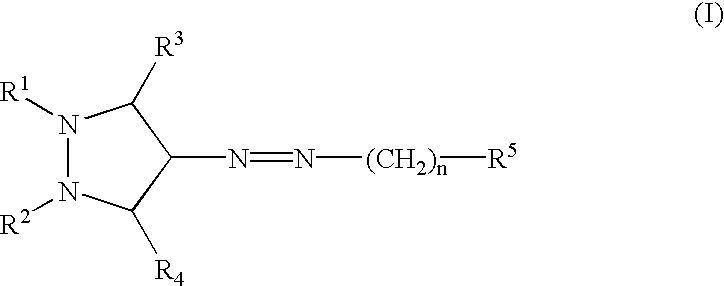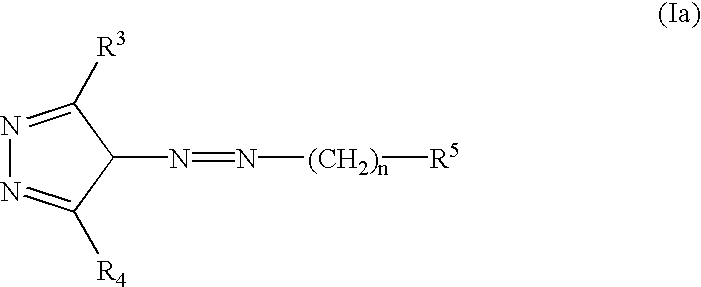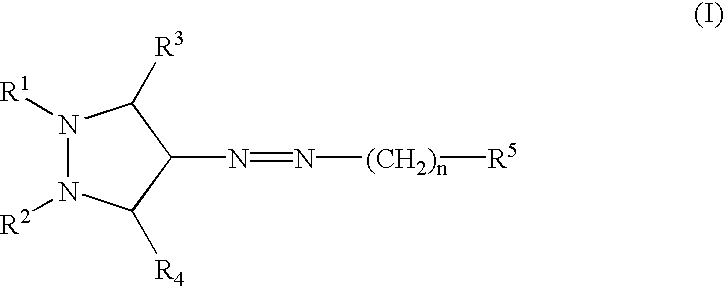Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Lymphoid tissue hyperplasia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Idiotypic vaccine
InactiveUS20100310586A1Substantial cross-reactivityIncrease biomassSsRNA viruses positive-senseViral antigen ingredientsADAMTS ProteinsLymphoid tissue hyperplasia
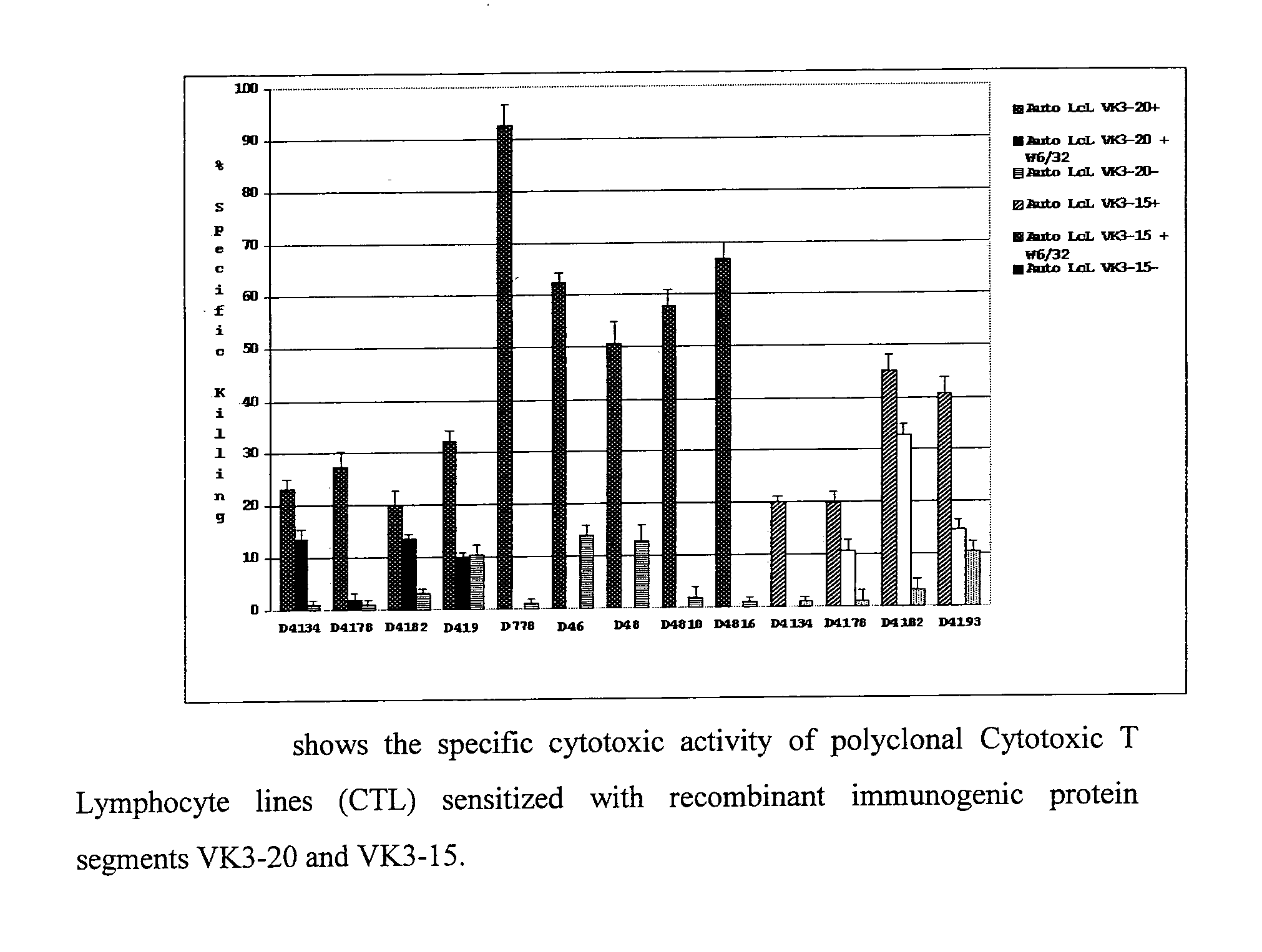
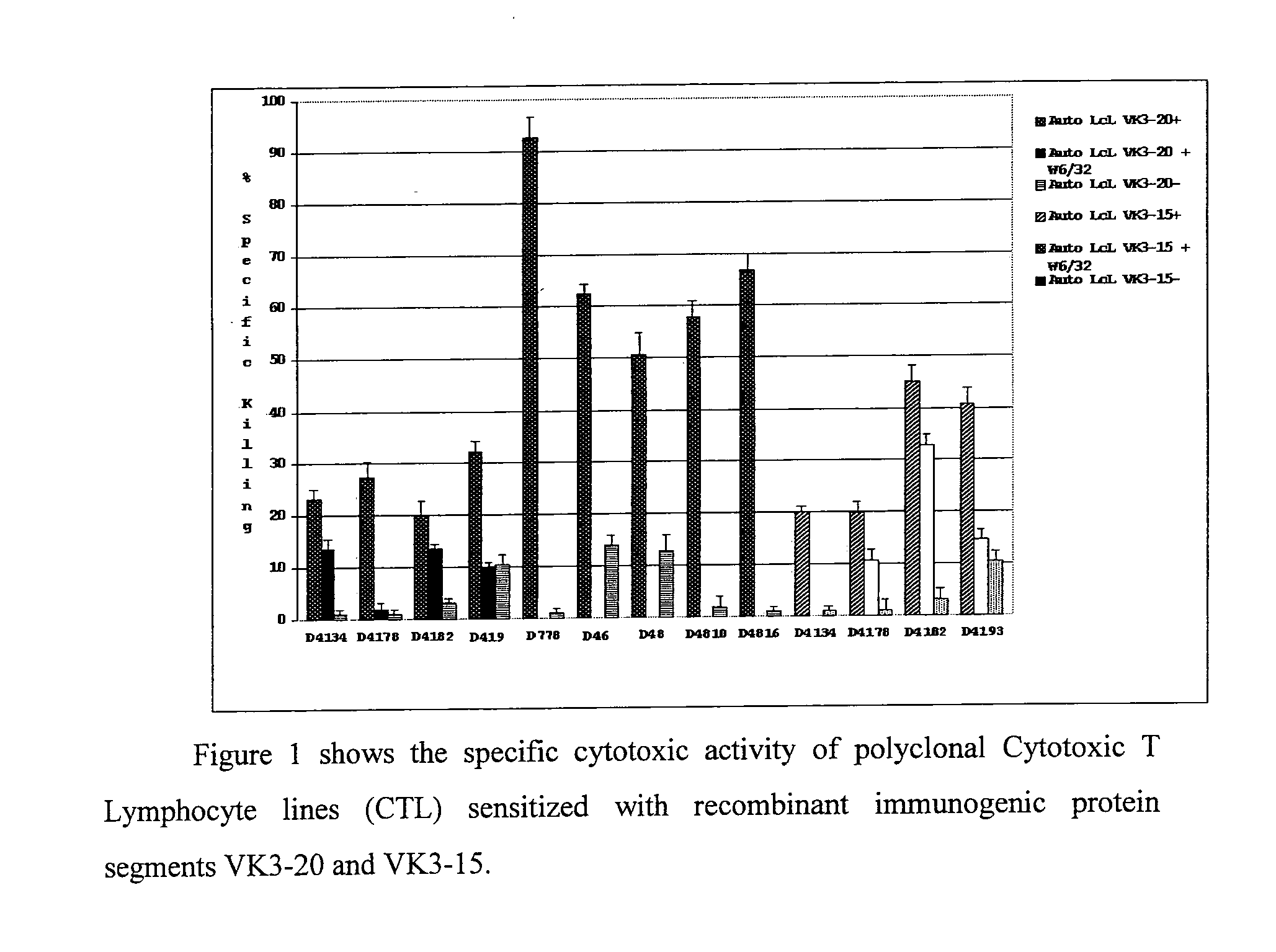
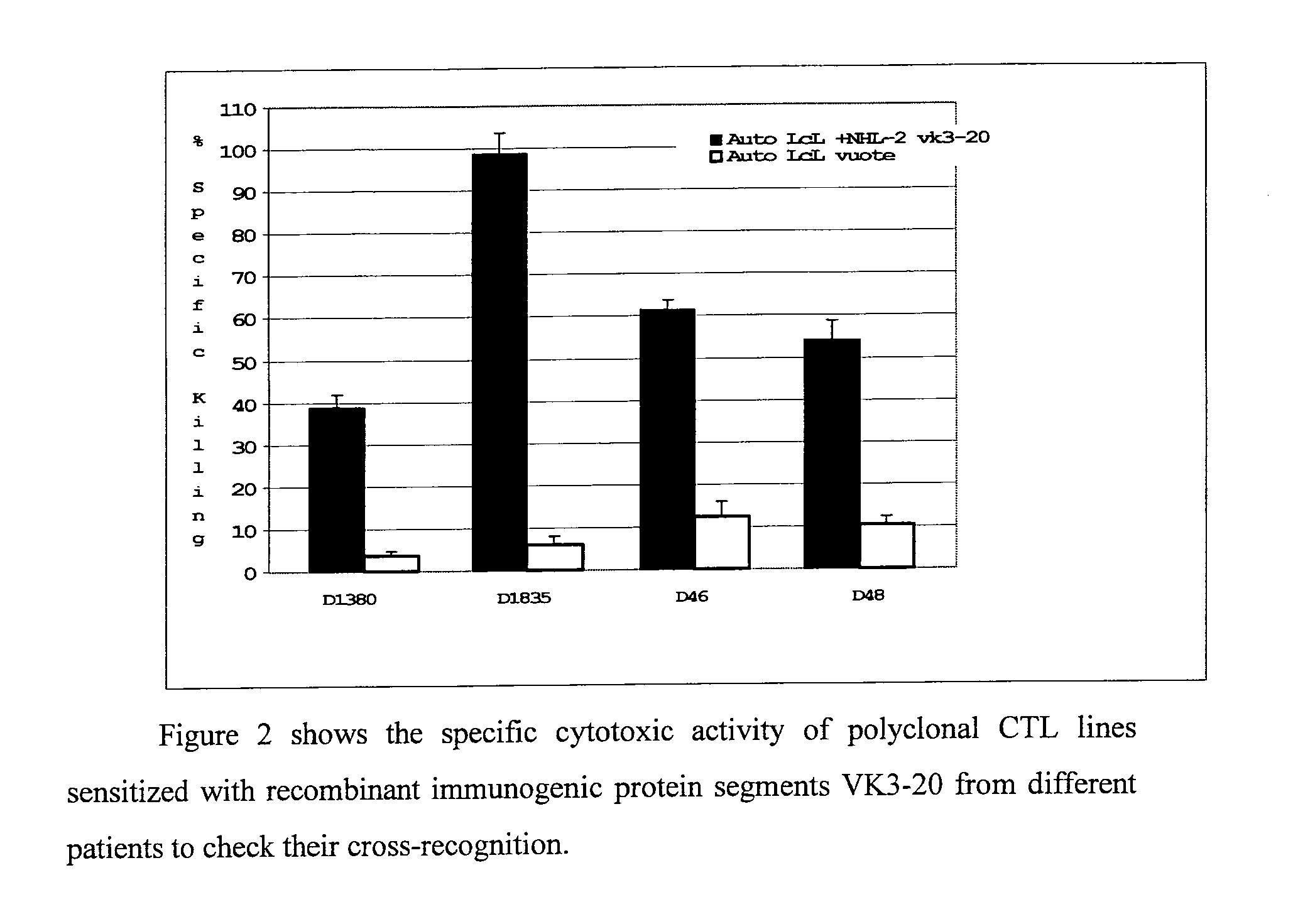
The invention concerns the use of recombinant clonotypic immunoglobulins (Ig) as a vaccine in the treatment of HCV-related and non HCV-related lymphoproliferations, in particular the use of recombinant proteins with immunogenic properties derived from protein segments VK3-20 and VK3-15 of Ig light chains derived from patients with lymphoproliferations.
