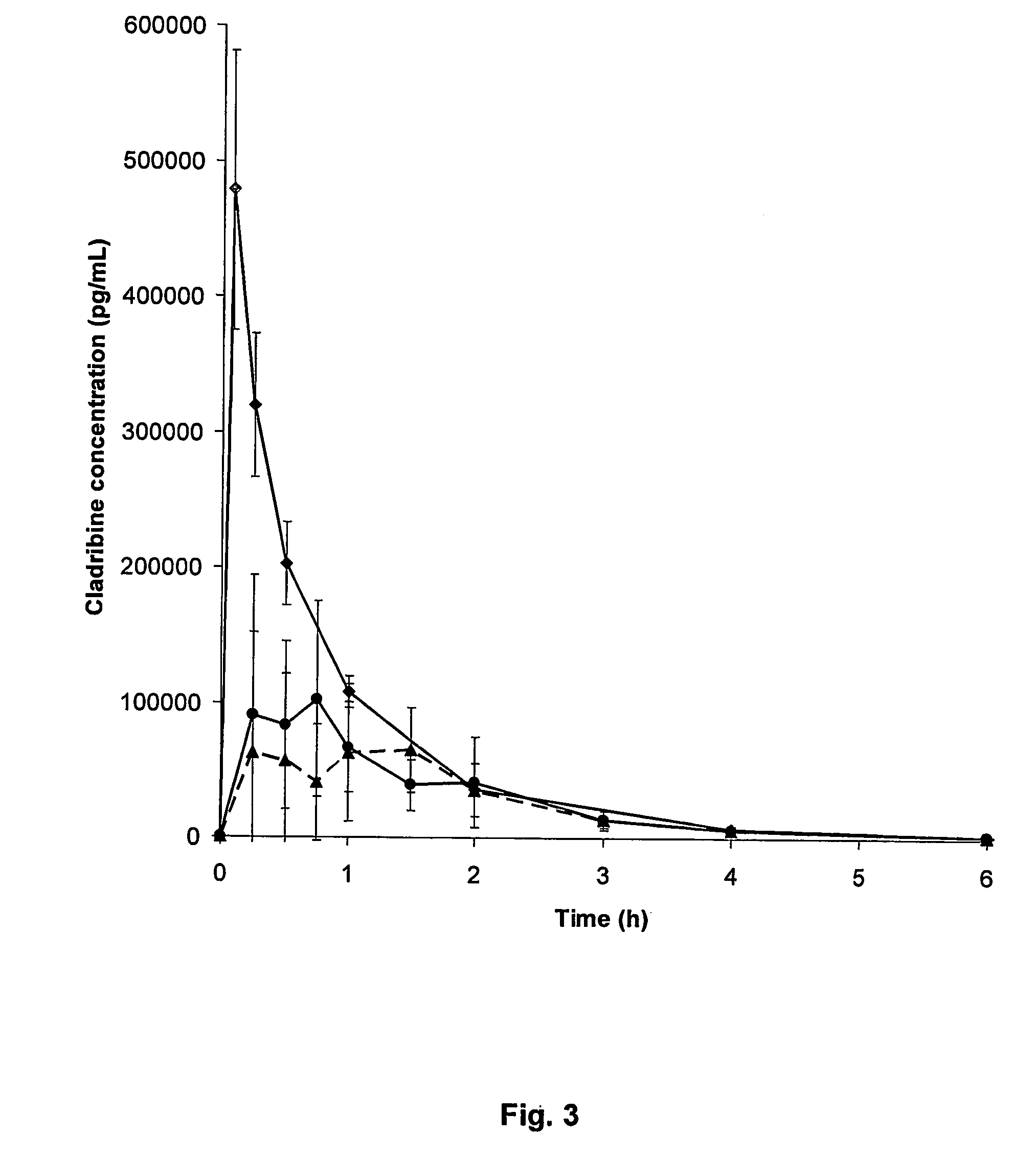
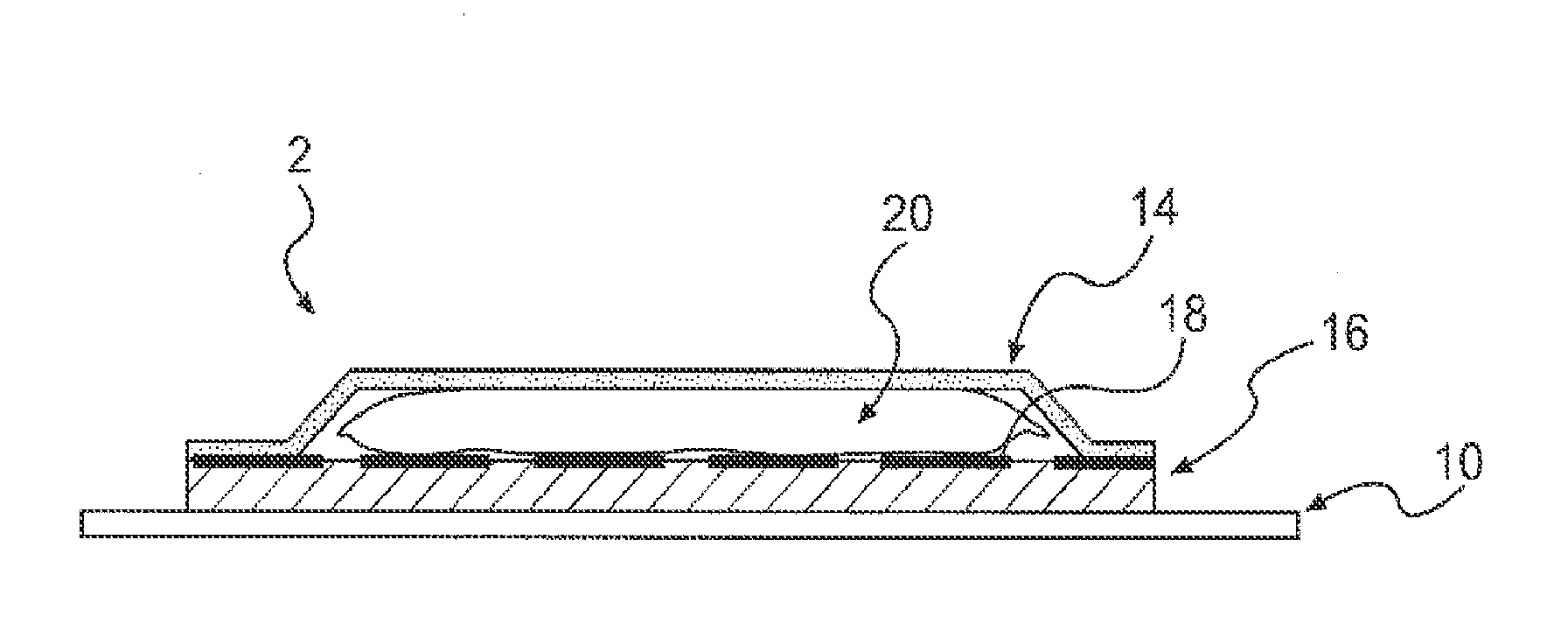
