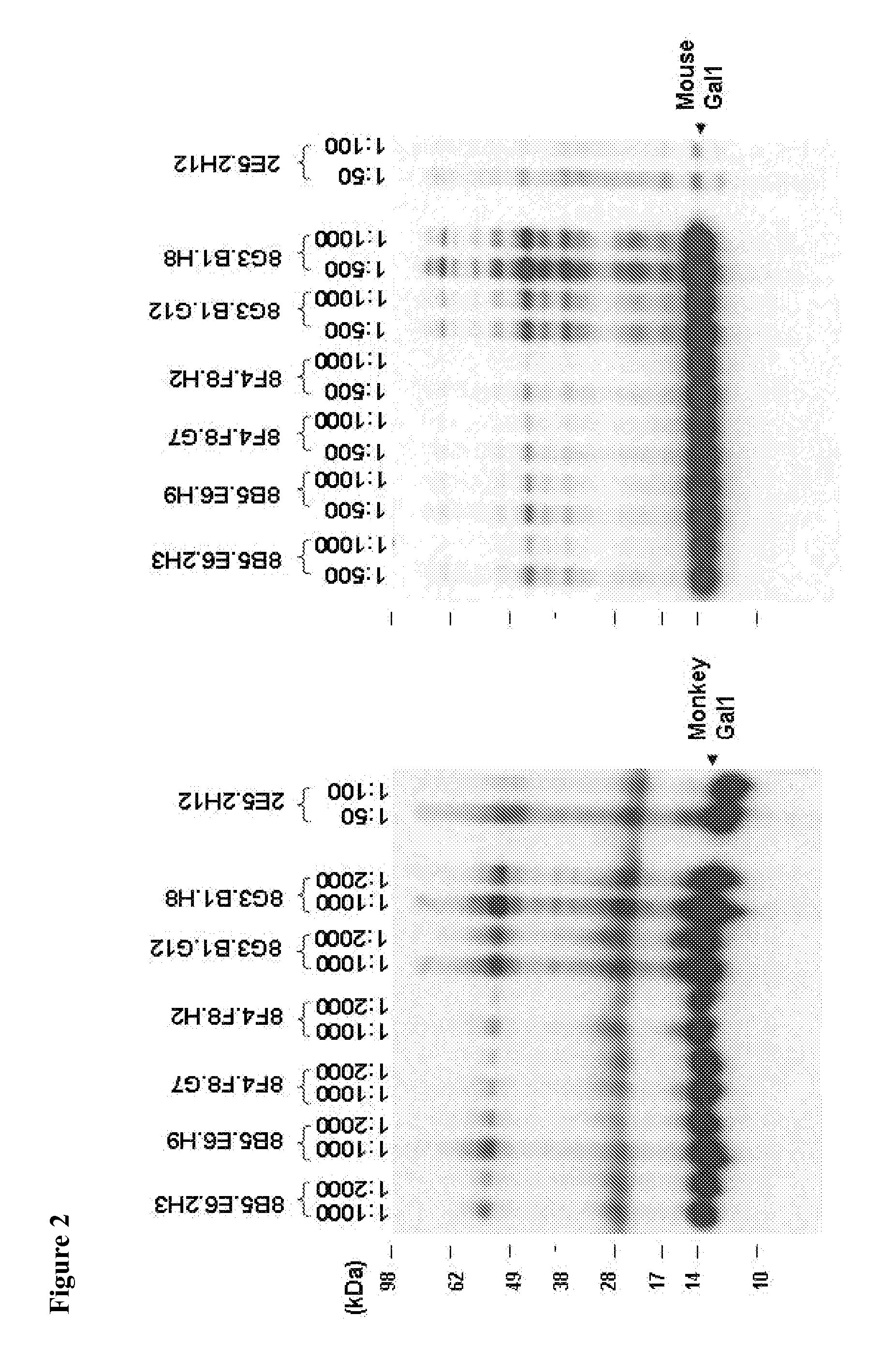
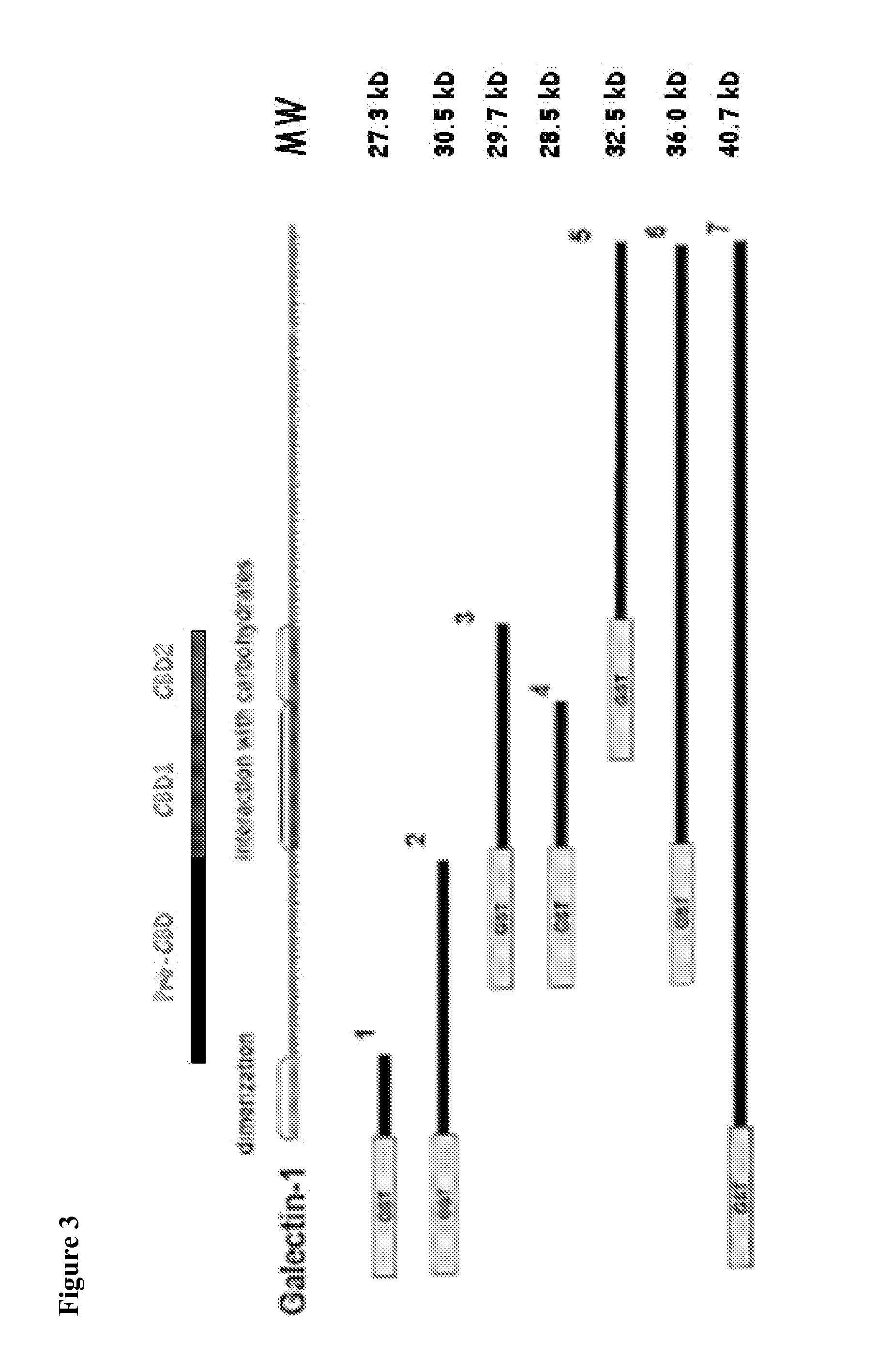
Compositions, Kits, and Methods for the Diagnosis, Prognosis, Monitoring, Treatment and Modulation of Post-Transplant Lymphoproliferative Disorders and Hypoxia Associated Angiogenesis Disorders Using Galectin-1
ActiveUS20130011409A1Modulate angiogenesisReduce the overall diameterOrganic active ingredientsFungiPost transplantAngiogenesis growth factor
The present invention is based, in part, on the discovery that galectin-1 (Gal1) plays a role in viral-associated PTLD, e.g., EBV-associated PTLD and hypoxia associated angiogenesis disorders. Accordingly, the invention relates to compositions, kits, and methods for diagnosing, prognosing, monitoring, treating and modulating viral-associated PTLD, e.g., EBV-associated PTLD and hypoxia associated angiogenesis disorders.