Owner:ARETA INT SRL
Transgenic mouse model of B cell malignancy
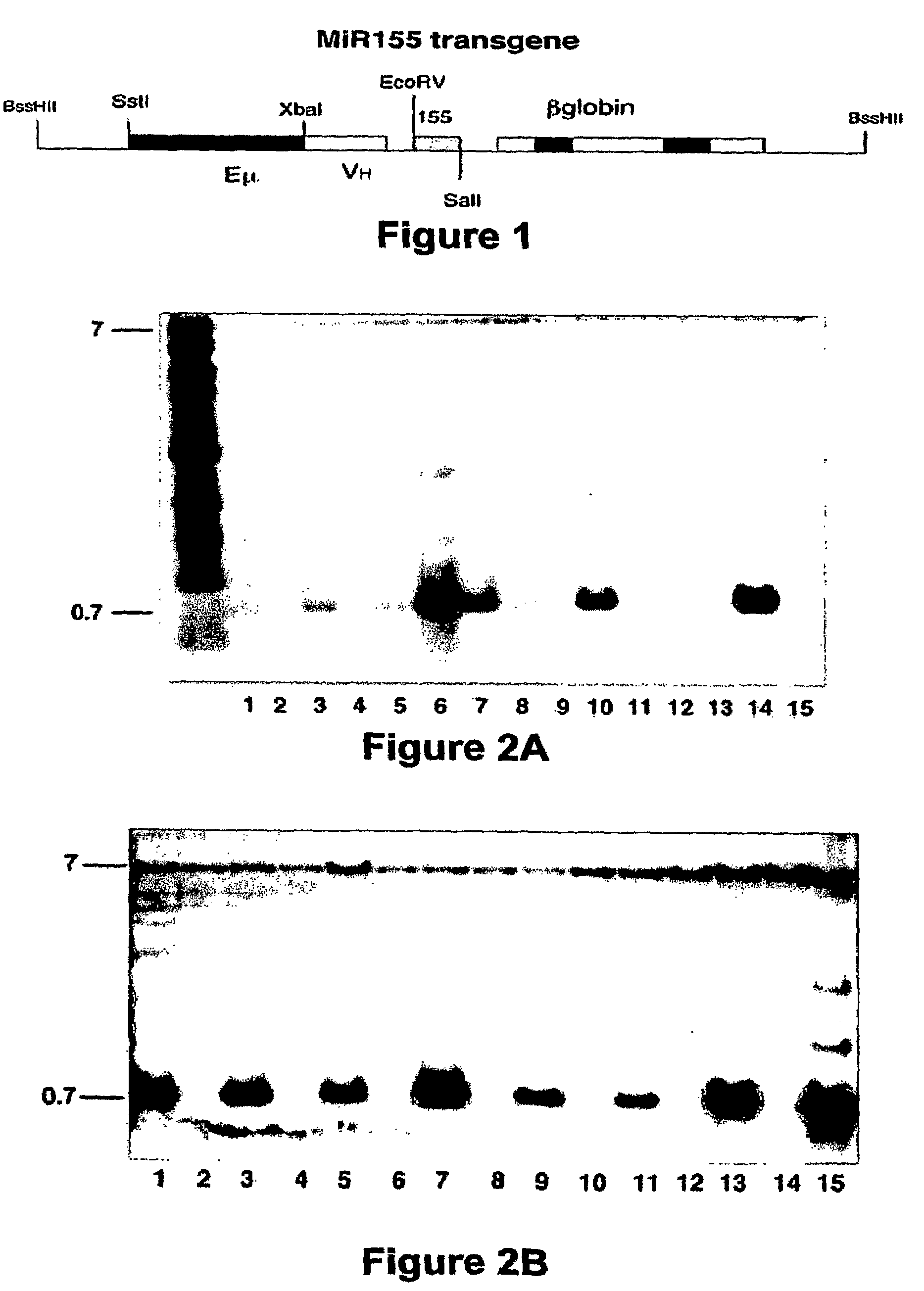
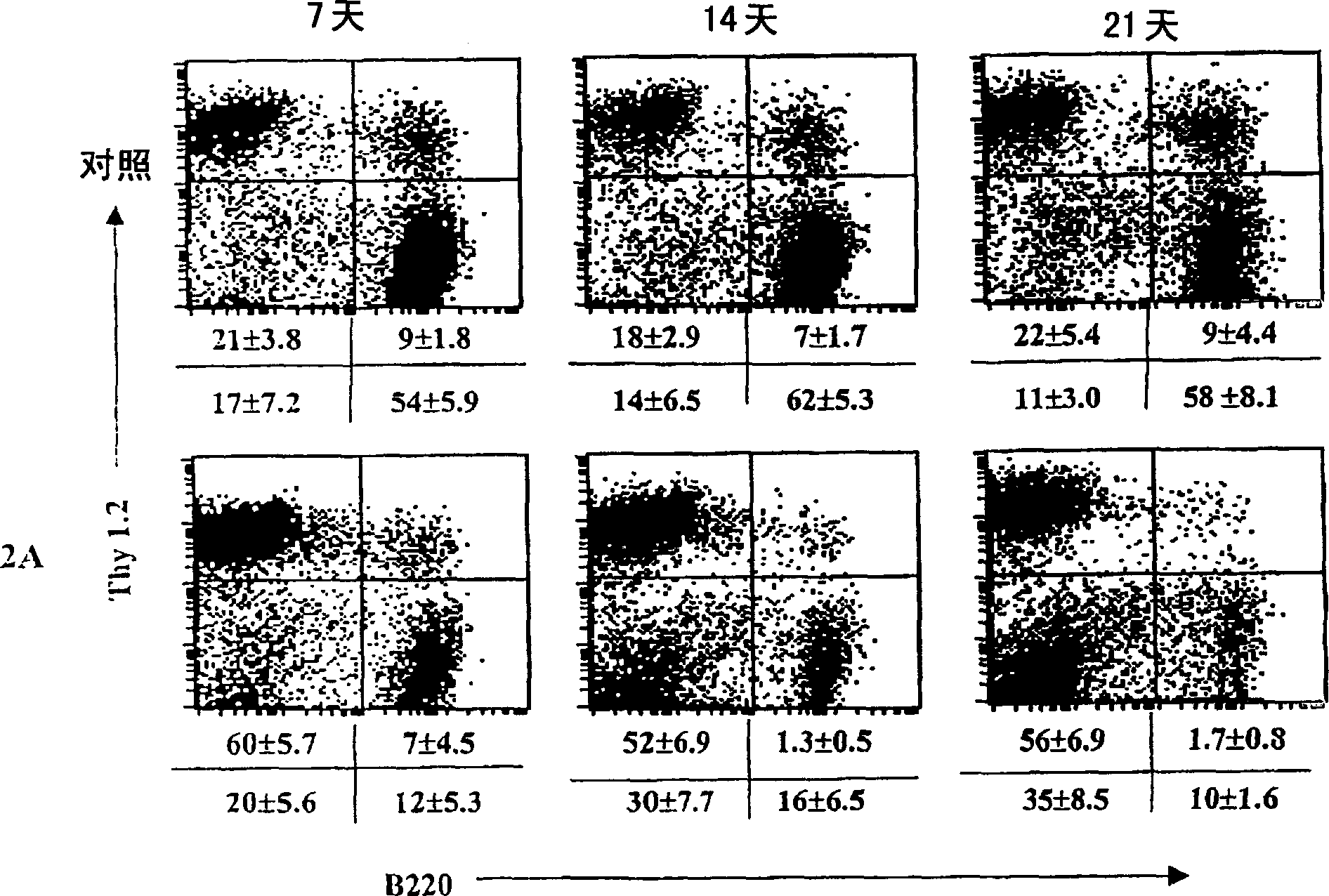
A transgenic non-human animal, such as a mouse, has a genome that include a nucleic acid construct having at least one transcriptional regulatory sequence capable of directing expression in B cells of the animal, wherein the transcriptional regulatory sequence is operably linked to a nucleic acid encoding a miR155 gene product. A method of testing the therapeutic efficacy of an agent in treating or preventing a lymphoproliferative condition includes assessing the effect(s) of the agent on a transgenic non-human animal.
Owner:THE OHIO STATE UNIV RES FOUND
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Methods for identifying, diagnosing, and predicting survival of lymphomas
ActiveUS20050164231A1Sugar derivativesMicrobiological testing/measurementLymphoid tissue hyperplasiaLymphocyte disorder
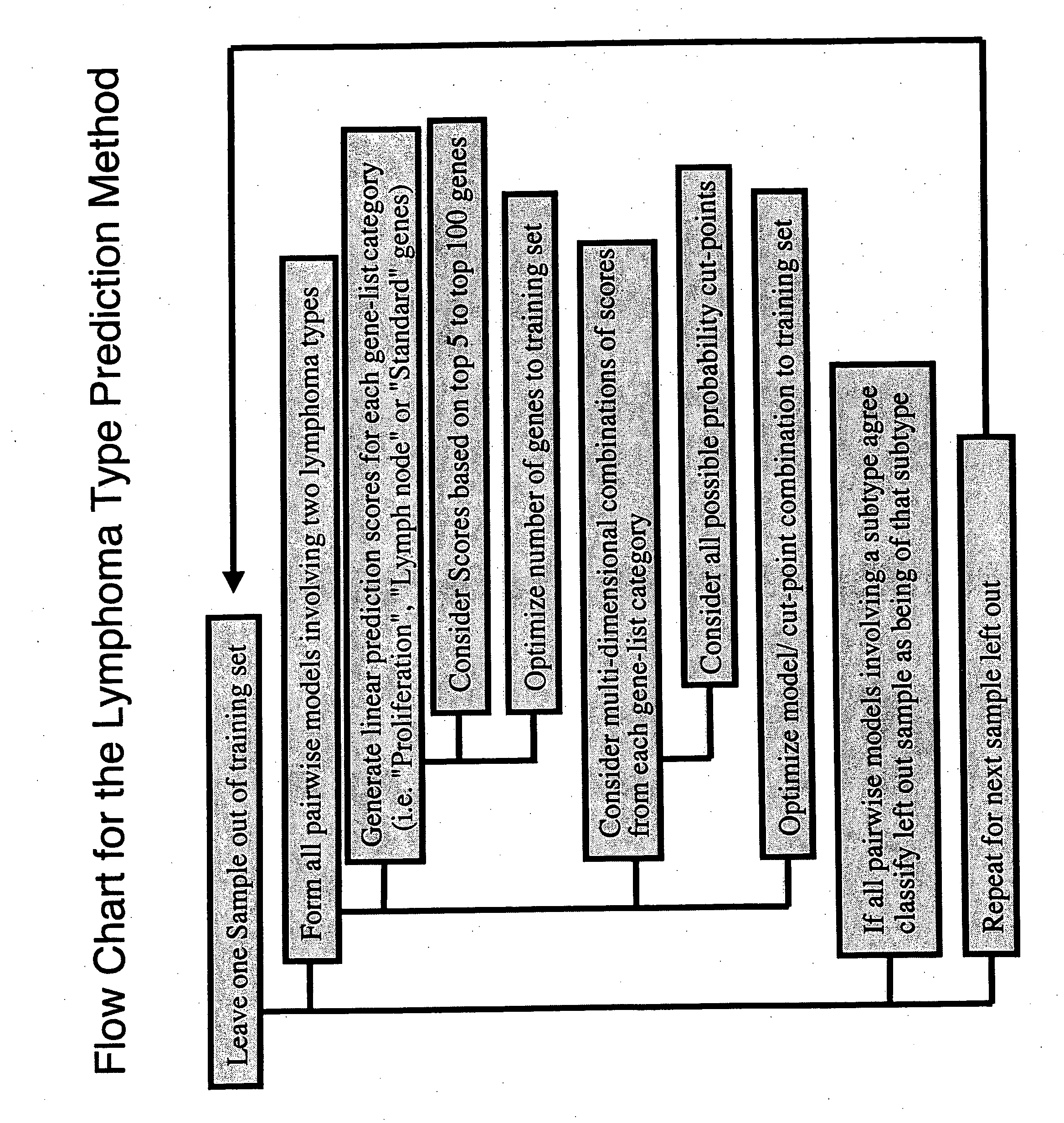
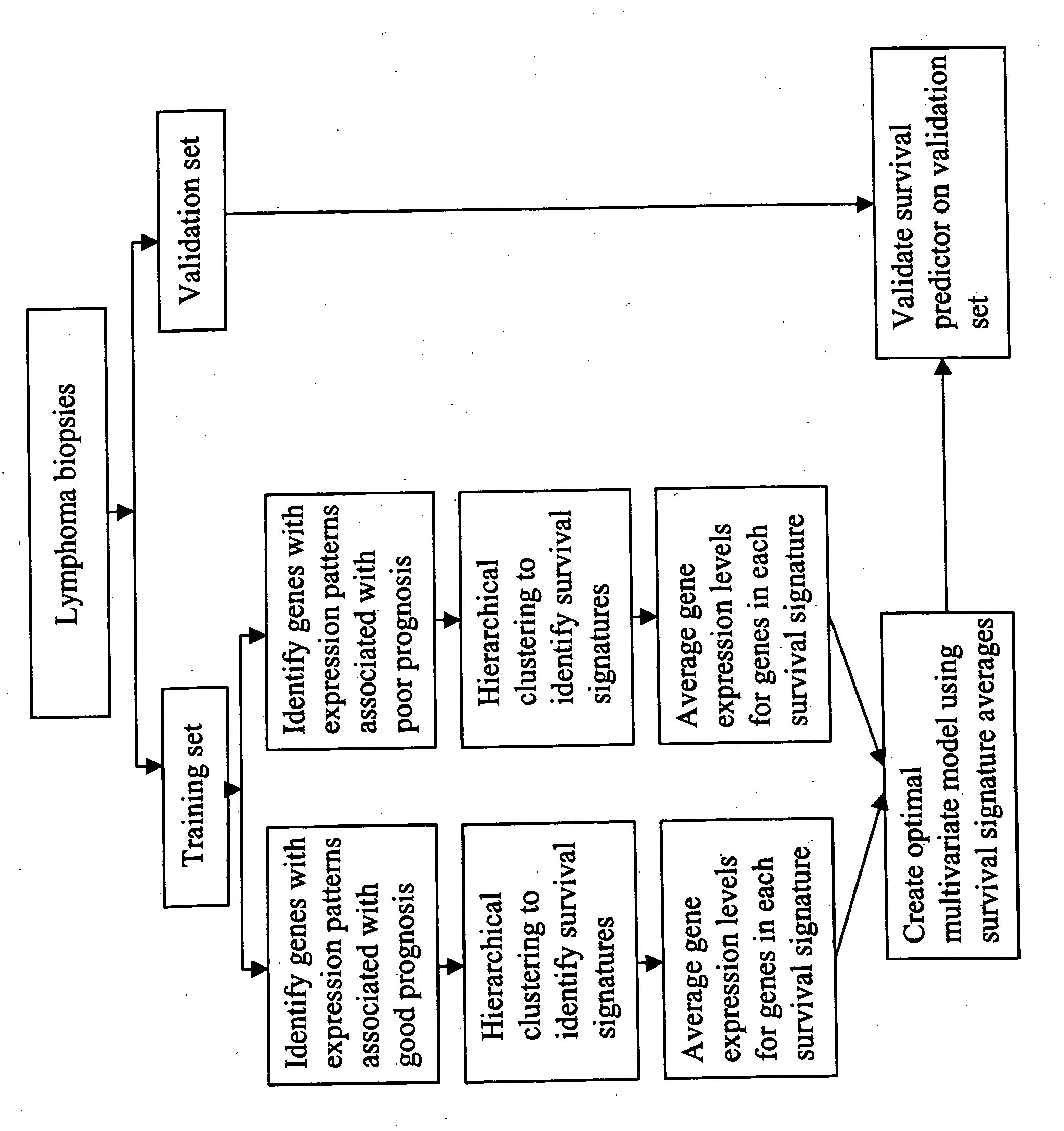
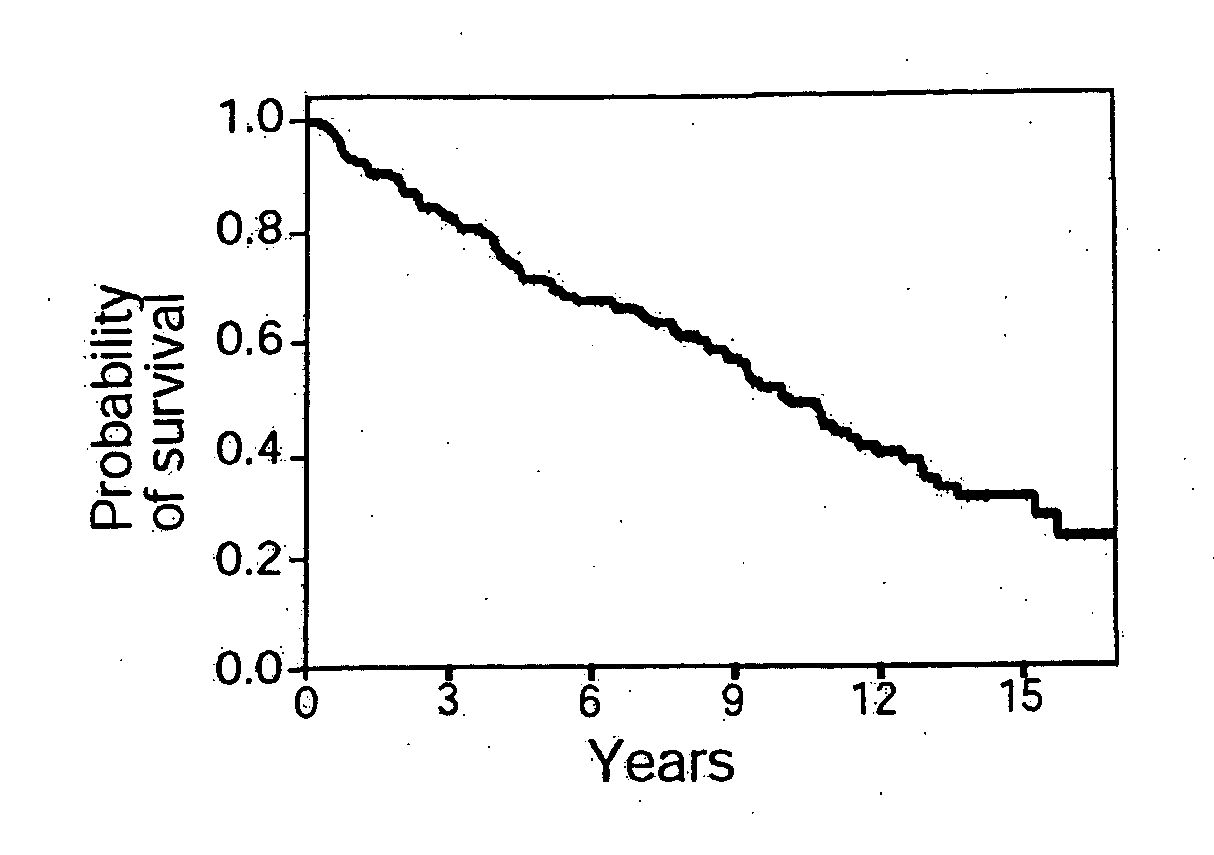
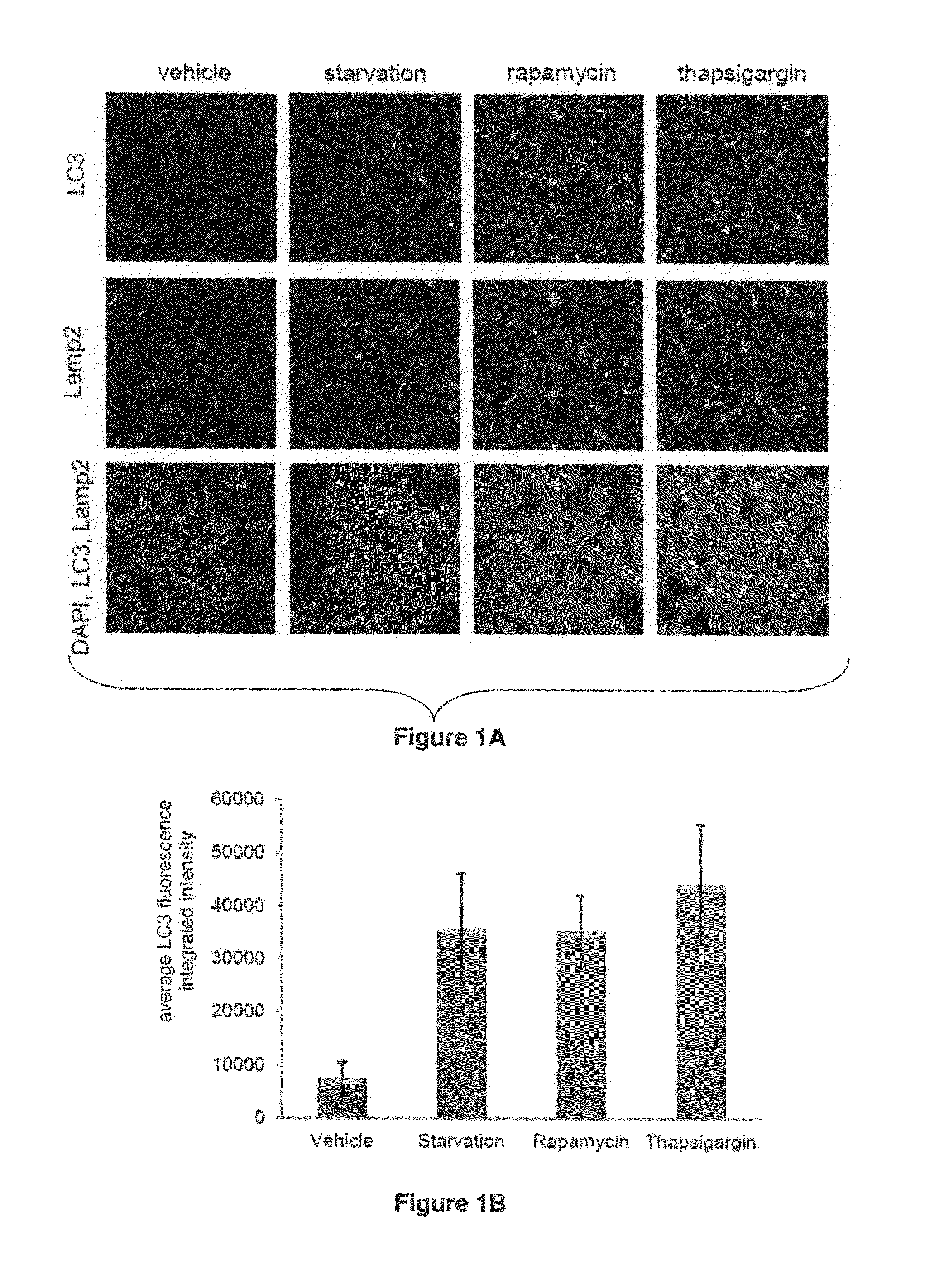
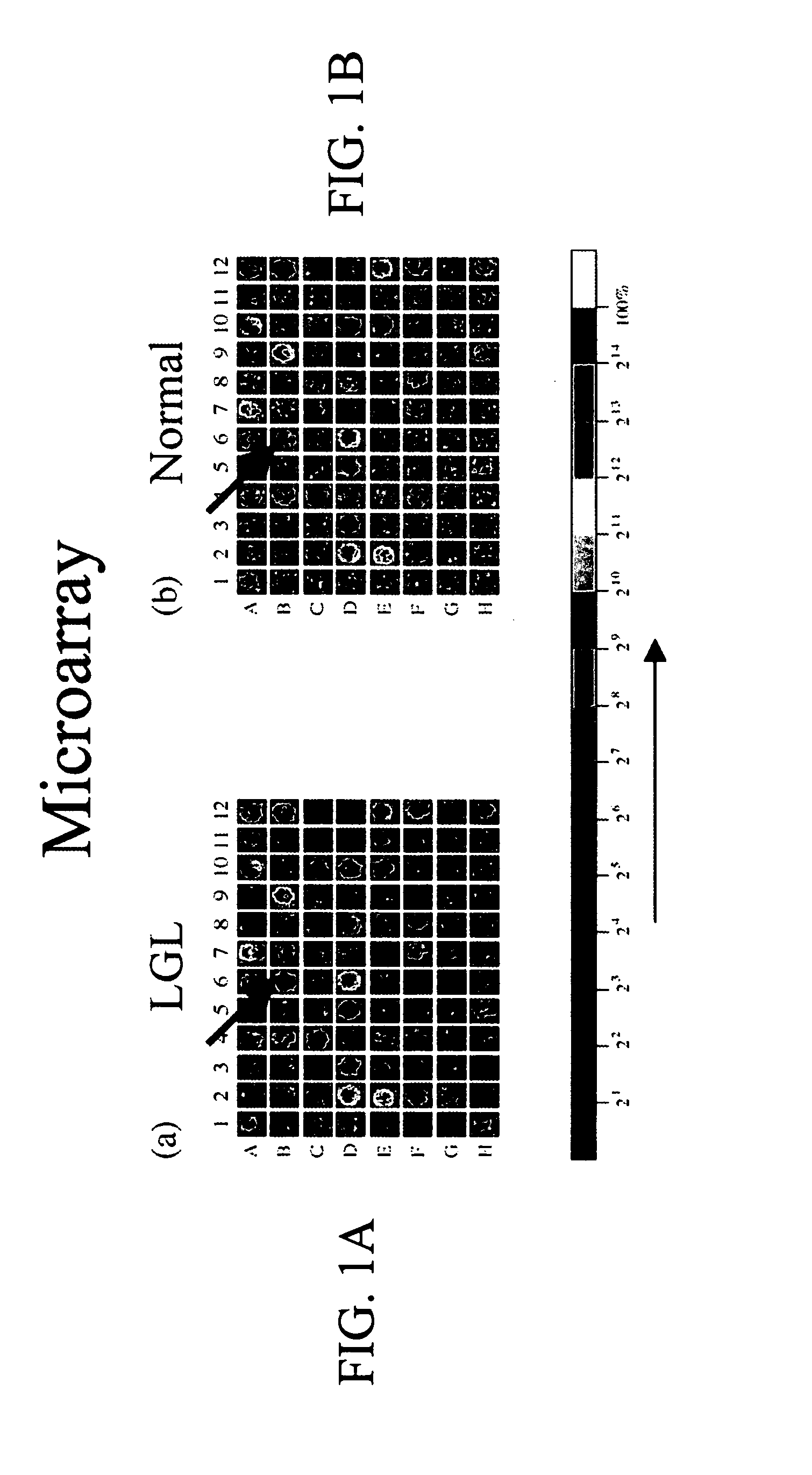
Gene expression data provides a basis for more accurate identification and diagnosis of lymphoproliferative disorders. In addition, gene expression data can be used to develop more accurate predictors of survival. The present invention discloses methods for identifying, diagnosing, and predicting survival in a lymphoma or lymphoproliferative disorder on the basis of gene expression patterns. The invention discloses a novel microarray, the Lymph Dx microarray, for obtaining gene expression data from a lymphoma sample. The invention also discloses a variety of methods for utilizing lymphoma gene expression data to determine the identity of a particular lymphoma and to predict survival in a subject diagnosed with a particular lymphoma. This information will be useful in developing the therapeutic approach to be used with a particular subject.