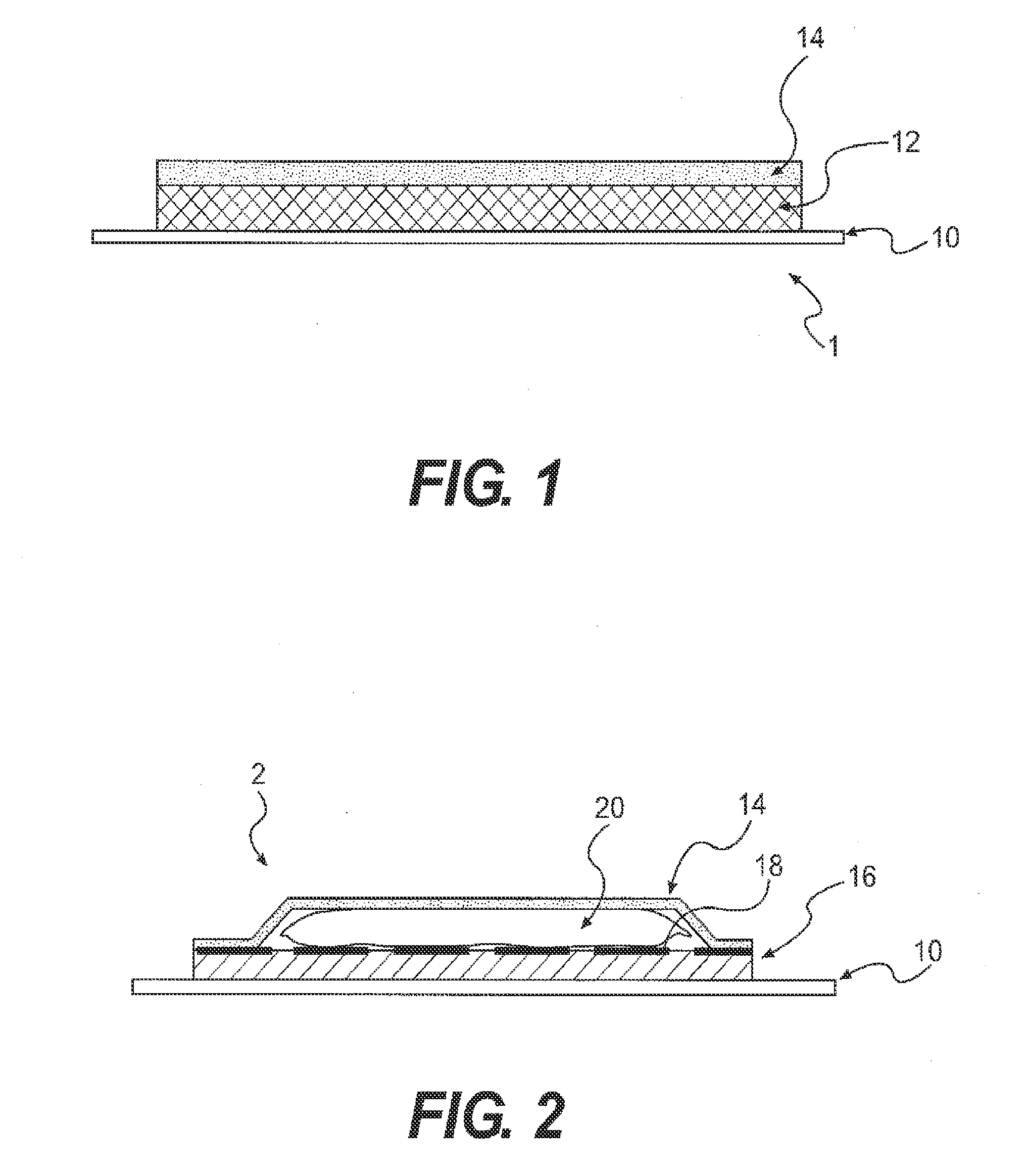
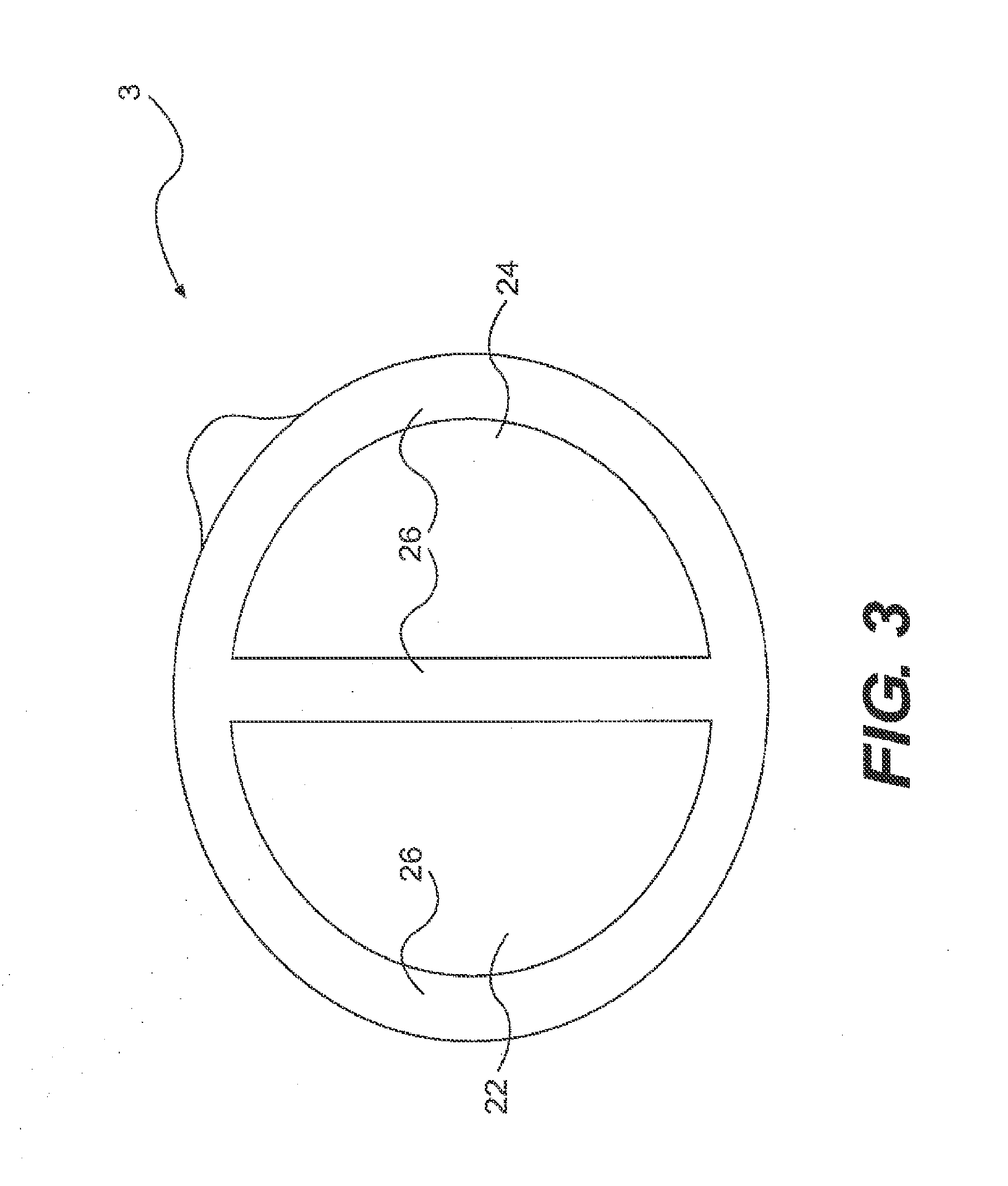