Owner:DANA FARBER CANCER INST INC +3
O-methylated rapamycin derivatives for alleviation and inhibition of lymphoproliferative disorders
The present invention relates to methods of alleviating and inhibiting a lymphoproliferative disorder in a mammal, the method comprising administering one or more rapamycin derivatives (including rapamycin) to the mammal. Further, the invention provides a method for identifying agents which are useful for alleviating and inhibiting a lymphoproliferative disorders, as well as a method for identifying agents which are capable of inhibiting metastasis of lymphatic tumors in a mammal.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Humanized anti-CD19 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
ActiveUS8323653B2Alleviation and mitigation and decreaseSenses disorderNervous disorderAntigenApoptosis
Owner:VIELA BIO INC
Novel therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
InactiveUS20050233987A1Improve toleranceImprove therapeutic potentialBiocideSugar derivativesDiseaseApoptosis
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
Anti-cd19 antibody therapy for transplantation
InactiveUS20090246195A1Reduce or deplete circulating B cellsReduce or deplete circulating immunoglobulin (Ig)Dead animal preservationAntibody ingredientsAntigenTherapeutic antibody
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Methods to Treat or Prevent Viral-Associated Lymphoproliferative Disorders
InactiveUS20090004182A1Prevents and treat and slows progressionMicrobiological testing/measurementBiological material analysisPost transplantPost-transplant lymphoproliferative disorder