Owner:HEALTH & HUMAN SERVICES NAT INST OF HEALTH GOVERNMENT OF THE UNITED STATES OF AMERICA THE DEPT OF
Pyrazole compounds
InactiveUS20030060453A1Useful in treatmentBiocideMonoazo dyesLymphoproliferative diseaseAngiogenesis growth factor
Pharmaceutical compositions and compounds are provided. The compounds of the invention demonstrate anti-proliferative activity, and may promote apoptosis in cells lacking normal regulation of cell cycle and death. In one embodiment of the invention, pharmaceutical compositions of the compounds in combination with a physiologically acceptable carrier are provided. The pharmaceutical compositions are useful in the treatment of hyperproliferative disorders, which disorders include tumor growth, lymphoproliferative diseases, angiogenesis. The compounds of the invention are substituted pyrazoles and pyrazolines.
Owner:DERMIRA CANADA
Methods and compositions for transducing lymphocytes and regulated expansion thereof
ActiveUS20170296678A1Increase proliferative activityReduce riskVirusesAntibody mimetics/scaffoldsAdoptive cellular therapyNatural Killer Cell Inhibitory Receptors
The present disclosure provides methods for genetically modifying lymphocytes and methods for performing adoptive cellular therapy that include transducing T cells and / or NK cells without prior ex vivo stimulation. The methods typically include engineered signaling polypeptides that can include a lymphoproliferative element, and / or a chimeric antigen receptor (CAR), for example a microenvironment restricted CAR. Additional elements of such engineered signaling polypeptides are provided herein, as well as vectors, such as retroviral vectors, packaging cell lines and methods of making the same. Furthermore, recombinant retroviruses and methods of making the same are provided. Numerous controls are provided, including riboswitches that are controlled, for example in vivo, by nucleoside analogues.