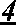Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Cladribine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cladribine is used to treat a certain type of cancer (hairy cell leukemia).
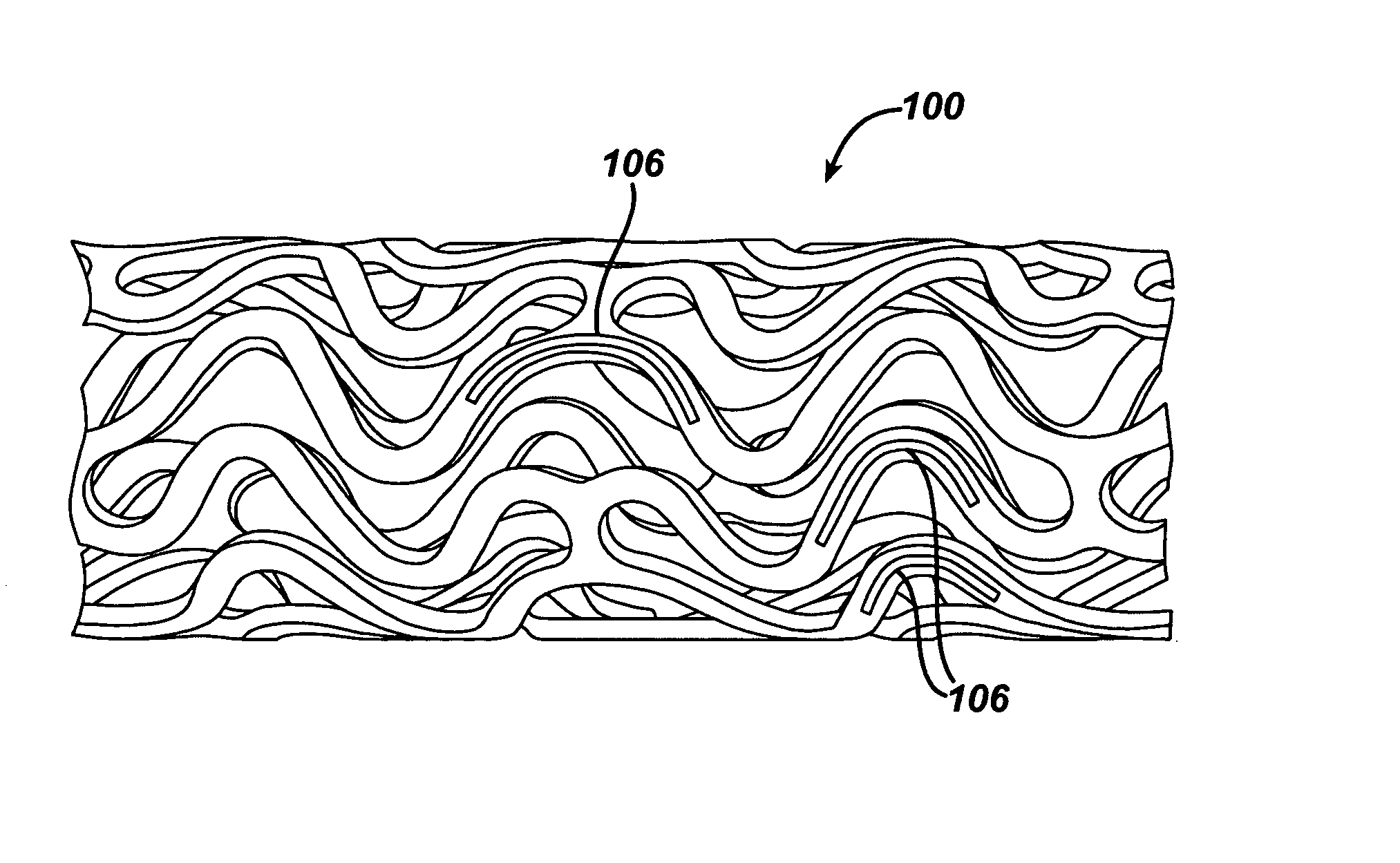