The disclosure relates to methods to prevent, treat, or slow the progression viral-associated lymphoproliferative disorders, EBV-associated lymphoproliferative disorders, and post-transplant lymphoproliferative disorders. In the methods, a TGF-β antagonist, e.g., an anti-TGF-β antibody is administered to a subject. Methods for treating viral-associated lymphoproliferative disorders and for enhancing T-cell responsiveness to a viral-associated lymphoproliferative disorder by administering a TGF-β antagonist are also described.
Owner:THE OHIO STATE UNIV RES FOUND
Compositions, kits, and methods for the diagnosis, prognosis, monitoring, treatment and modulation of post-transplant lymphoproliferative disorders and hypoxia associated angiogenesis disorders using galectin-1
ActiveUS8968740B2Extensive remodelingIncreased influx and expansionPeptide/protein ingredientsImmunoglobulins against growth factorsLymphoproliferative diseasePost transplant
Owner:DANA FARBER CANCER INST INC +3
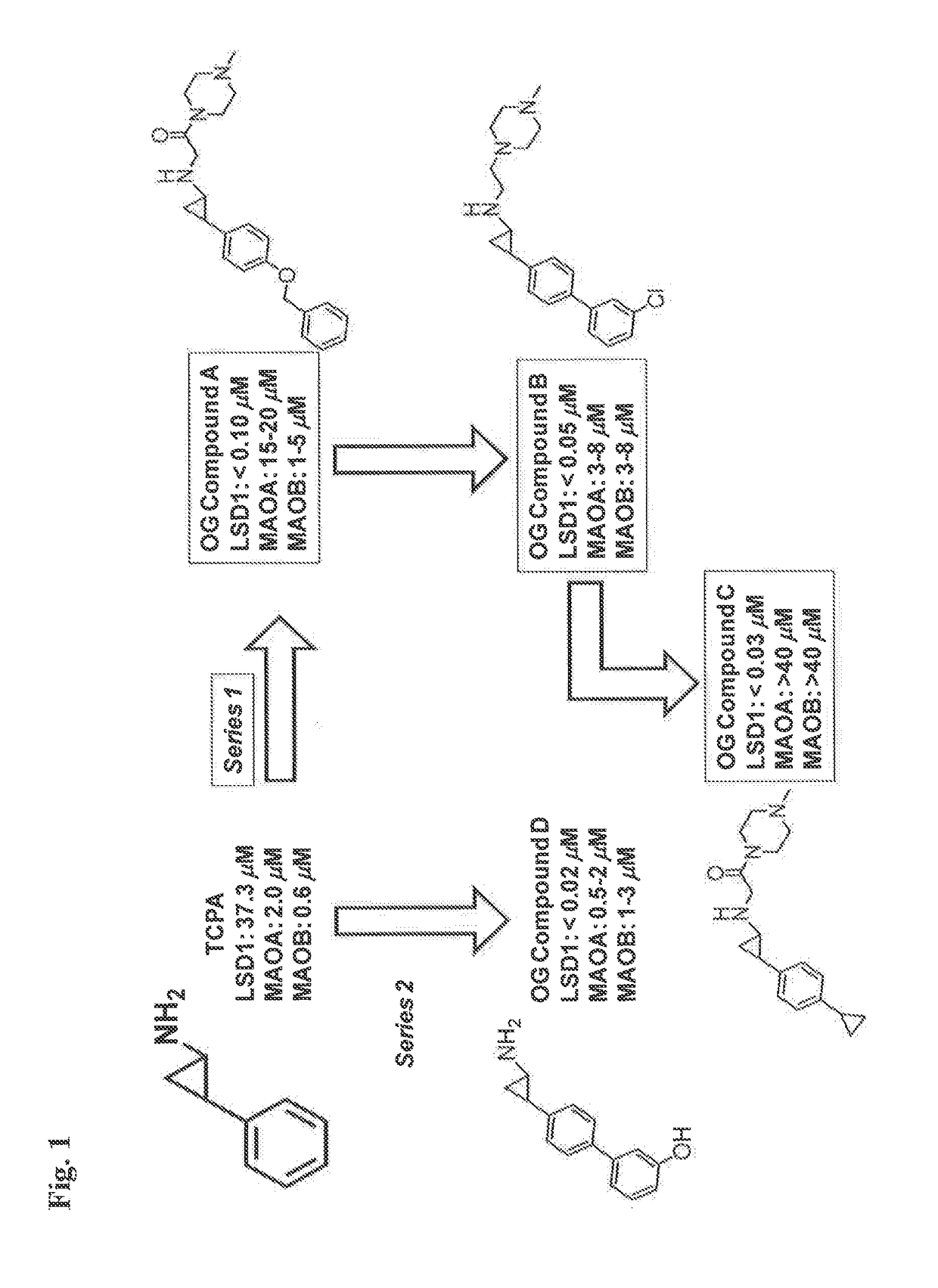
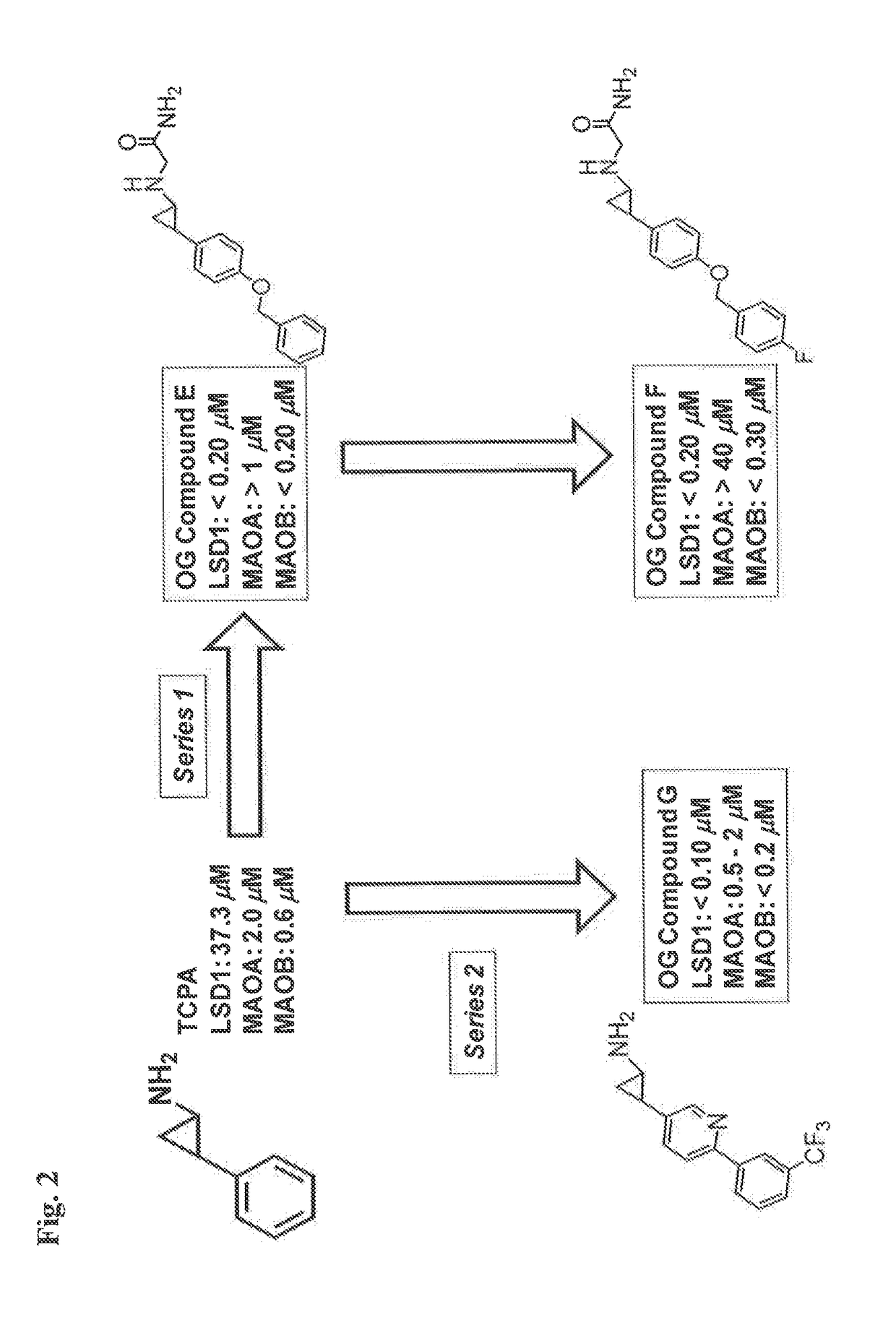
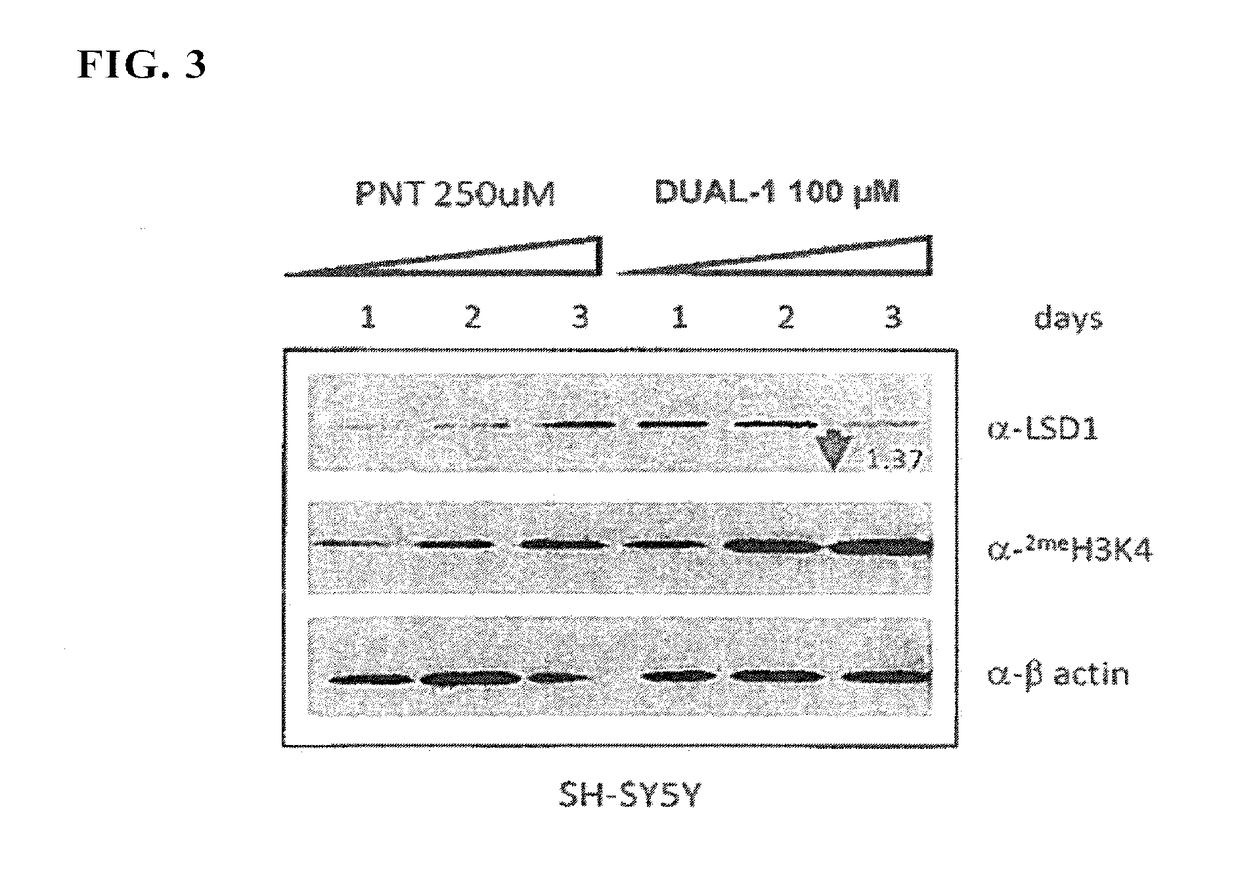
Lysine demethylase inhibitors for myeloproliferative or lymphoproliferative diseases or disorders
InactiveUS20170209432A1Reduce platelets and other blood cellsReduce impactOrganic chemistryAntineoplastic agentsLymphoproliferative diseaseLymphoid tissue hyperplasia
The present invention relates to methods and compositions for the treatment or prevention of diseases and disorder associated with myeloproliferative and lymphoproliferative disorders. In particular, the invention relates to an LSD1 inhibitor for use in treating or preventing diseases and disorder associated with myeloproliferative and lymphoproliferative disorders.