Owner:EXUMA BIOTECH CORP
Diagnosis and treatment of myeloid and lymphoid cell cancers
InactiveUS20110311445A1Prevent degradationOrganic active ingredientsHeavy metal active ingredientsCell cancerLymphocyte
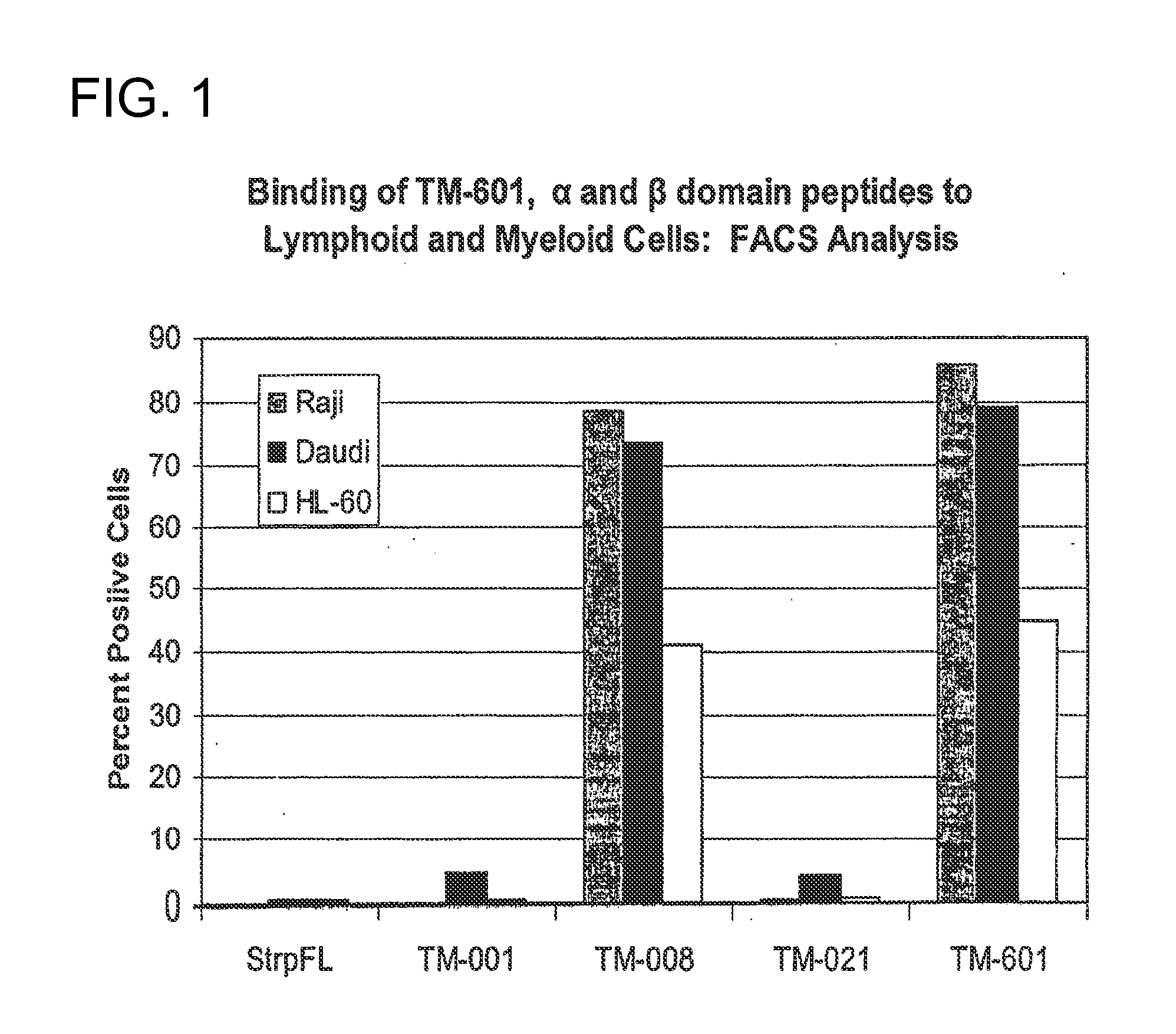
Disclosed is a method of diagnosing and treating myeloproliferative or lymphoproliferative cell disorders, such as cancer, with chlorotoxin and / or derivatives, analogs or fragments thereof, which are effective to bind to an inhibit abnormal myeloid or lymphoid cell growth.
Owner:MORPHOTEX INC
Compositions of boswellic acids derived from Boswellia serrata gum resin, for treating lymphoproliferative and autoimmune conditions
InactiveUS20060234990A1Growth inhibitionInhibit synthesisBiocideOrganic chemistryAutoimmune conditionBeta-boswellic acid
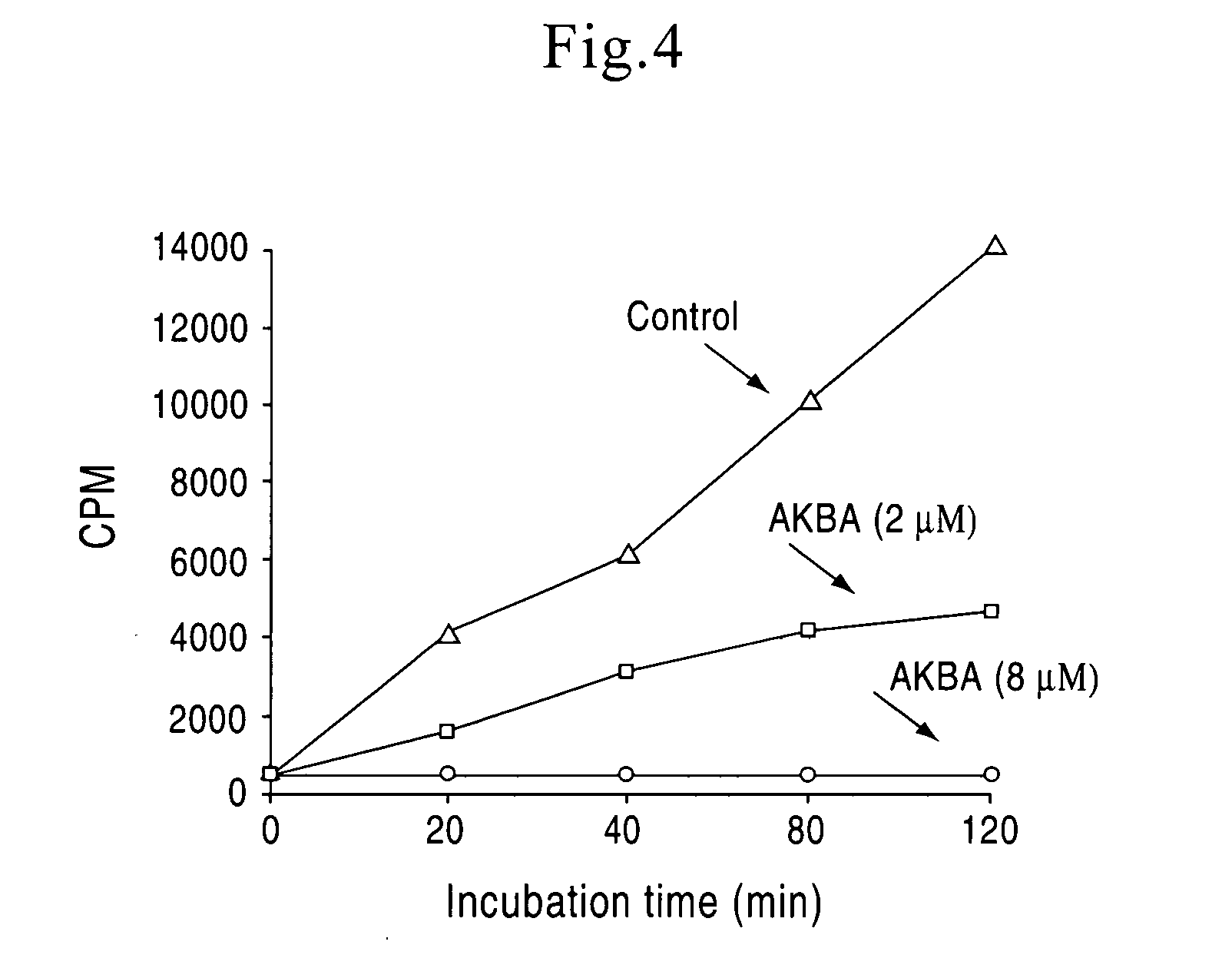
A method of treatment of lymphoproliferative and autoimmune disorders with a new composition of four boswellic acids including β-boswellic acid, 3-O-acetyl-β-boswellic acid, 11-keto-β-boswellic acid, and 3-O-acetyl-11-keto-β-boswellic acid. Boswellic acids of invention have been obtained in a novel industrial process from the gum resin of Boswellia serrata tree, providing standardized composition which inhibits DNA, RNA and protein synthesis of the target cell without cytotoxic effects. The composition of invention provides advantage of irreversible cytostatic therapy, equivalent to biological effects of a cytotoxic therapy without killing body cells.
Owner:SAMI LABS LTD
Pharmaceutical compositions capable of inducing apoptosis in tumour cells, useful for diagnosis and treatment of B-chronic lymphocytic leukaemia
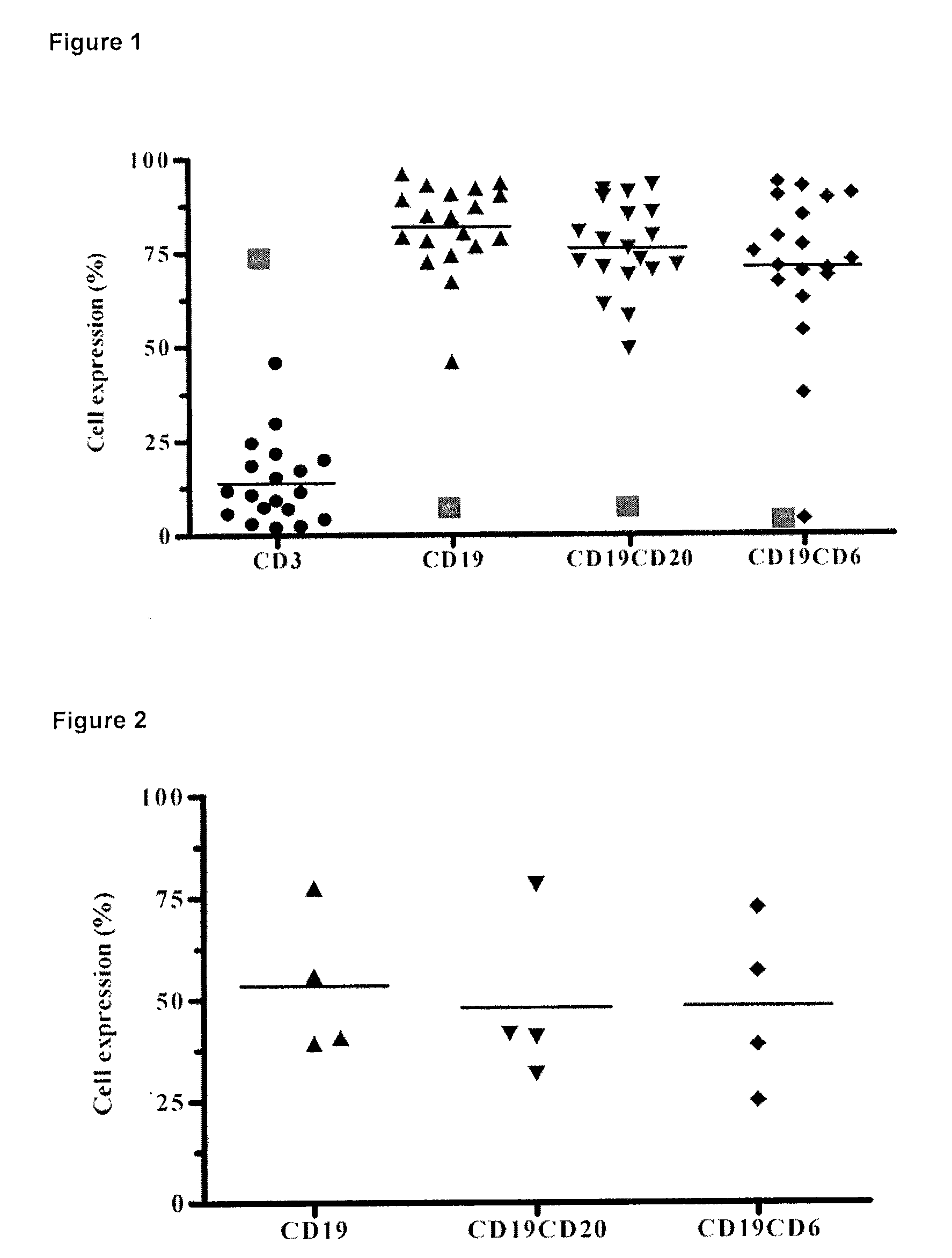
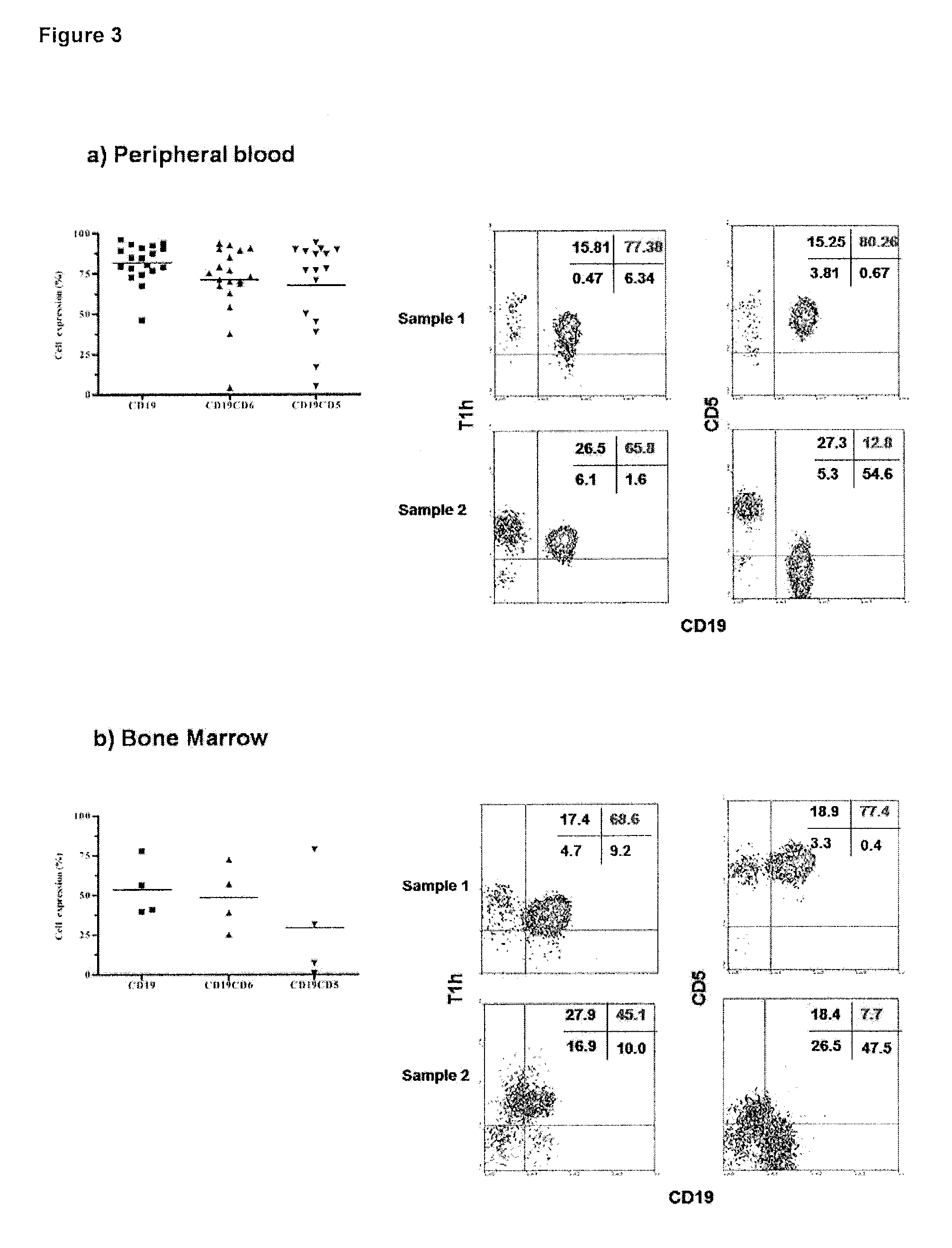
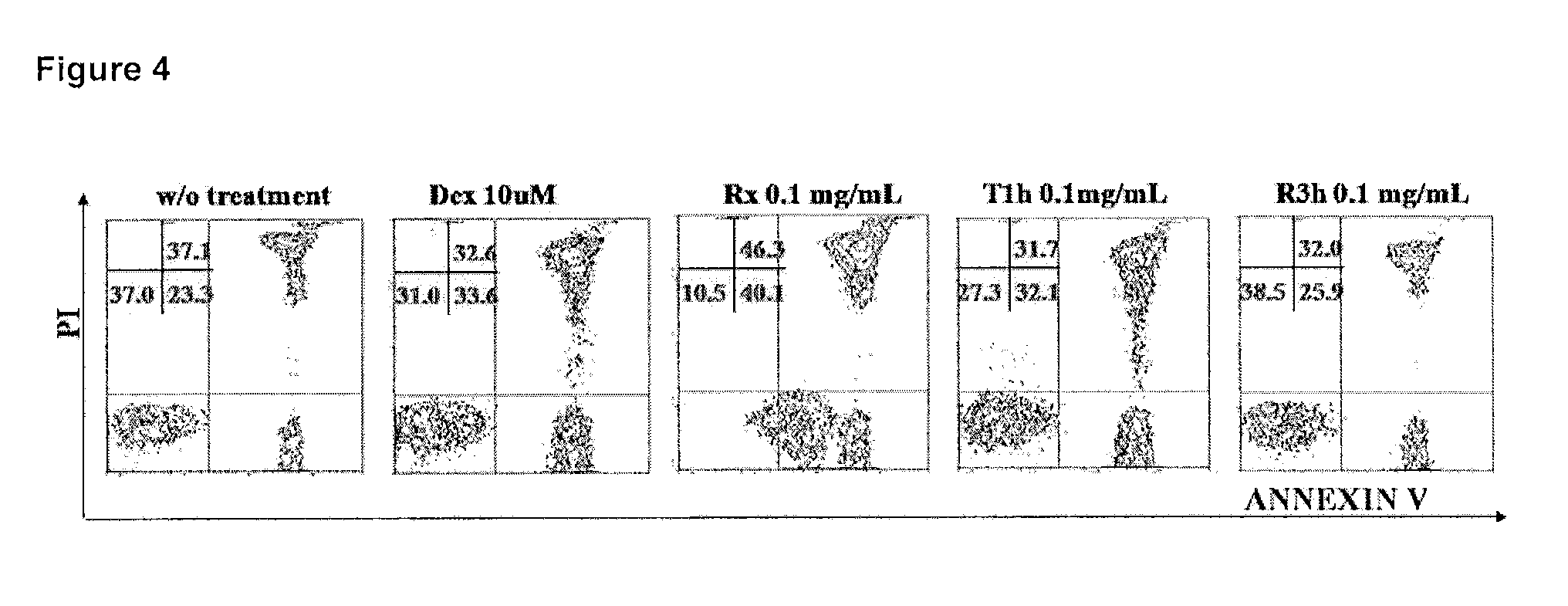
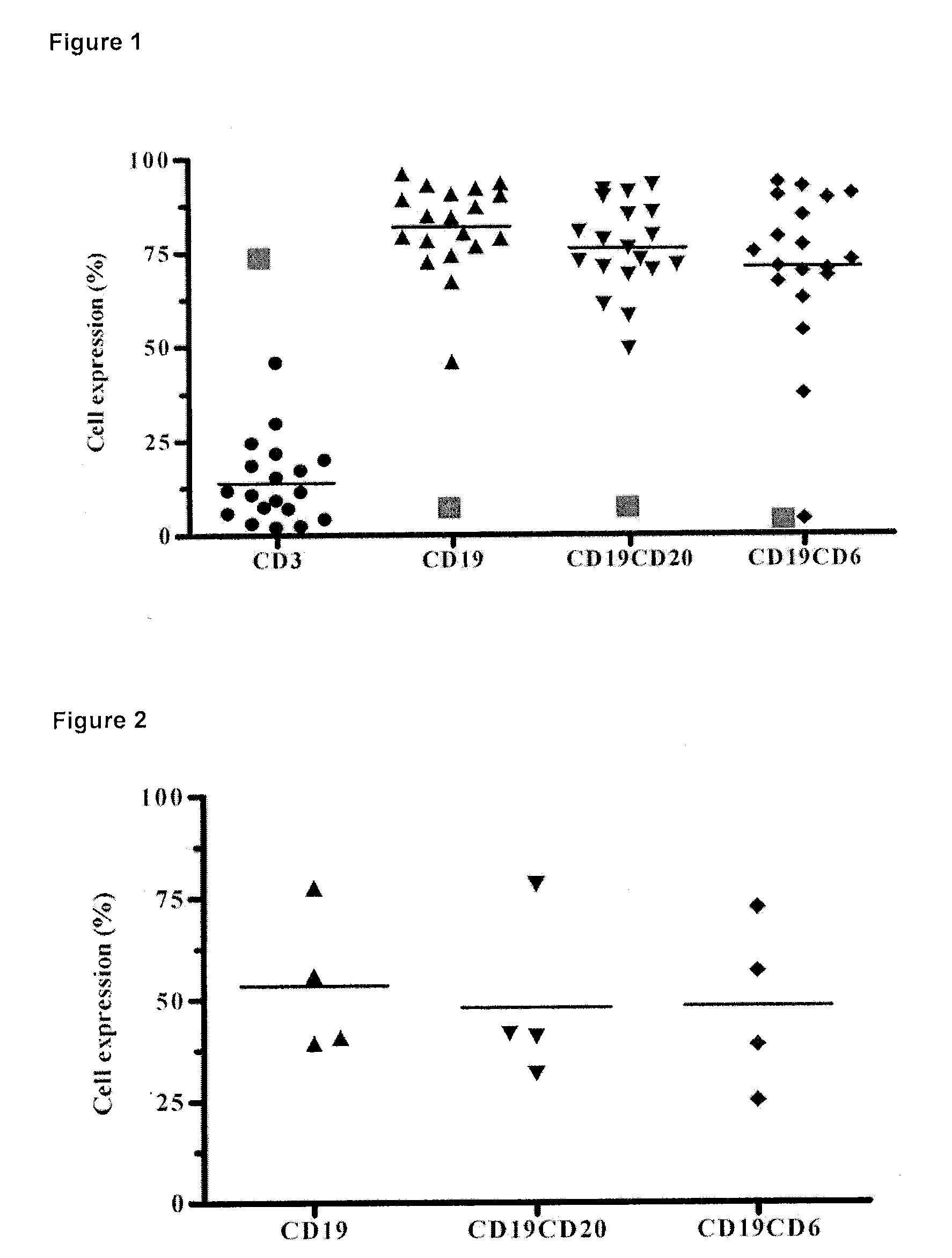
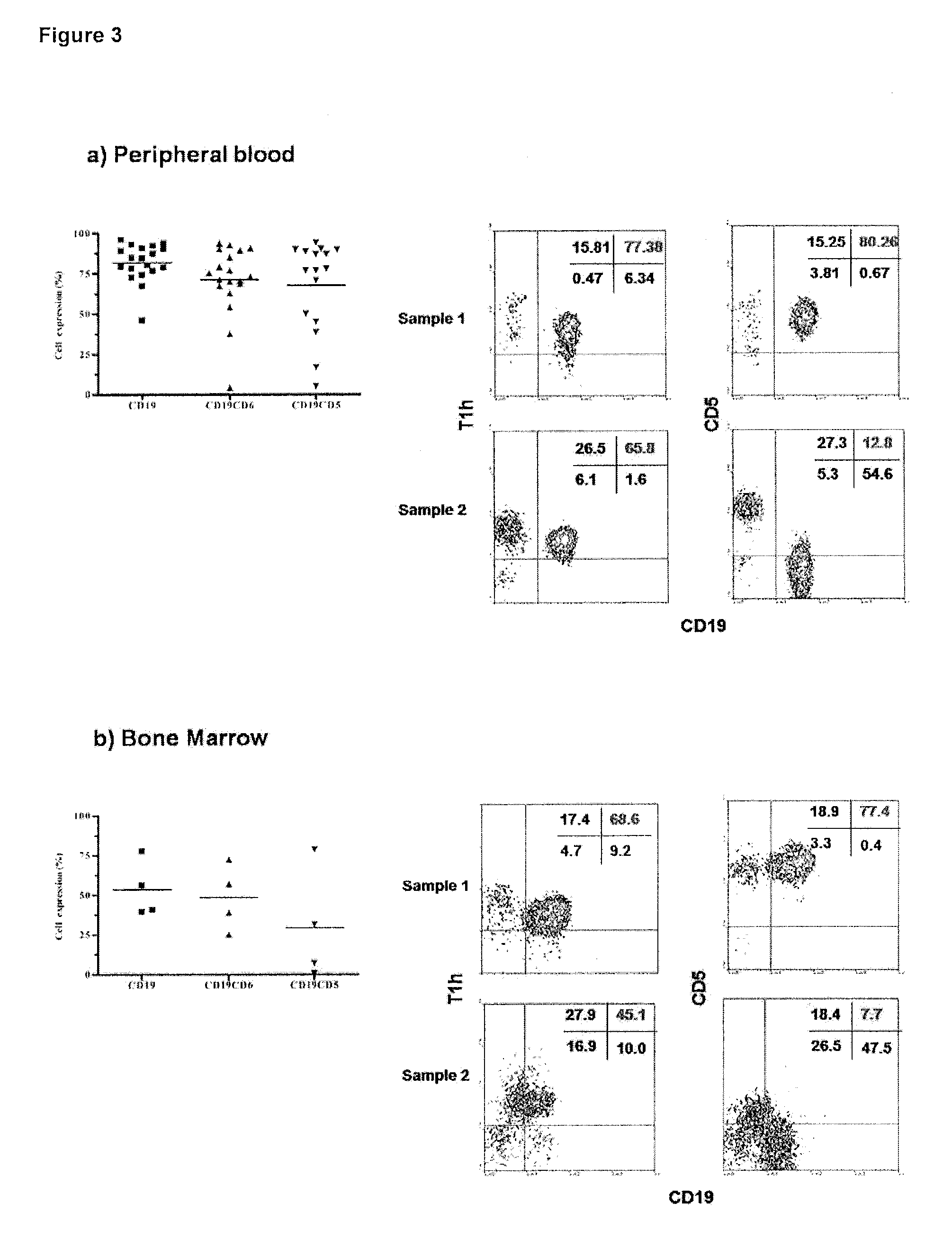
The present invention is related to the branch of immunology and particularly with the generation of pharmaceutical compositions comprising a humanized monoclonal antibody recognizing the leukocyte differentiation antigen CD6. Accordingly with that statement, the purpose of this invention is to provide pharmaceutical compositions comprising a humanized anti-CD6 monoclonal antibody for the diagnosis and treatment of Lymphoproliferative Syndromes and particularly the B-Cell Chronic Lymphocytic Leukemia. The essence of the invention consist in the application of a humanized Monoclonal Antibody that recognizes the CD6 antigen, the generation of pharmaceutical compositions comprising that antibody being able to induce apoptosis of malignant cells from B-Cell Chronic Lymphocytic Leukemia patients, reaching a clinical and a histological antitumor efficacy. The field of application of the present invention extends to the Oncology.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Pharmaceutical compositions capable of inducing apoptosis in tumour cells, useful for diagnosis and treatment of b-chronic lymphocytic leukaemia
The present invention is related to the branch of immunology and particularly with the generation of pharmaceutical compositions comprising a humanized monoclonal antibody recognizing the leukocyte differentiation antigen CD6. Accordingly with that statement, the purpose of this invention is to provide pharmaceutical compositions comprising a humanized anti-CD6 monoclonal antibody for the diagnosis and treatment of Lymphoproliferative Syndromes and particularly the B-Cell Chronic Lymphocytic Leukemia. The essence of the invention consist in the application of a humanized Monoclonal Antibody that recognizes the CD6 antigen, the generation of pharmaceutical compositions comprising that antibody being able to induce apoptosis of malignant cells from B-Cell Chronic Lymphocytic Leukemia patients, reaching a clinical and a histological antitumor efficacy. The field of application of the present invention extends to the Oncology.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Lysine demethylase inhibitors for myeloproliferative or lymphoproliferative diseases or disorders
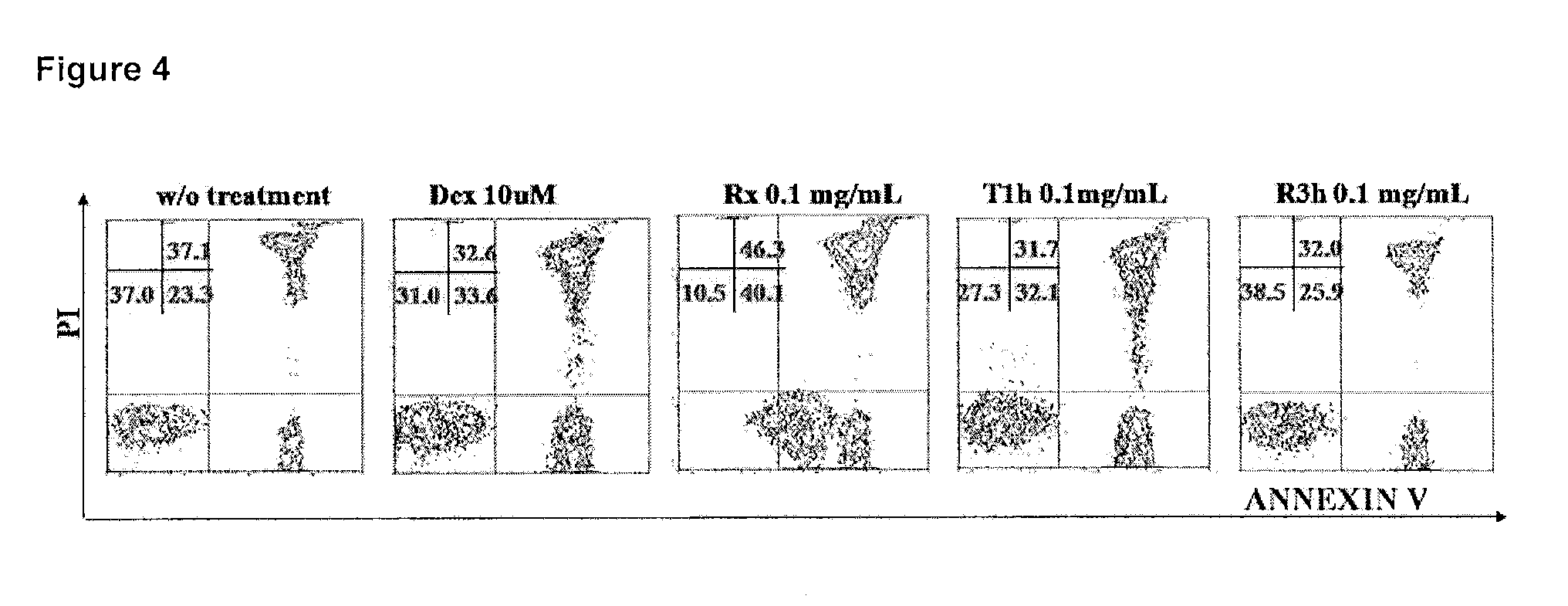
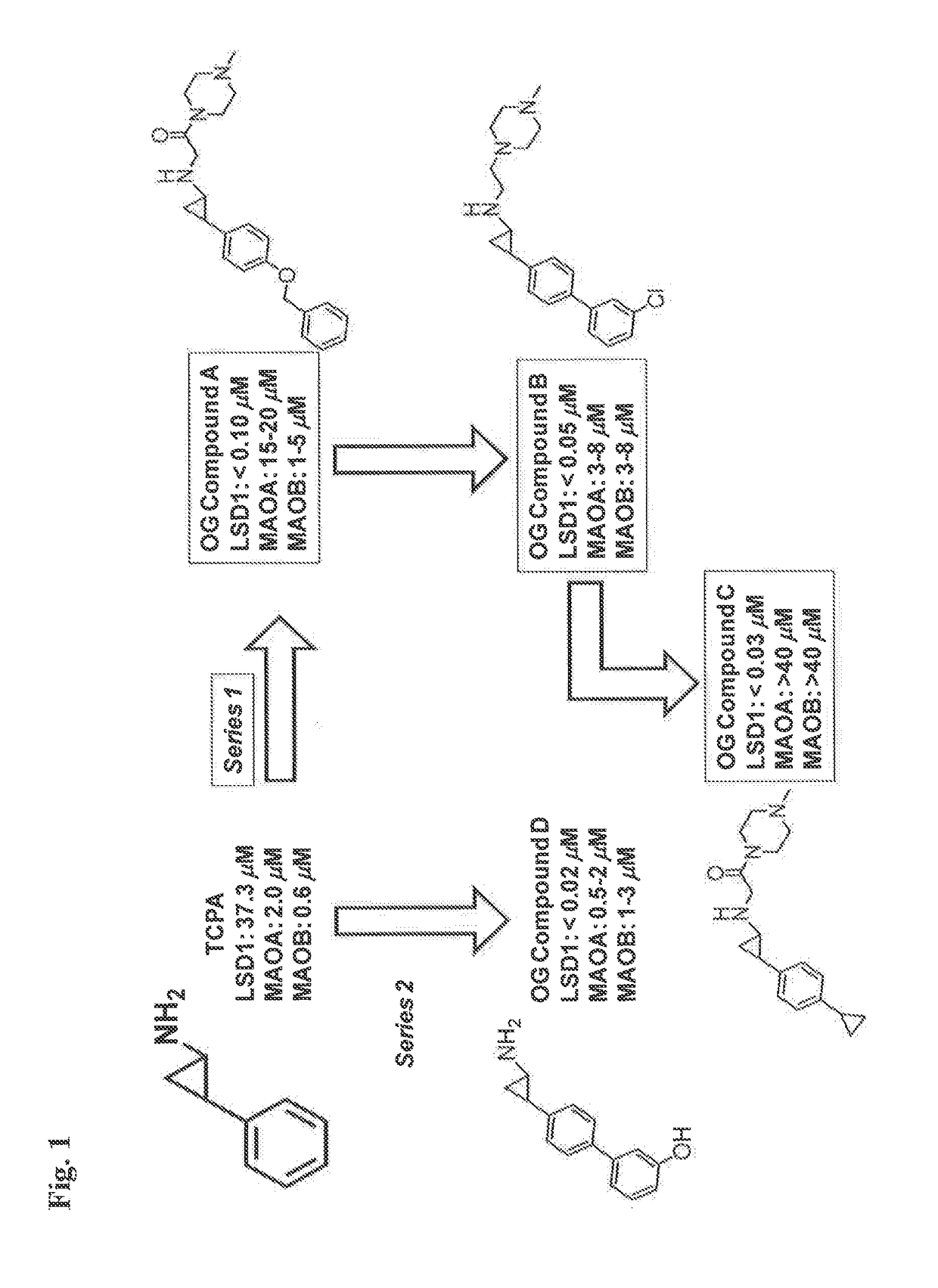
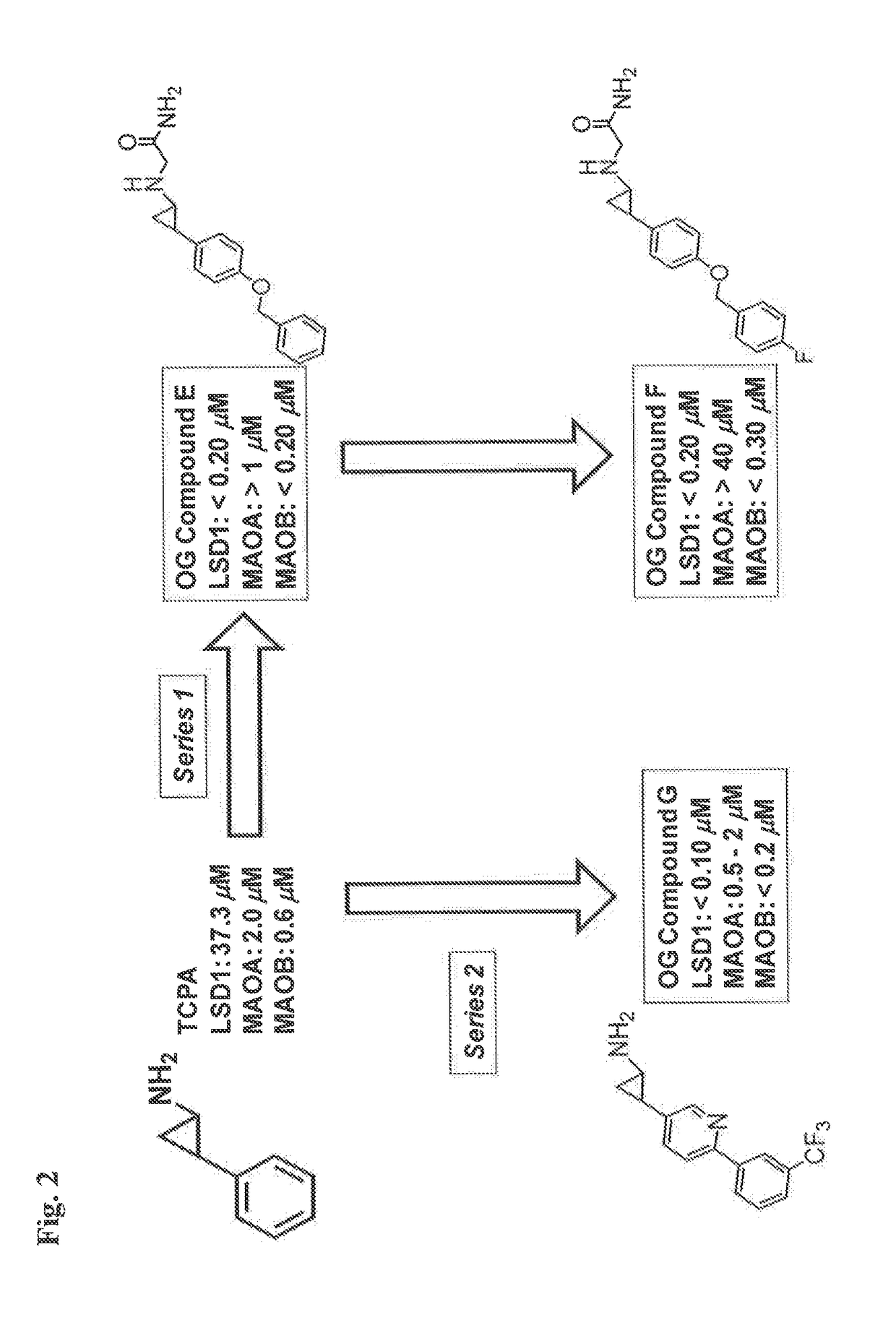
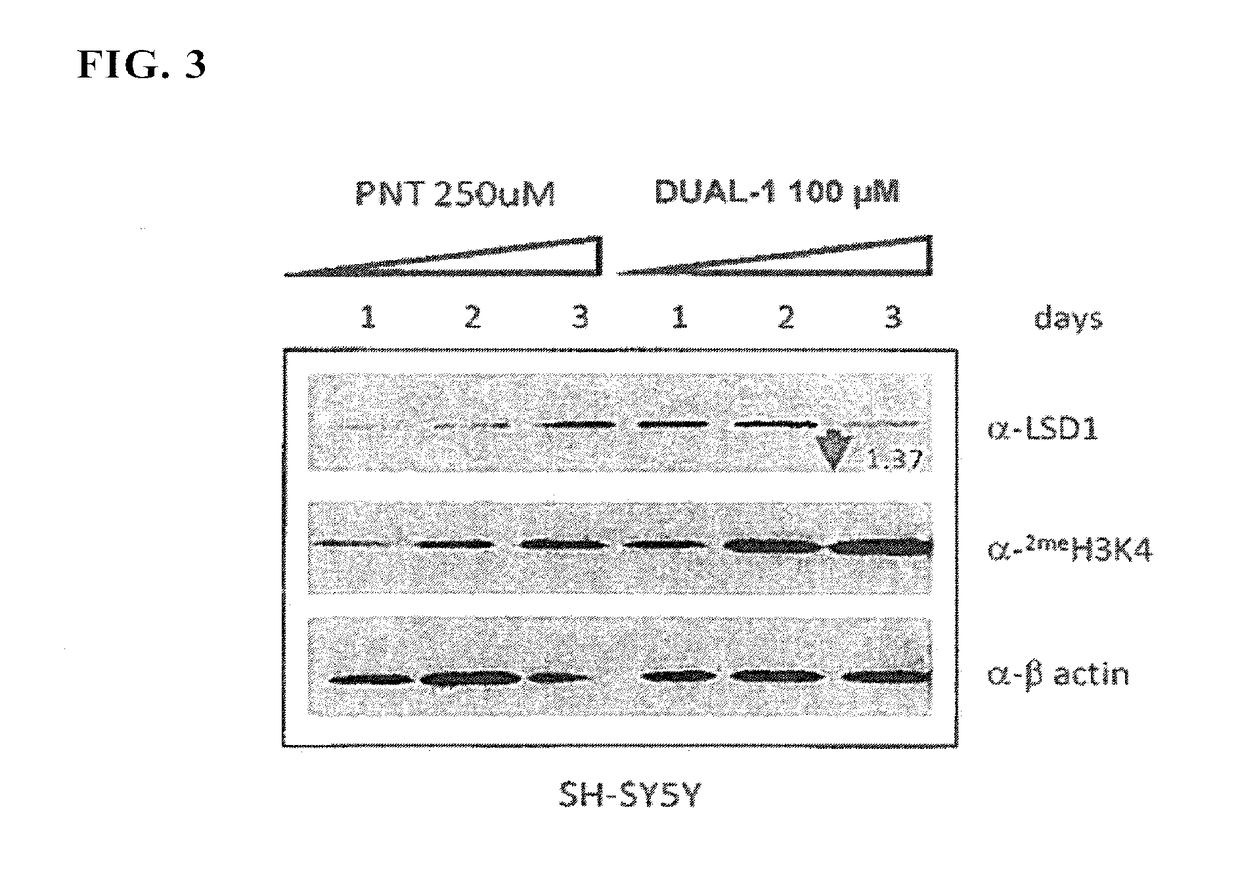