Owner:ORYZON GENOMICS SA
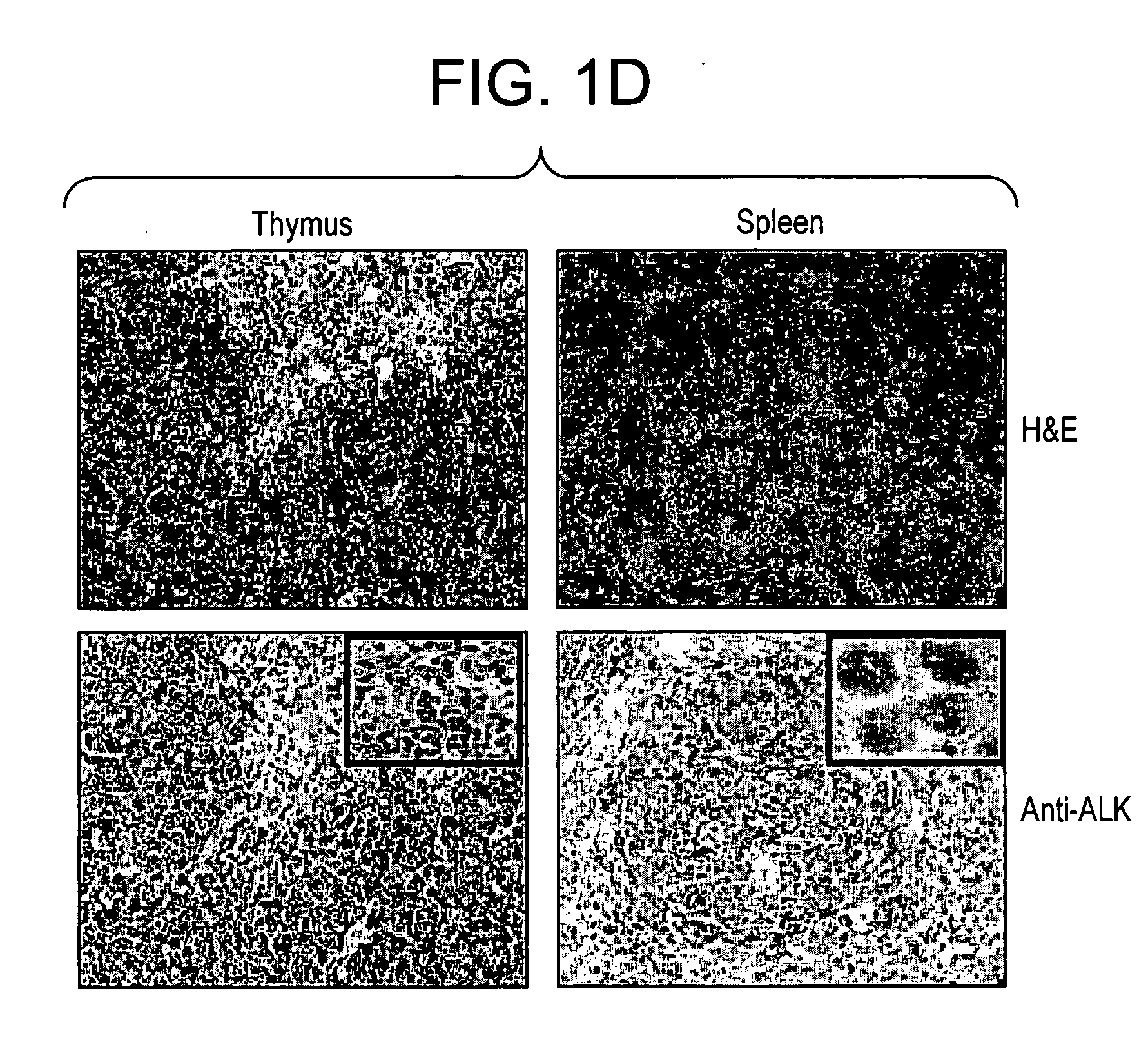
ALK protein tyrosine kinase, cells and methods embodying and using same
ActiveUS20050005314A1Inhibit expressionStable expressionVectorsSugar derivativesProtein-Tyrosine KinasesPlasma Cell Tumors
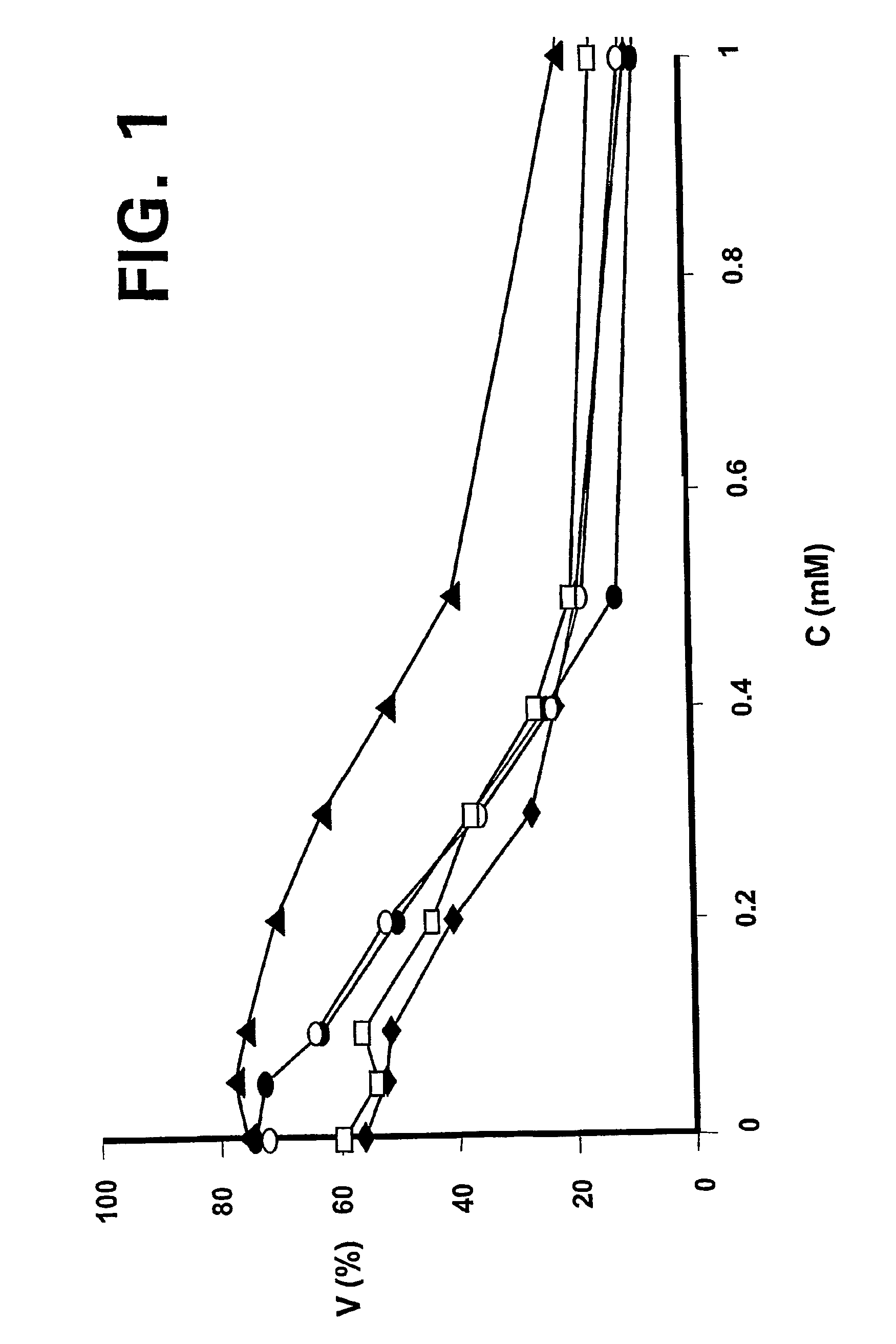
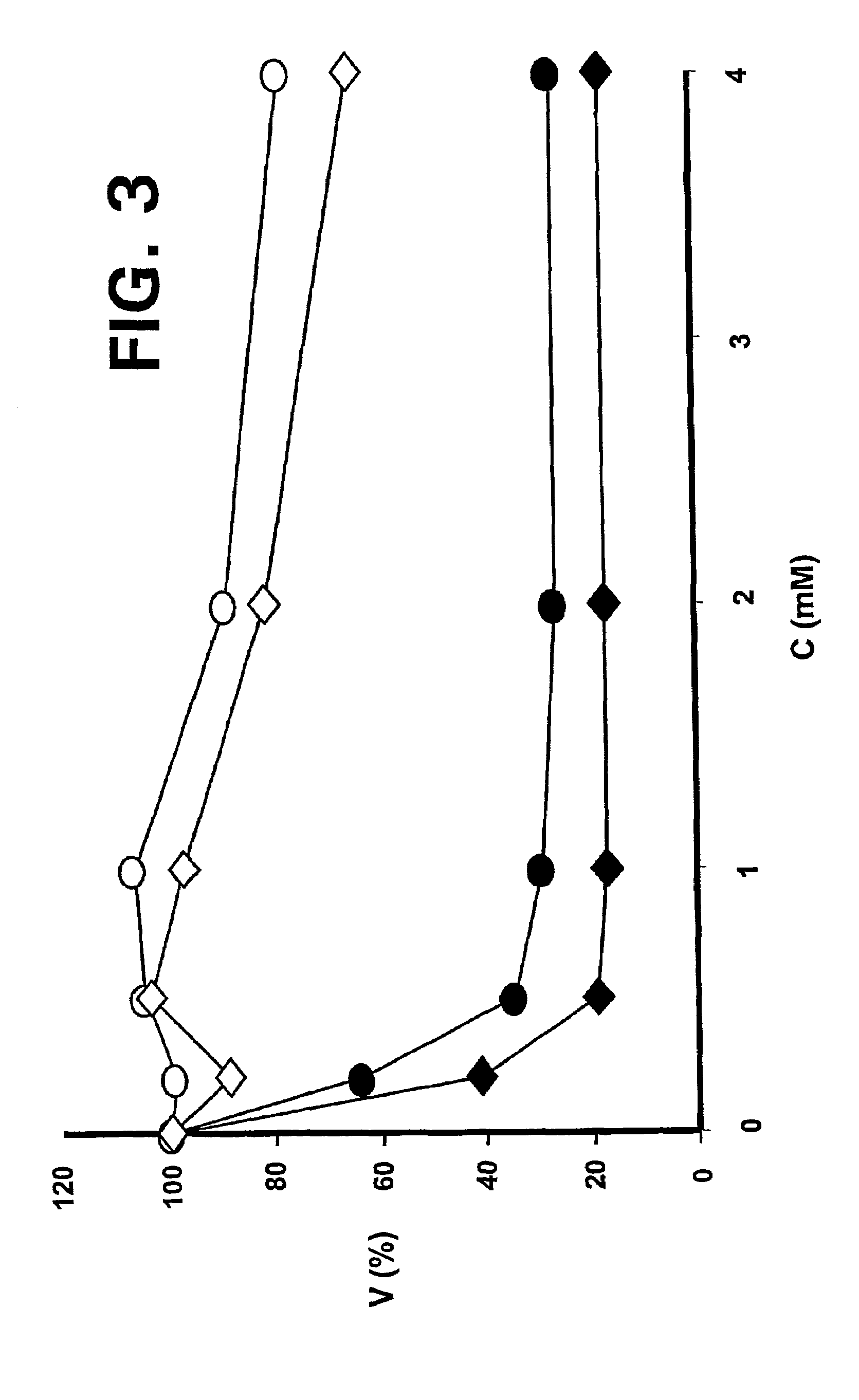
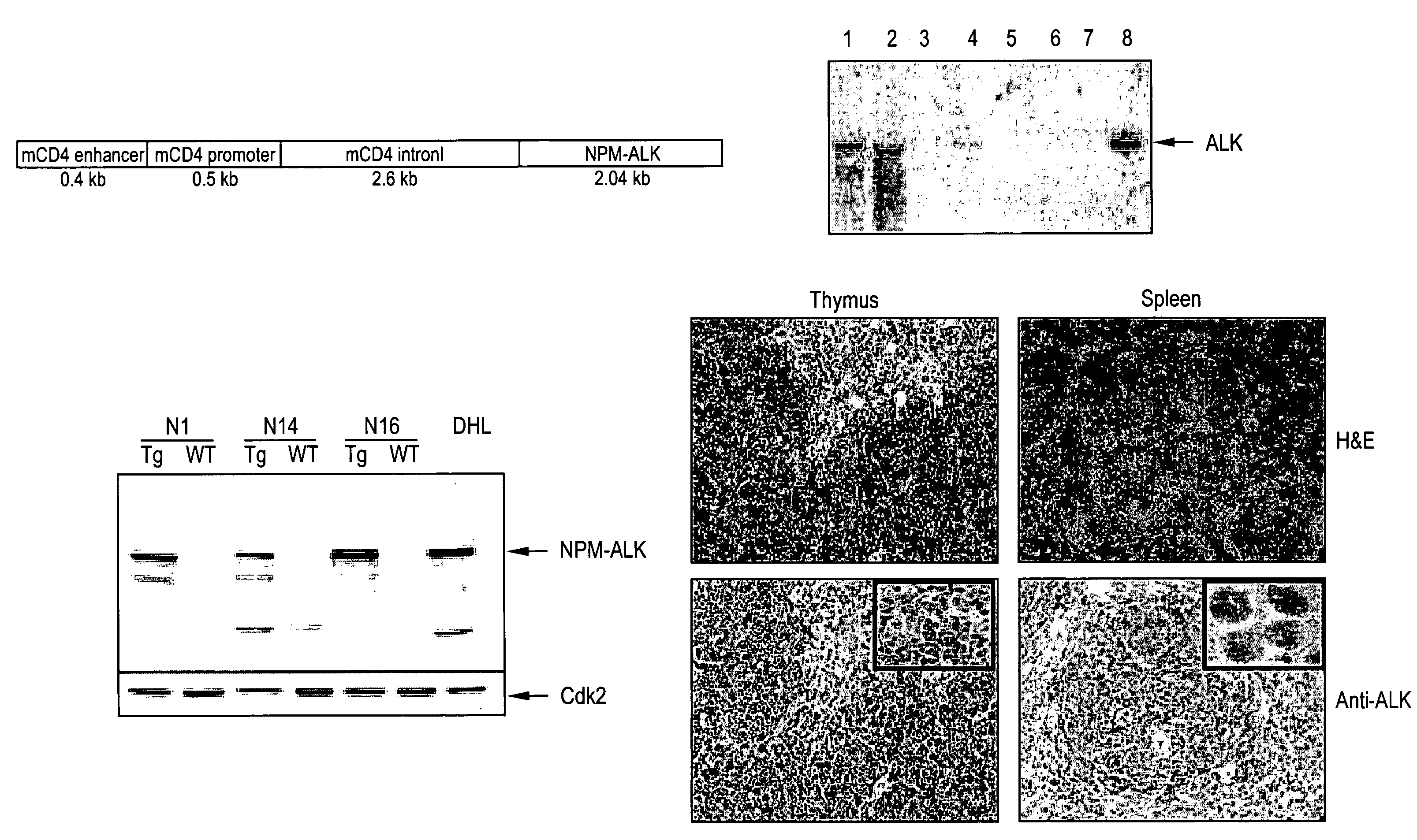
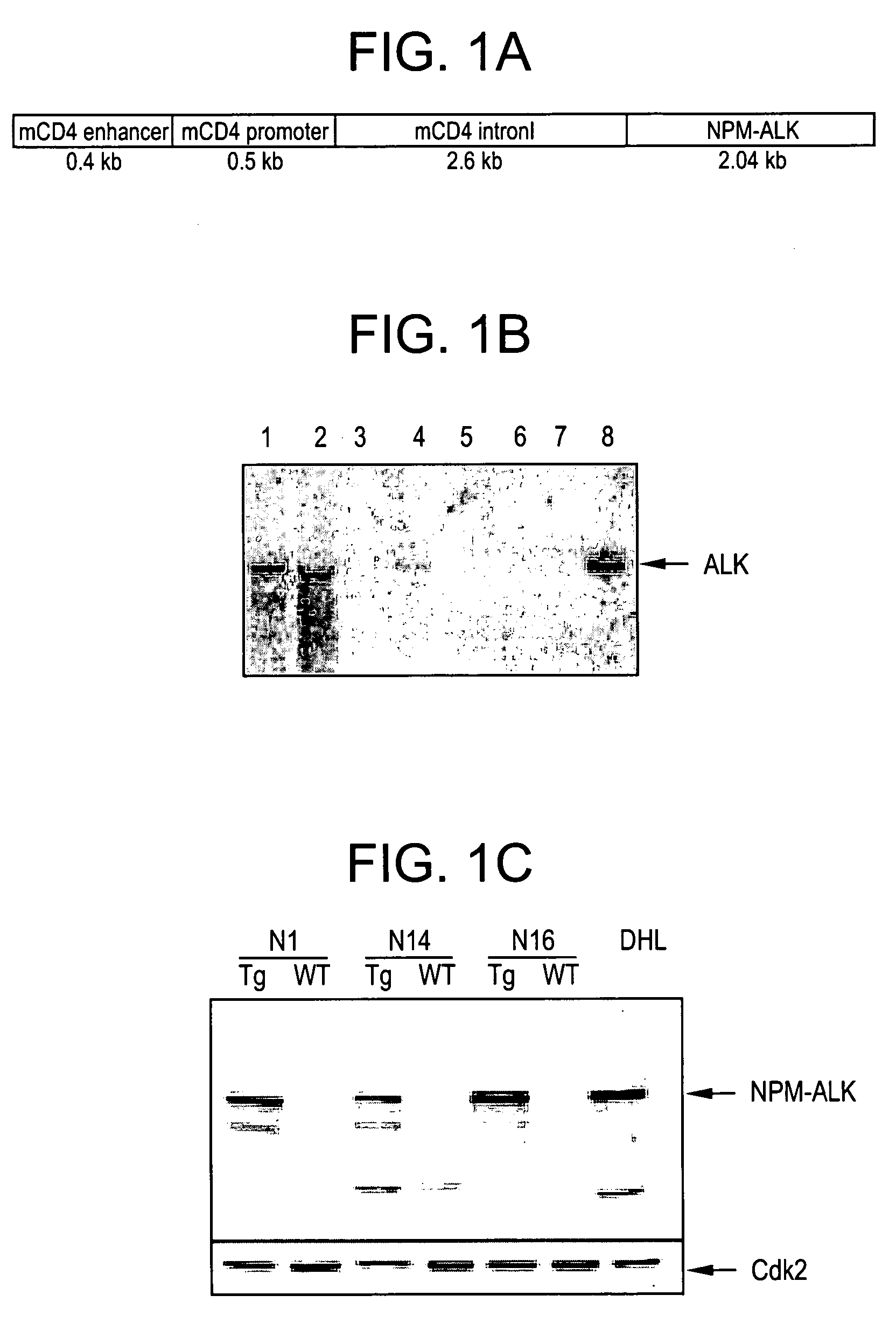
The present invention provides for a transgenic animal model that constitutively expresses a protein encoded by the NPM-ALK gene in lymphoid tissue, and exhibits enhanced and accelerated development of a T cell lymphoproliferative disorder or B cell plasma cell tumor, together with the identification of cells transduced with the ALK tyrosine kinase gene or fusion proteins thereof, and methods for using this animal model and cells for screening compounds or treatments for antitumor activity. In preferred embodiments, the animal is a transgenic mouse that expresses a human NPM-ALK gene operably linked to human regulatory sequences, and the cells of the mouse have at least one copy of the NPM-ALK transgene, whereby the mouse constitutively expresses a protein encoded by the NPM-ALK transgene. The animals and cells of the invention are useful in the study of NPM-ALK-dependent lymphomagenesis and plasma cell tumors and in the development of treatments for these conditions.
Owner:NEW YORK UNIV
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
Therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
The present disclosure relates to a method of treatment of a human patient suffering from a B-cell lymphoproliferative disorders such as B-cell chronic lymphocytic leukemia (B-CLL), splenic marginal zone lymphoma (SMZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), lymphoplasmacytic lymphoma (LPL), and Waldenström syndrome (WS), by the administration of a therapeutically effective amount of 5-aminoimidazole-4-carboxamide riboside (acadesine) or its precursors (eg. its mono-, di- and tri-5′-phosphates). This makes acadesine and its bioprecursors (eg. its mono-, di- and tri-5′-phosphates) useful as therapeutic agents for B-cell lymphoproliferative disorders in humans. The surprising feature that T cells are virtually not affected means that the side effect (immunosuppression) is minor, what represents a therapeutical advantage of acadesine over cladribine, fludarabine and other nucleosides known in the art.
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
ALK protein tyrosine kinase, cells and methods embodying and using same
ActiveUS7288529B2Inhibit expressionStable expressionVectorsSugar derivativesProtein-Tyrosine KinasesPlasma Cell Tumors
The present invention provides for a transgenic animal model that constitutively expresses a protein encoded by the NPM-ALK gene in lymphoid tissue, and exhibits enhanced and accelerated development of a T cell lymphoproliferative disorder or B cell plasma cell tumor, together with the identification of cells transduced with the ALK tyrosine kinase gene or fusion proteins thereof, and methods for using this animal model and cells for screening compounds or treatments for antitumor activity. In preferred embodiments, the animal is a transgenic mouse that expresses a human NPM-ALK gene operably linked to human regulatory sequences, and the cells of the mouse have at least one copy of the NPM-ALK transgene, whereby the mouse constitutively expresses a protein encoded by the NPM-ALK transgene. The animals and cells of the invention are useful in the study of NPM-ALK-dependent lymphomagenesis and plasma cell tumors and in the development of treatments for these conditions.
Owner:NEW YORK UNIV
Transgenic zebra fish embryo model for hematopoiesis and lymphoproliferative disorders
A transgenic zebrafish animal model is disclosed. The model can be used for study of hematopoetic cell differentiation, control, and screening of therapeutic agents and can include a transgenic zebrafish expressing a heterologous Ikaros protein.
Owner:UCKUN FATIH M
Epstein Barr virus antibodies, vaccines, and uses of the same
ActiveUS11116835B2Reduce riskReduce infectionViral antigen ingredientsImmunoglobulins against virusesEpstein-Barr Virus AntibodyMononucleosis
Anti-Epstein Barr Virus (EBV) antibodies and vaccines are described herein. The antibodies and vaccines can be used to treat and / or reduce the risk of EBV infection and to treat and / or reduce the risk of complications associated with EBV infection, such as infectious mononucleosis, lymphoproliferative disorders, carcinomas, and smooth muscle tumors.
Owner:FRED HUTCHINSON CANCER CENT
Transgenic zebra fish embryo model for hematopoiesis and lymphoproliferative disorders
ActiveUS20050198696A1High affinityGuaranteed functionVectorsAnimals/human peptidesFish embryoLymphoid tissue hyperplasia
A transgenic zebrafish animal model for the study of haemopoietic cell differentiation, control, and screening of therapeutic agents.
Owner:PARKER HUGHES INST
Epstein barr virus antibodies, vaccines, and uses of the same
ActiveUS20200164059A1Reduce EBV infectionReduce riskViral antigen ingredientsImmunoglobulins against virusesEpstein-Barr Virus AntibodyMononucleosis
Anti-Epstein Barr Virus (EBV) antibodies and vaccines are described herein. The antibodies and vaccines can be used to treat and / or reduce the risk of EBV infection and to treat and / or reduce the risk of complications associated with EBV infection, such as infectious mononucleosis, lymphoproliferative disorders, carcinomas, and smooth muscle tumors.
Owner:FRED HUTCHINSON CANCER CENT
Method for the diagnosis of lymphoproliferative diseases
InactiveUS7803532B2Extending remissionSugar derivativesMaterial analysis by observing effect on chemical indicatorT cellLymphocyte disorder
The present invention relates to novel methods for the diagnosis and therapy of lymphoproliferative diseases. Specifically, the present invention relates to novel methods for the diagnosis and therapy taking advantage of the detection of chromosomal breakpoints in chromosome 12 and / or translocation of chromosomal material from chromosome 12, said chromosomal breakpoints and / or translocation(s) being associated with lymphoproliferative diseases, such as primary cutaneous T-cell lymphomas (CTCL). The present invention further relates to the use of neuron navigator 3 gene (NAV3) or an equivalent or functional fragment thereof involved in chromosomal breakpoints in chromosome 12 and / or translocations thereof, said gene and / or translocations thereof being associated with lymphoproliferative diseases, such as primary cutaneous T-cell lymphomas (CTCL), as a diagnostic and therapeutic agent. The present invention also relates to the development of therapy.