InactiveUS20170209432A1Reduce platelets and other blood cellsReduce impactOrganic chemistryAntineoplastic agentsLymphoproliferative diseaseLymphoid tissue hyperplasia
The present invention relates to methods and compositions for the treatment or prevention of diseases and disorder associated with myeloproliferative and lymphoproliferative disorders. In particular, the invention relates to an LSD1 inhibitor for use in treating or preventing diseases and disorder associated with myeloproliferative and lymphoproliferative disorders.
Owner:ORYZON GENOMICS SA
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
Antiproliferative 1,2,3-thiadiazole compounds
Pharmaceutical compositions and compounds are provided. The compounds of the invention have anti-proliferative activity, and may promote apoptosis in cells lacking normal regulation of cell cycle and death. In one embodiment of the invention, formulations of the compounds in combination with a physiologically acceptable carrier are provided. The pharmaceutical formulations are useful in the treatment of hyperproliferative disorders, which disorders include tumor growth, lymphoproliferative diseases, angiogenesis. The compounds of the invention are 1,2,3-thiadiazoles having the structure: and including stereoisomers, solvates, and pharmaceutically acceptable salts thereof, wherein each of R1, R22 , R3 and R4 is independently selected from hydrogen, R5R6, and R7, R5 is selected from alkyl, heteroalkyl, aryl and heteroaryl; R6 is selected from (R5)n-alkylene, (R5)n-heteroalkylene, (R5)n-arylene and (R5)n-heteroarylene; R7 is selected from (R6)n-alkylene, (R6)n-heteroalkylene, (R6)n-arylene, and (R6)n-heteroarylene; and n is selected from 0, 1, 2, 3, 4 and 5, where R1 and R2 may together form a heterocyclic structure including the nitrogen to which they are both attached, and R3 and R4 may together form a heterocyclic structure including the nitrogen to which they are both attached; and each of L1 and L2 is independently selected from -A1-A2-A3- where each of Al, A2, and A3 is independently selected from a direct bond, alkylene, heteroalkylene, arylene and heteroarylene.