Owner:VALIPHARMA
Antibody composition and application thereof in screening of post-transplant lymphoproliferative disorders (PTLD)
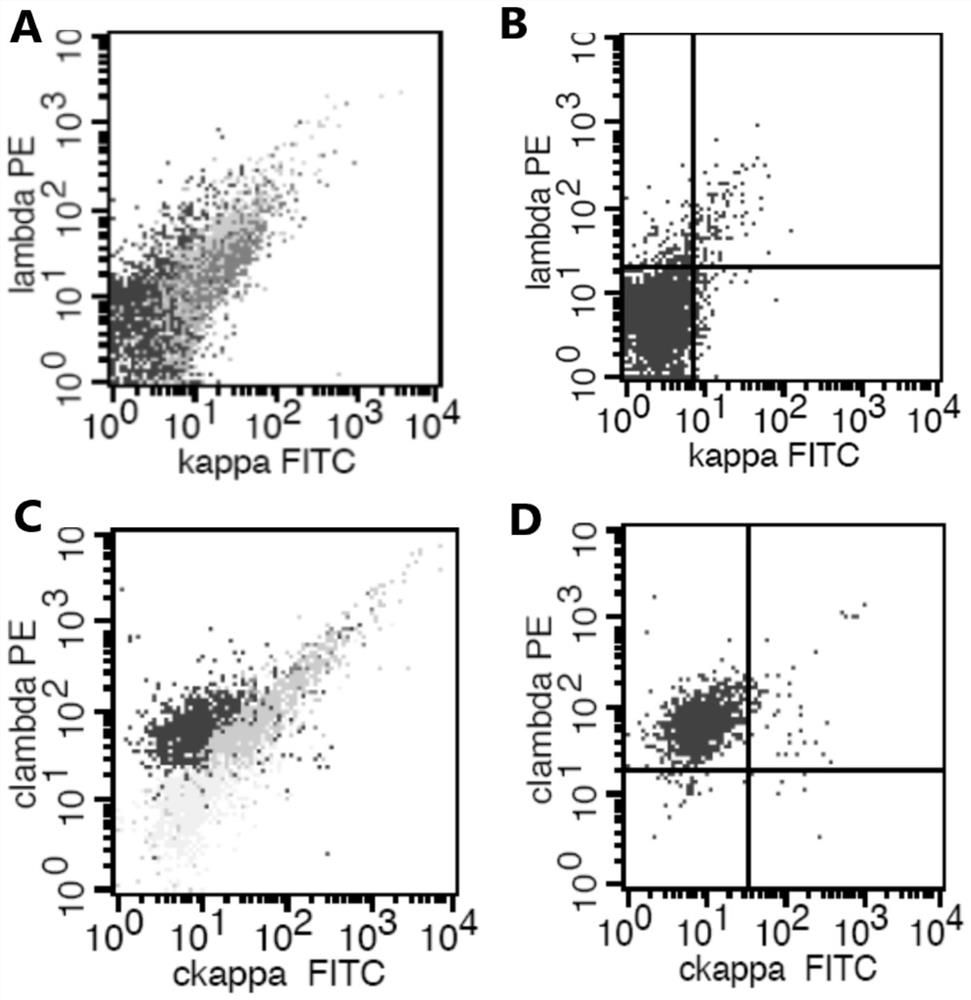
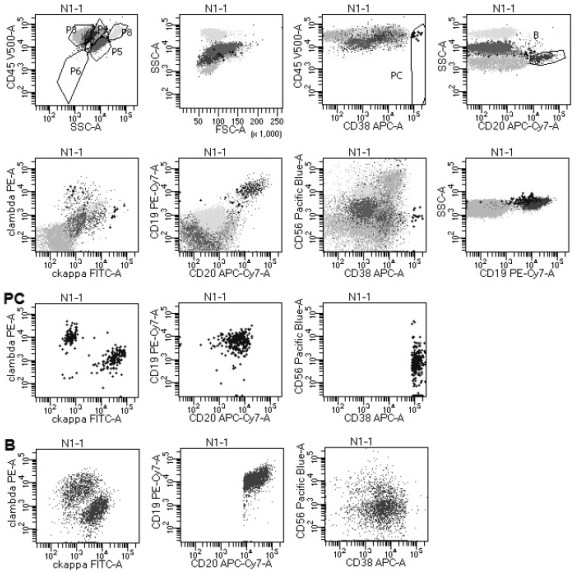
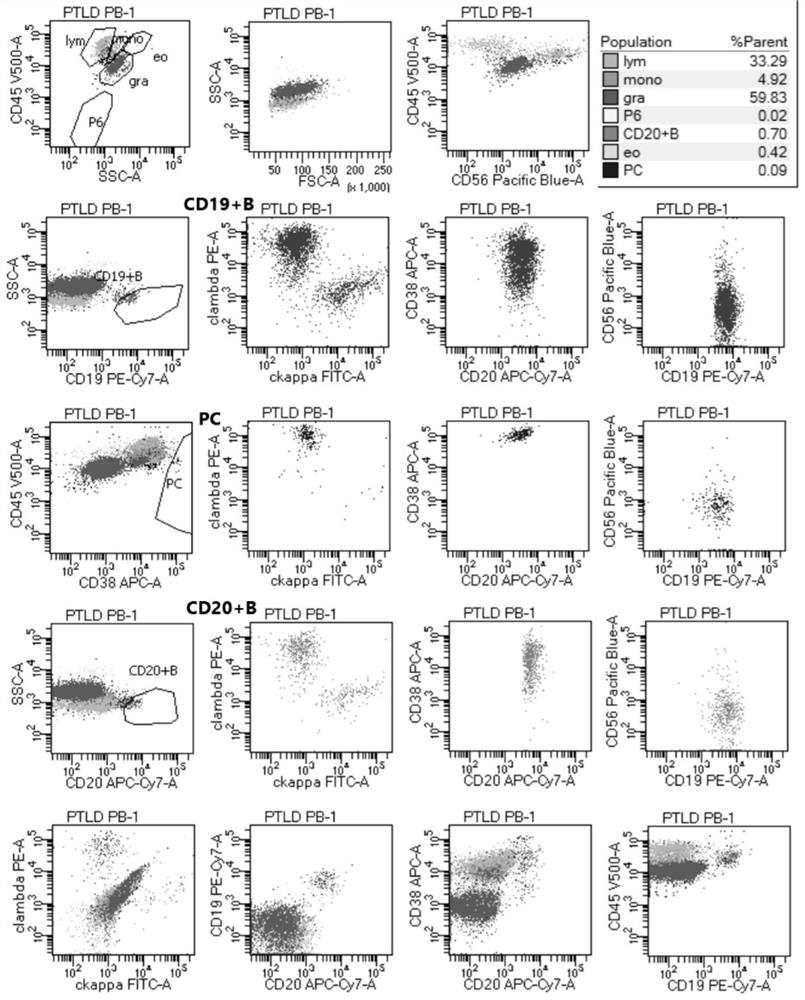
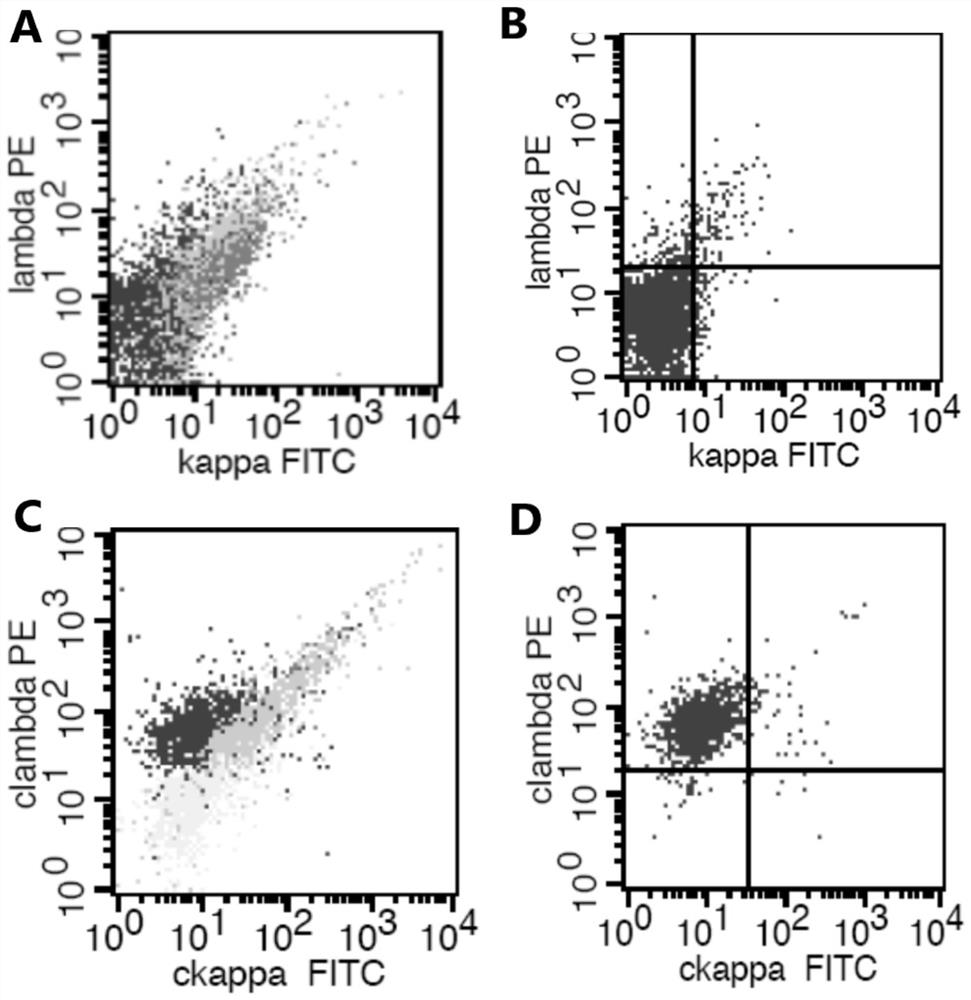
ActiveCN112578117AWide portfolio coverageWon't missBiological material analysisIndividual particle analysisDiseaseAntiendomysial antibodies
The invention provides an antibody composition and an application thereof in screening of post-transplant lymphoproliferative disorders (PTLD). The antibody composition comprises a first group of antibodies and a second group of antibodies, wherein the first group of antibodies comprises an anti-CD19 antibody, an anti-CD38 antibody, an anti-CD20 antibody, an anti-CD56 antibody and an anti-CD45 antibody; and the second group of antibodies comprises an anti-cytoplasm (cytoplasmatic, c) kappa antibody and an anti-cytoplasm lambda antibody. The antibody composition disclosed by the invention can be used for PTLD screening of patients suffering from fever and lymphadenitis after transplantation, can be used for monitoring PTLD in the early stage, and is high in sensitivity, good in specificity,convenient to operate, simple, convenient, economical and high in cost performance.