Owner:DERMIRA CANADA
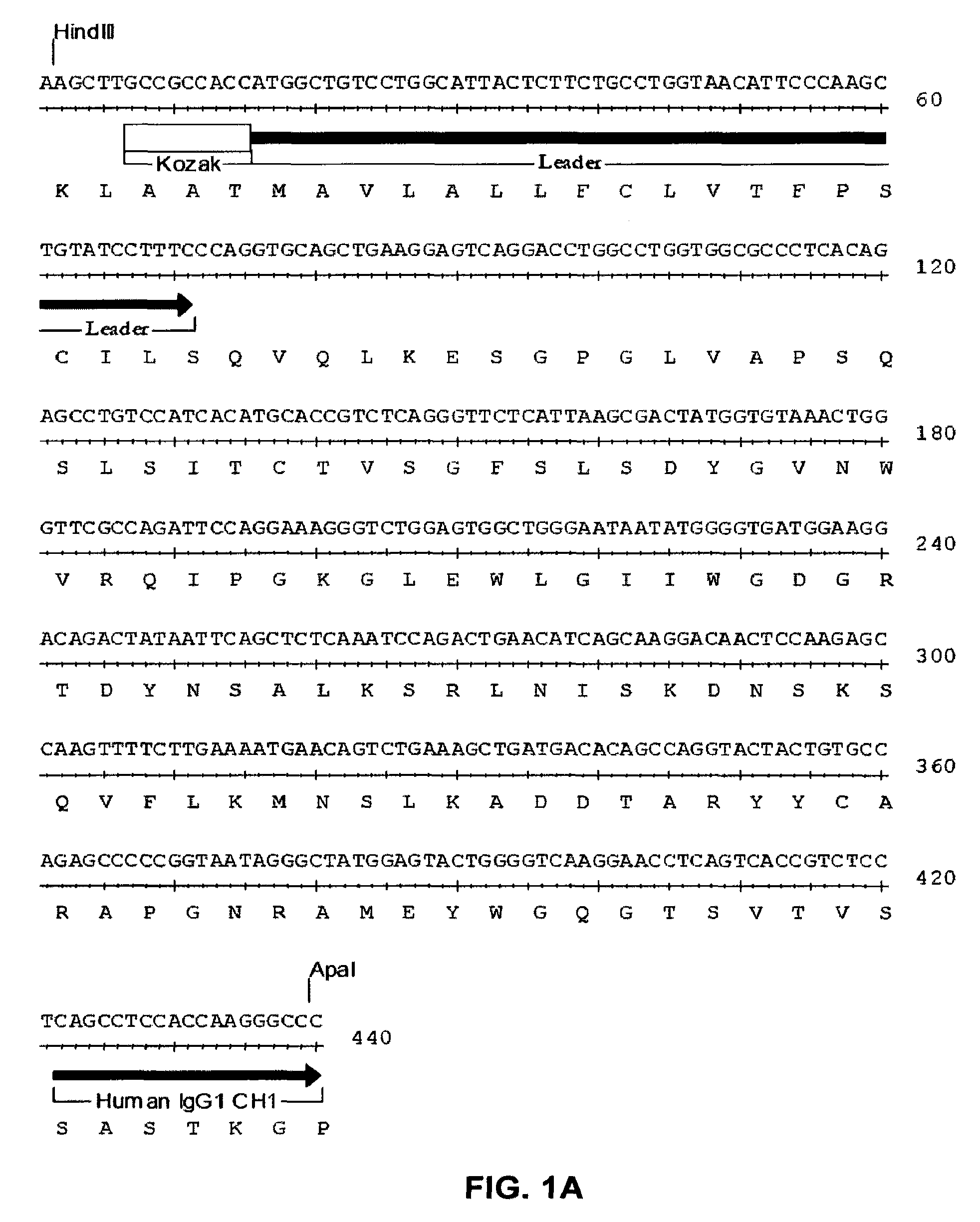
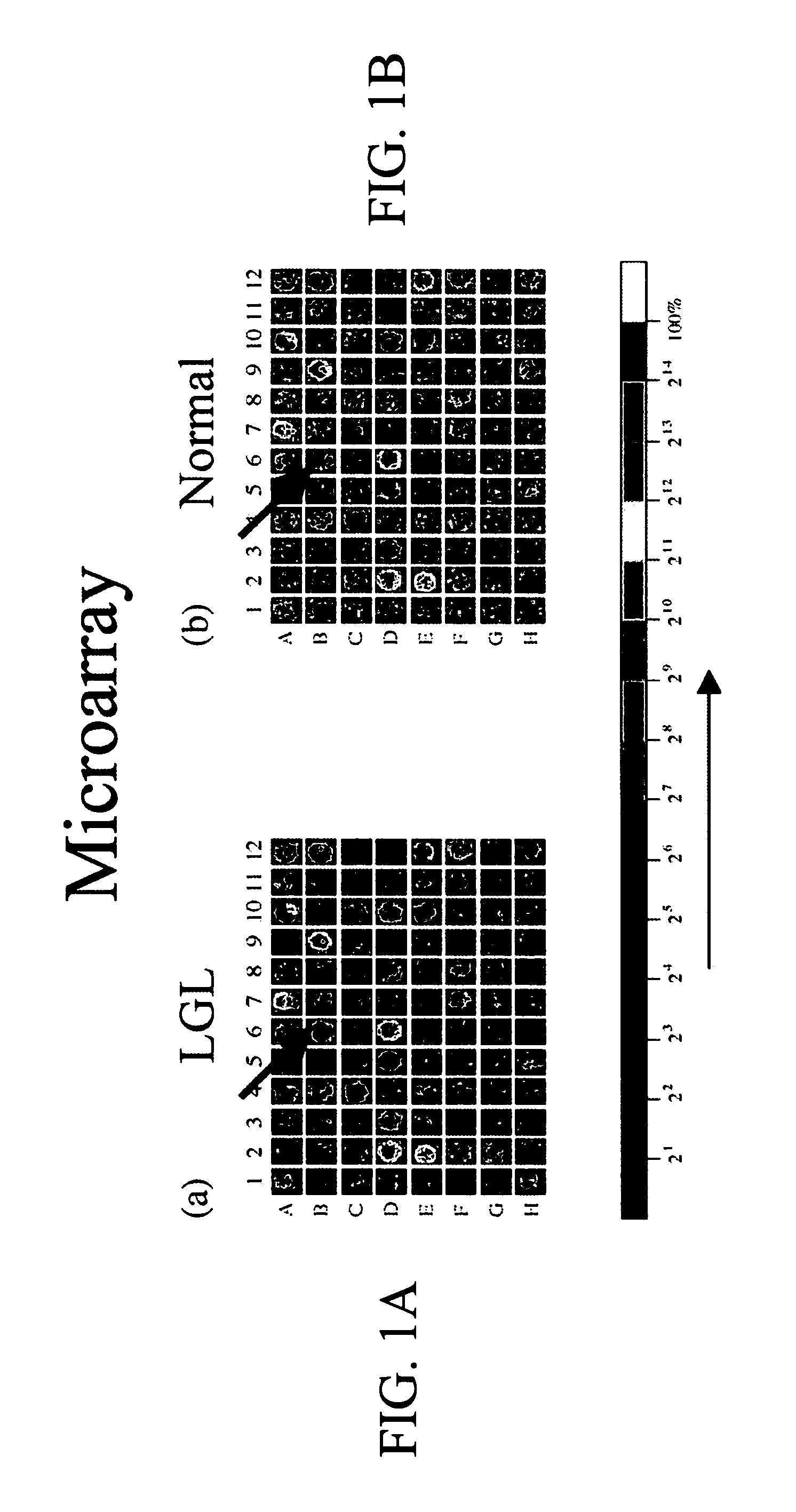
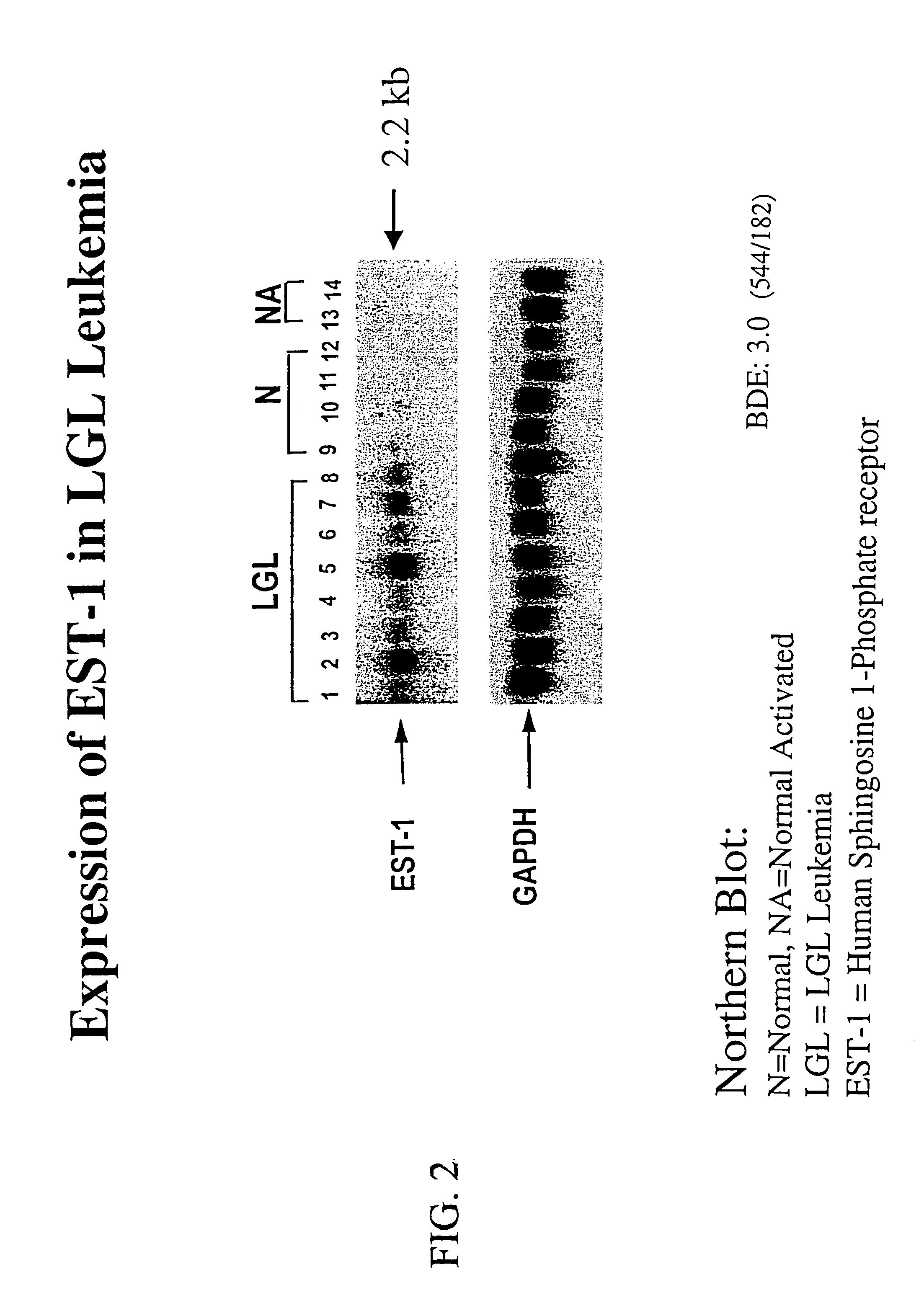
Sphingosine 1-phosphate receptor gene, sppr
InactiveUS7220580B2Produce in largeAvoid small quantitiesCell receptors/surface-antigens/surface-determinantsSugar derivativesDiseaseAutoimmune disease
A novel sphingosine 1-phosphate receptor gene, herein termed sppr, and its splice variants. Sppr is up-regulated in LGL and is useful, for example, in the diagnosis and treatment of certain lymphoproliferative, neurodegenerative and autoimmune diseases.