Owner:信纳克(北京)生化标志物检测医学研究有限责任公司
Methods of treating malignant lymphoproliferative disorders
Owner:ACTUATE THERAPEUTICS INC
Methods of treating Epstein-Barr virus-associated lymphoproliferative disorders by T cell therapy
The invention relates to methods of treating an EBV-LPD (Epstein-Barr Virus-associated lymphoproliferative disorder) in a human patient who has failed combination chemotherapy to treat the EBV-LPD and / or radiation therapy to treat the EBV-LPD, comprising administering to the human patient a population of allogeneic T cells comprising EBV-specific T cells.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Treatment with Anti-alpha2 integrin antibodies
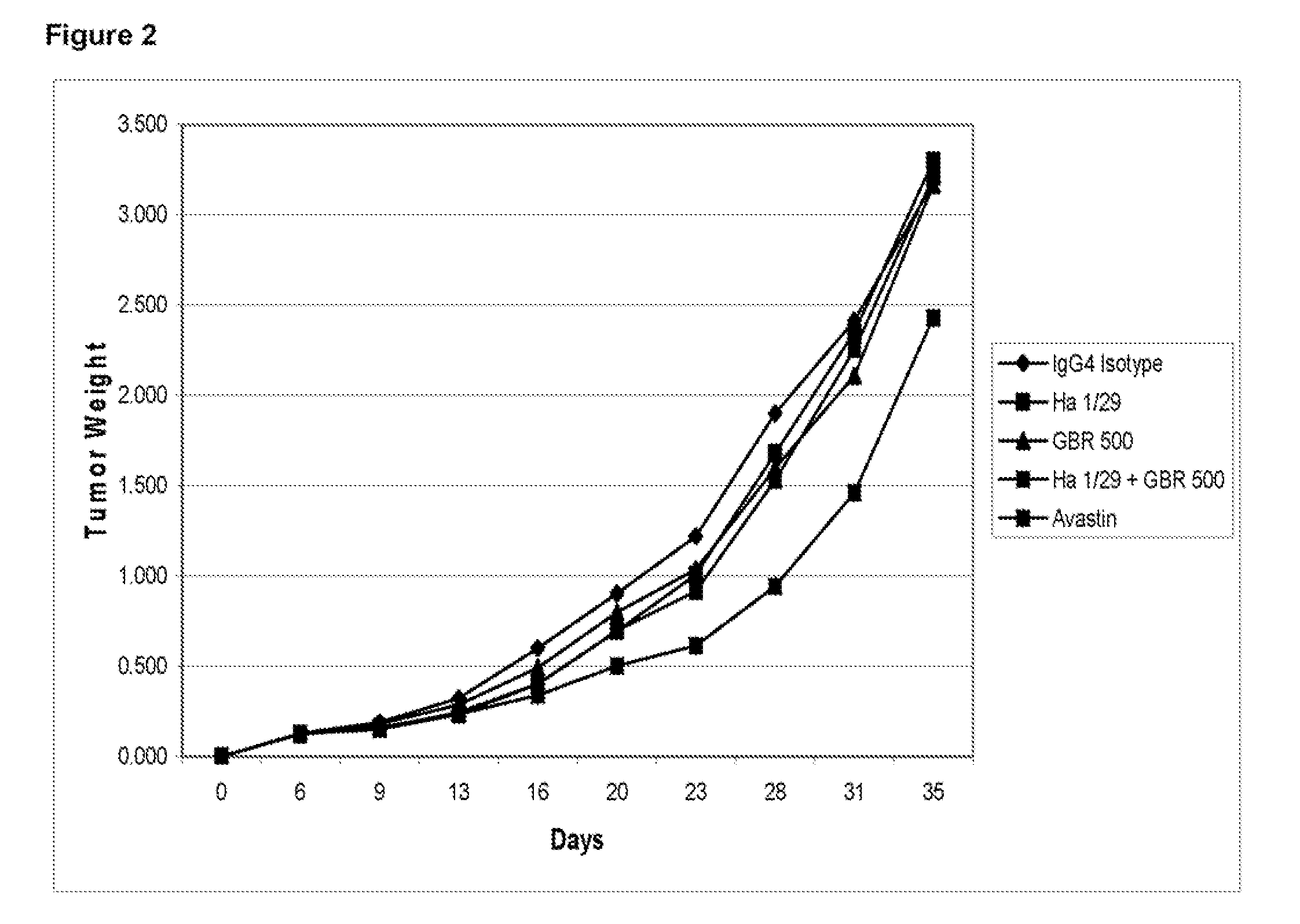
ActiveUS20100158904A1Inhibit bindingAvoid stickingNervous disorderMuscular disorderUterine carcinomaLymphoid Tumor
The invention relates to treatment of cancer. More specifically the invention relates to methods of treating cancer selected from the group consisting of squamous cell cancer, lung cancer including small-cell lung cancer, non-small cell lung cancer, adenocarcinoma of the lung, and squamous carcinoma of the lung, cancer of the peritoneum, hepatocellular cancer, gastric or stomach cancer including gastrointestinal cancer, pancreatic cancer, glioblastoma, cervical cancer, ovarian cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer, colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma, kidney or renal cancer, liver cancer, prostate cancer, vulval cancer, thyroid cancer, hepatic carcinoma and various types of head and neck cancer, as well as B-cell lymphoma including low grade / follicular non-Hodgkin's lymphoma (NHL); small lymphocytic (SL) NHL; intermediate grade / follicular NHL; intermediate grade diffuse NHL; high grade immunoblastic NHL; high grade lymphoblastic NHL; high grade small non-cleaved cell NHL; bulky disease NHL; mantle cell lymphoma; AIDS-related lymphoma; and Waldenstrom's Macroglobulinemia; chronic lymphocytic leukemia (CLL); acute lymphoblastic leukemia (ALL); Hairy cell leukemia; chronic myeloblastic leukemia; and post-transplant lymphoproliferative disorder (PTLD), as well as abnormal vascular proliferation associated with phakomatoses, edema such as that associated with brain tumors, Meigs' syndrome, melanoma, mesothelioma, multiple myeloma, fibrosarcoma, osteosarcoma and epidermoid carcinoma, by administering antibodies directed to α2β1 integrin.
Owner:ICHNOS SCI SA
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20130028888A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAutoimmune conditionAutoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
Methods for identifying inducers and inhibitors of propteolytic antibodies, compositions and their uses
Covalently reactive antigen analogs are disclosed herein. The antigens of the invention may be used to stimulate production of catalytic antibodies specific for predetermined antigens associated with particular medical disorders. The antigen analogs may also be used to permanently inactivate endogenously produced catalytic antibodies produced in certain autoimmune diseases as well as in certain lymphoproliferative disorders.
Owner:内布拉斯卡大学评议会 +1
Means and methods for counteracting myeloproliferative or lymphoproliferative disorders
ActiveCN106062002ASerum immunoglobulinsBiological material analysisLymphoid tissue hyperplasiaLymphocyte disorder
The invention provides human AML-specific binding compounds that are able to bind a cell surface component of AML cells. Therapeutic uses of binding compounds against AML are also provided.
Owner:科凌生物医疗有限公司
Antibody composition and its application in screening lymphoproliferative diseases after transplantation
ActiveCN112578117BWide portfolio coverageWon't missBiological material analysisIndividual particle analysisDiseaseAntiendomysial antibodies
The invention provides an antibody composition and its application for screening post-transplant lymphoproliferative disorders (PTLD). The antibody composition includes a first group of antibodies and a second group of antibodies, the first group of antibodies includes: anti-CD19 antibodies, anti-CD38 antibodies, anti-CD20 antibodies, anti-CD56 antibodies and anti-CD45 antibodies; the second group of antibodies includes: anti- Cytoplasmic (cytoplasmatic, c) kappa antibody and anti-cytoplasmic lambda antibody. The antibody composition of the present invention can be used for PTLD screening of patients with fever and enlarged lymph nodes after transplantation, can monitor PTLD at an early stage, has high sensitivity, good specificity, convenient operation, and is simple, economical and cost-effective.
Owner:信纳克(北京)生化标志物检测医学研究有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com