Owner:UNIV OF SOUTH FLORIDA
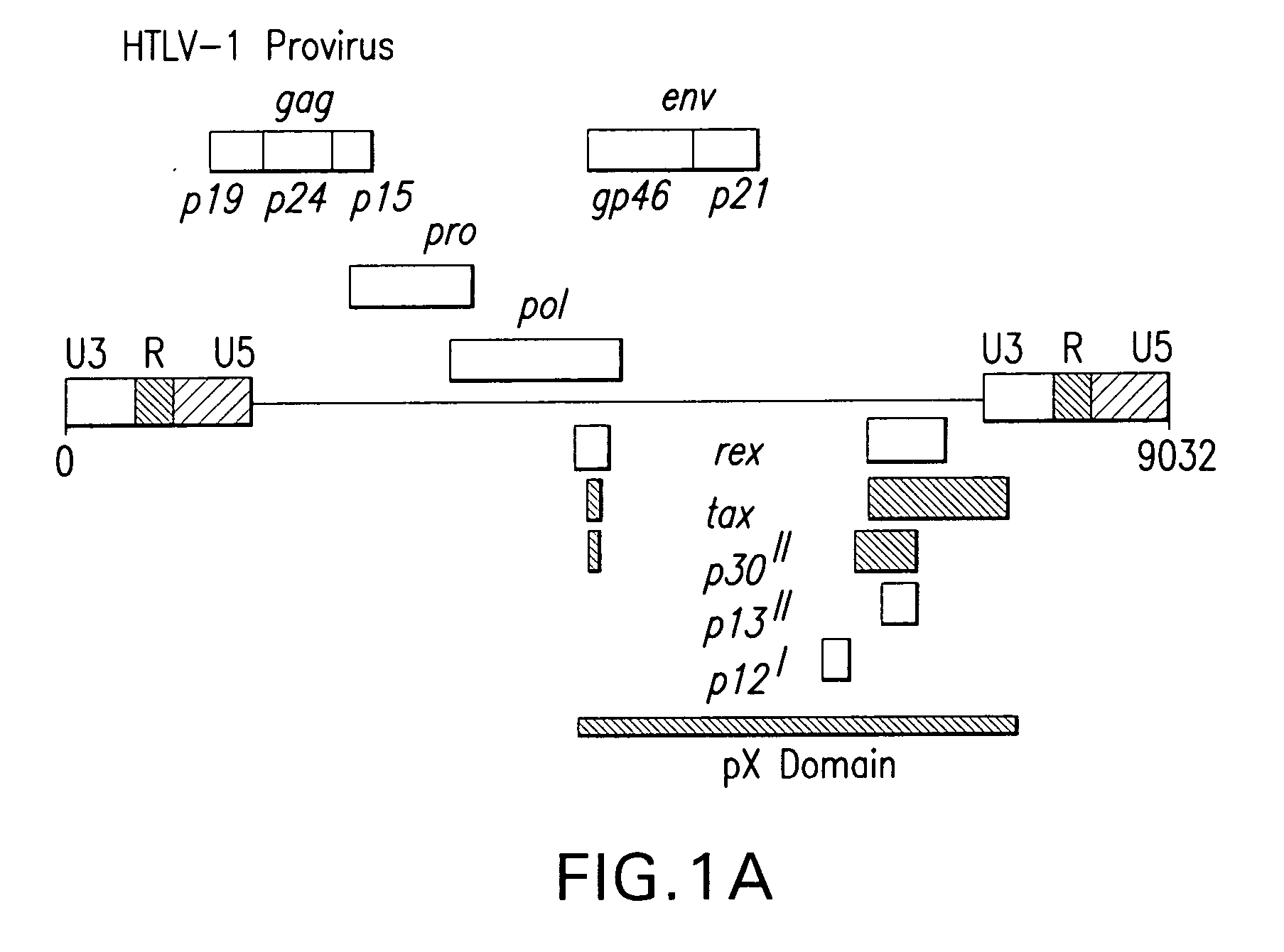
Aberrant Myc/TIP60 interactions as a target for anti-cancer therapeutics
InactiveUS20050186604A1Enhance transforming potentialSpeed upMicrobiological testing/measurementGenetic material ingredientsStructural proteinEnhancer Elements
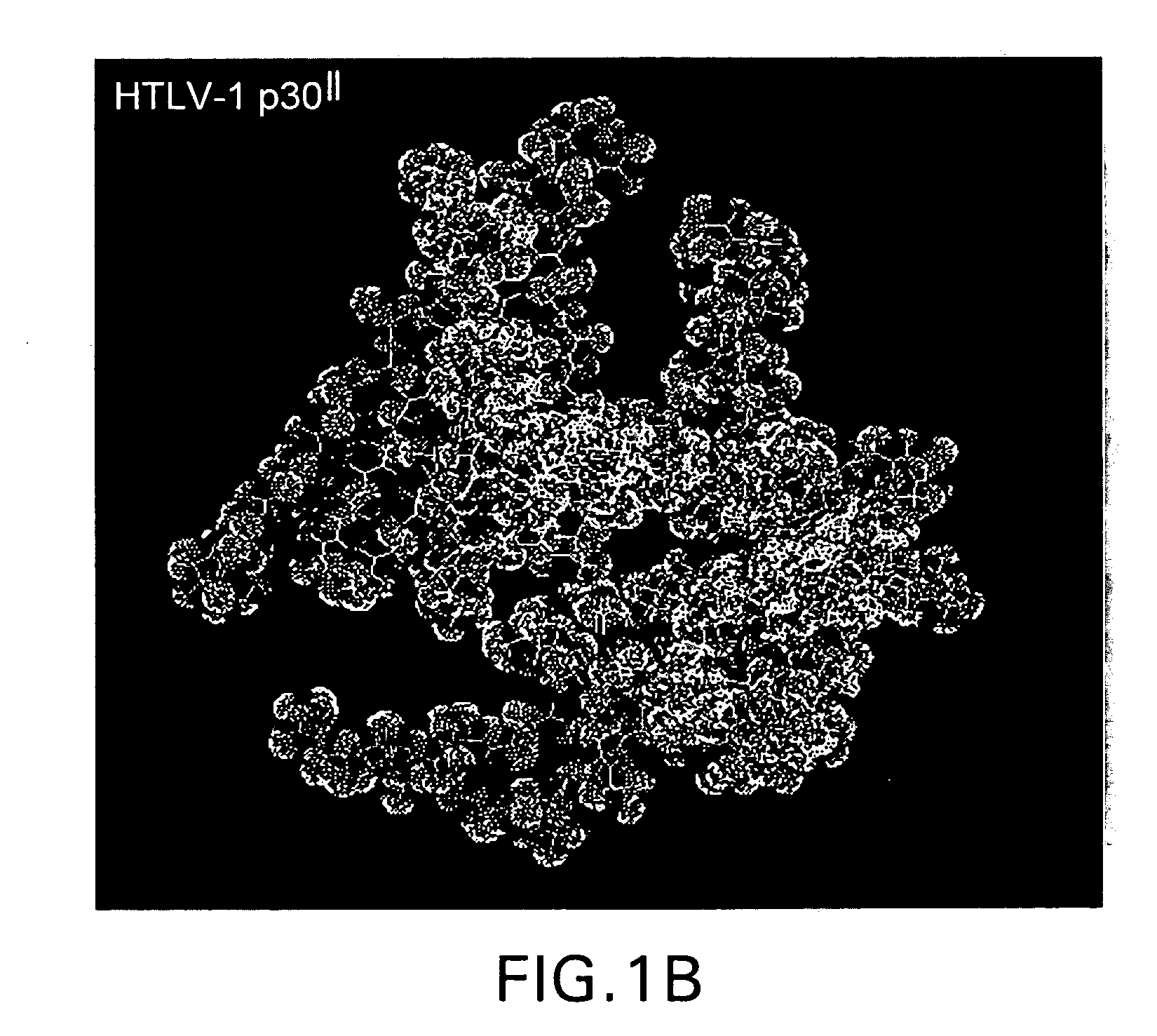
Human T-cell lymphotropic virus type-1 (HTLV-1) infects and transforms CD4+ lymphocytes and causes Adult T-cell Leukemia / Lymphoma (ATLL), an aggressive, often fatal, lymphoproliferative disease. A conserved HTLV-1 3+ regulatory domain, pX, encodes at least five non-structural proteins, including the alternative splice-variant p30II. HTLV-1 p30II may enhance the transforming activity of Myc and transcriptionally activate the human cyclin D2 promoter, dependent upon its conserved Myc-responsive enhancer elements, associated with markedly increased S-phase entry and multi-nucleation. Enhancement of Myc transforming activity by HTLV-1 p30II may be dependent upon the transcriptional coactivators, TRRAP / p4346-8 and TIP60, require TIP60 histone acetyltransferase activity, and strongly correlate with interactions between HTLV-1 p30II and Myc-TIP60 complexes in HTLV-1-infected ATLL patient-derived lymphocytes. Thus, p30II may function as a novel retroviral modulator of Myc-transforming interactions that may prominently contribute to adult T-cell leukemogenesis. Thus, the present invention provides methods and compositions for screening and identifying agents that interfere with transformation.
Owner:SOUTHERN METHODIST UNIVERSITY
Inhibition of expansion and function of pathogenic age-associated b cells and use for the prevention and treatment of autoimmune disease
InactiveUS20180153922A1Abolishing or decreasing pathogenic ABCsHigh expressionOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune responsesBasic research
This current invention provides methods and agents for preventing and treating autoimmune and lymphoproliferative disease by targeting pathogenic age-associated B cells as well as methods of detecting these pathogenic age-associated B cells as a method of diagnosing and predicting autoimmune disease and other lymphoproliferative and chronic inflammatory disorders.The current invention also provides targets for drug development and basic research for autoimmune diseases and other lymphoproliferative and chronic inflammatory disorders.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Transgenic zebra fish embryo model for hematopoiesis and lymphoproliferative disorders
ActiveUS20050198696A1High affinityGuaranteed functionVectorsAnimals/human peptidesFish embryoLymphoid tissue hyperplasia
A transgenic zebrafish animal model for the study of haemopoietic cell differentiation, control, and screening of therapeutic agents.
Owner:PARKER HUGHES INST
Methods of cancer therapy
InactiveUS20170065561A1Suppress clonal B cell proliferationReduce generationAntineoplastic agentsHeterocyclic compound active ingredientsEliglustat tartrateLymphoid tissue hyperplasia
Owner:CAMBRIDGE ENTERPRISE LTD
Method for the diagnosis of lymphoproliferative diseases
InactiveUS7803532B2Extending remissionSugar derivativesMaterial analysis by observing effect on chemical indicatorT cellLymphocyte disorder
The present invention relates to novel methods for the diagnosis and therapy of lymphoproliferative diseases. Specifically, the present invention relates to novel methods for the diagnosis and therapy taking advantage of the detection of chromosomal breakpoints in chromosome 12 and / or translocation of chromosomal material from chromosome 12, said chromosomal breakpoints and / or translocation(s) being associated with lymphoproliferative diseases, such as primary cutaneous T-cell lymphomas (CTCL). The present invention further relates to the use of neuron navigator 3 gene (NAV3) or an equivalent or functional fragment thereof involved in chromosomal breakpoints in chromosome 12 and / or translocations thereof, said gene and / or translocations thereof being associated with lymphoproliferative diseases, such as primary cutaneous T-cell lymphomas (CTCL), as a diagnostic and therapeutic agent. The present invention also relates to the development of therapy.
Owner:VALIPHARMA
Compositions and Methods for Increasing Drug Efficacy in Cancer
ActiveUS20140005250A1Shorten the progressAvoid developmentBiocideArtificial cell constructsEfficacyPharmaceutical medicine
A method of (a) treating a lymphoproliferative disease in a subject in need thereof; (b) slowing the progression of lymphoproliferative disease in a subject who has been diagnosed with a lymphoproliferative disease; or (c) preventing or delaying development of a lymphoproliferative disease in a subject who is at risk of developing a lymphoproliferative disease. The method generally comprises administering to the individual an effective amount of a combination therapy comprising: i) at least one autophagy modulating agent, or a derivative, pharmaceutically acceptable salt thereof, or prodrug thereof; and ii) at least one CDK inhibitor agent, or a derivative, pharmaceutically acceptable salt thereof, or prodrug thereof, wherein the i) agent and the ii) agents are administered in amounts sufficient to enhance the cytotoxicity of the combination relative to the CDK inhibitor agent treatment alone.
Owner:THE OHIO STATES UNIV
Sphingosine 1-phosphate receptor gene, SPPR
InactiveUS20070105148A1Produce in largeAvoid small quantitiesCell receptors/surface-antigens/surface-determinantsMicrobiological testing/measurementAutoimmune conditionS1P Receptor
A novel sphingosine 1-phosphate receptor gene, herein termed sppr, and its splice variants. Sppr is up-regulated in LGL and is useful, for example, in the diagnosis and treatment of certain lymphoproliferative, neurodegenerative and autoimmune diseases.
Owner:UNIV OF SOUTH FLORIDA
Methods of treating Epstein-Barr virus-associated lymphoproliferative disorders by T cell therapy
The invention relates to methods of treating an EBV-LPD (Epstein-Barr Virus-associated lymphoproliferative disorder) in a human patient who has failed combination chemotherapy to treat the EBV-LPD and / or radiation therapy to treat the EBV-LPD, comprising administering to the human patient a population of allogeneic T cells comprising EBV-specific T cells.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Treatment and prophylaxis with 4-1BB-binding agents
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
PD-1 related cancer therapy
ActiveUS11110171B2Peptide/protein ingredientsMass spectrometric analysisLymphoproliferative diseaseLymphocyte
Provided are compositions and methods for identifying individuals with cancer who will benefit from PD-1 inhibitor therapy. The method comprises determining levels of signaling lymphocyte activation molecule-associated protein (SAP) in an individual and based on the SAP levels, determining if the individual is suitable for PD-1 inhibitor therapy. Also provided is a method of treatment of X-linked lymphoproliferative disease comprising administering to an individual PD-1 inhibitory therapy, with or without SHP2 inhibitors.
Owner:NEW YORK UNIV
Method for the diagnosis of lymphoproliferative diseases
InactiveUS20050221309A1Extending remissionSugar derivativesMaterial analysis by observing effect on chemical indicatorLymphoproliferative diseaseNEURON NAVIGATOR 3
The present invention relates to novel methods for the diagnosis and therapy of lymphoproliferative diseases. Specifically, the present invention relates to novel methods for the diagnosis and therapy taking advantage of the detection of chromosomal breakpoints in chromosome 12 and / or translocation of chromosomal material from chromosome 12, said chromosomal breakpoints and / or translocation(s) being associated with lymphoproliferative diseases, such as primary cutaneous T-cell lymphomas (CTCL). The present invention further relates to the use of neuron navigator 3 gene (NAV3) or an equivalent or functional fragment thereof involved in chromosomal breakpoints in chromosome 12 and / or translocations thereof, said gene and / or translocations thereof being associated with lymphoproliferative diseases, such as primary cutaneous T-cell lymphomas (CTCL), as a diagnostic and therapeutic agent. The present invention also relates to the development of therapy.
Owner:VALIPHARMA
Inhibition of expansion and function of pathogenic age-associated b cells and use for the prevention and treatment of autoimmune disease
ActiveUS20200188422A1Abolishing or decreasing pathogenic ABCsHigh expressionOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsImmunologic disordersAutoimmune condition
This current invention provides methods and agents for preventing and treating autoimmune and lymphoproliferative disease by targeting pathogenic age-associated B cells as well as methods of detecting these pathogenic age-associated B cells as a method of diagnosing and predicting autoimmune disease and other lymphoproliferative and chronic inflammatory disorders.The current invention also provides targets for drug development and basic research for autoimmune diseases and other lymphoproliferative and chronic inflammatory disorders.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20130028888A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAutoimmune conditionAutoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
Methods for identifying inducers and inhibitors of propteolytic antibodies, compositions and their uses
Covalently reactive antigen analogs are disclosed herein. The antigens of the invention may be used to stimulate production of catalytic antibodies specific for predetermined antigens associated with particular medical disorders. The antigen analogs may also be used to permanently inactivate endogenously produced catalytic antibodies produced in certain autoimmune diseases as well as in certain lymphoproliferative disorders.
Owner:内布拉斯卡大学评议会 +1
Means and methods for counteracting myeloproliferative or lymphoproliferative disorders
ActiveCN106062002ASerum immunoglobulinsBiological material analysisLymphoid tissue hyperplasiaLymphocyte disorder
The invention provides human AML-specific binding compounds that are able to bind a cell surface component of AML cells. Therapeutic uses of binding compounds against AML are also provided.
Owner:科凌生物医疗有限公司
Proteomic screening for diseases
PendingUS20210285965A1Promote resultsReliable diagnosisImmunoglobulins against animals/humansOmicsAtaxia-telangiectasiaHemophagocytic lymphohistiocytosis
Clinical diagnosis and newborn screening for primary immunodeficiencies (PIDDs) is described. The PIDDs include X-linked chronic granulomatous disease (X-CGD), X-linked lymphoproliferative syndrome (XLP1; SH2D1A deficiency), familial hemophagocytic lymphohistiocytosis 2 (FHL2), ataxia telangiectasia (AT), common variable immunodeficiency (CVID; B-cell dysfunctions), severe combined immunodeficiency (SCID), adenosine deaminase (ADA) deficiency, and dedicator of cytokinesis 8 (DOCKS) deficiency. Additionally, cell specific markers for platelets (CD42), natural killer (NK) cells (CD56), and T cells (CD3epsilon and CD3delta) can be used as secondary markers to provide support for diagnosis of disease.
Owner:SEATTLE CHILDRENS HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com