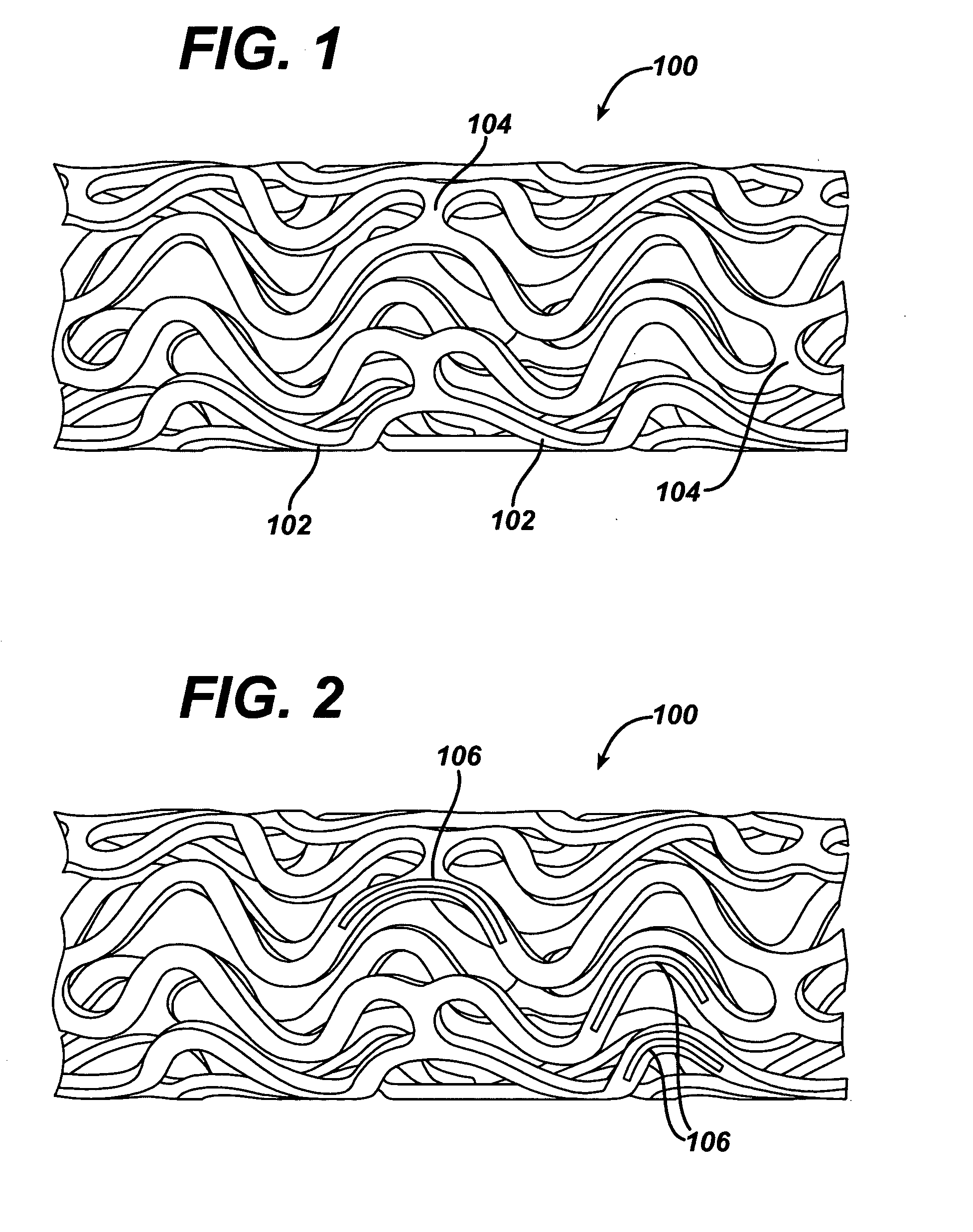
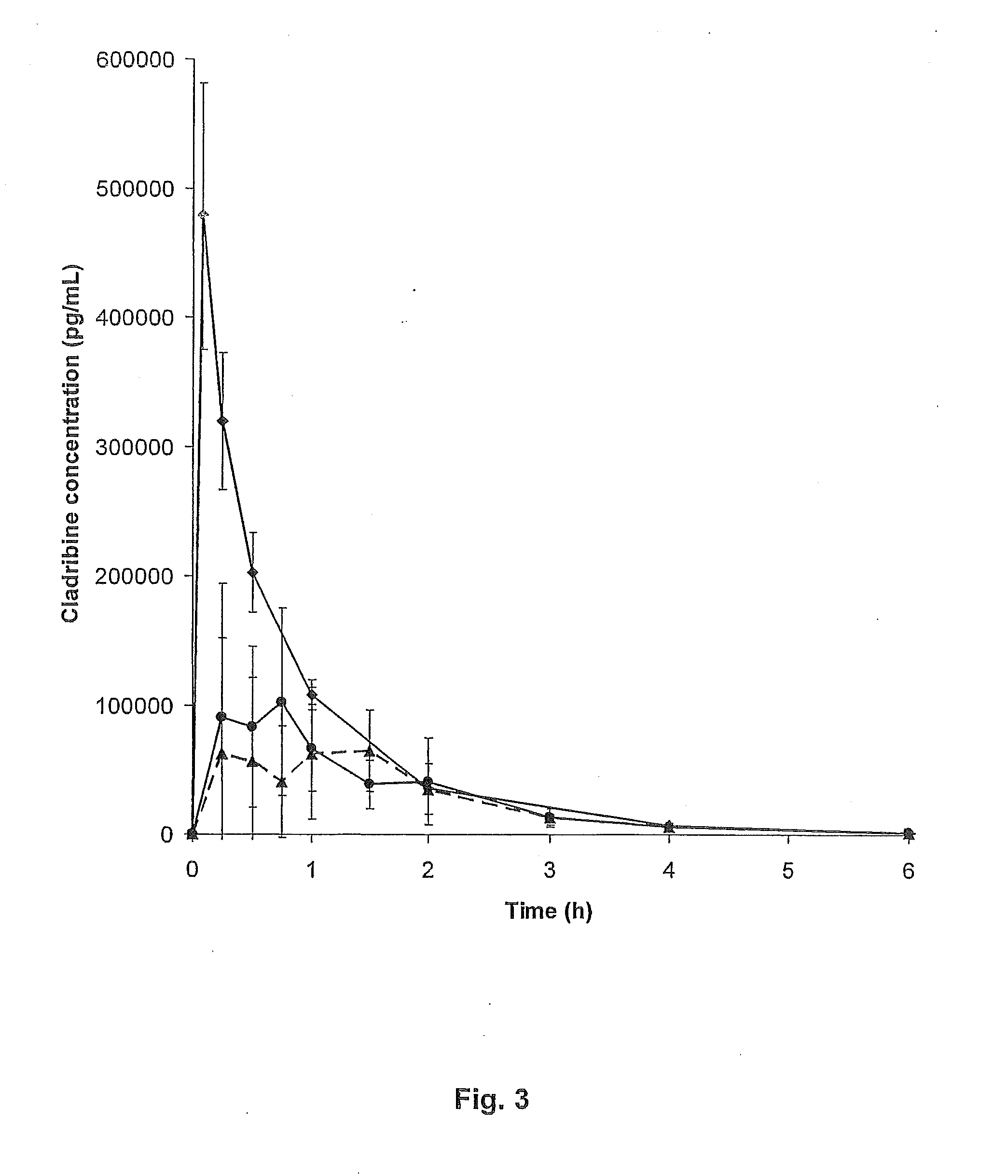
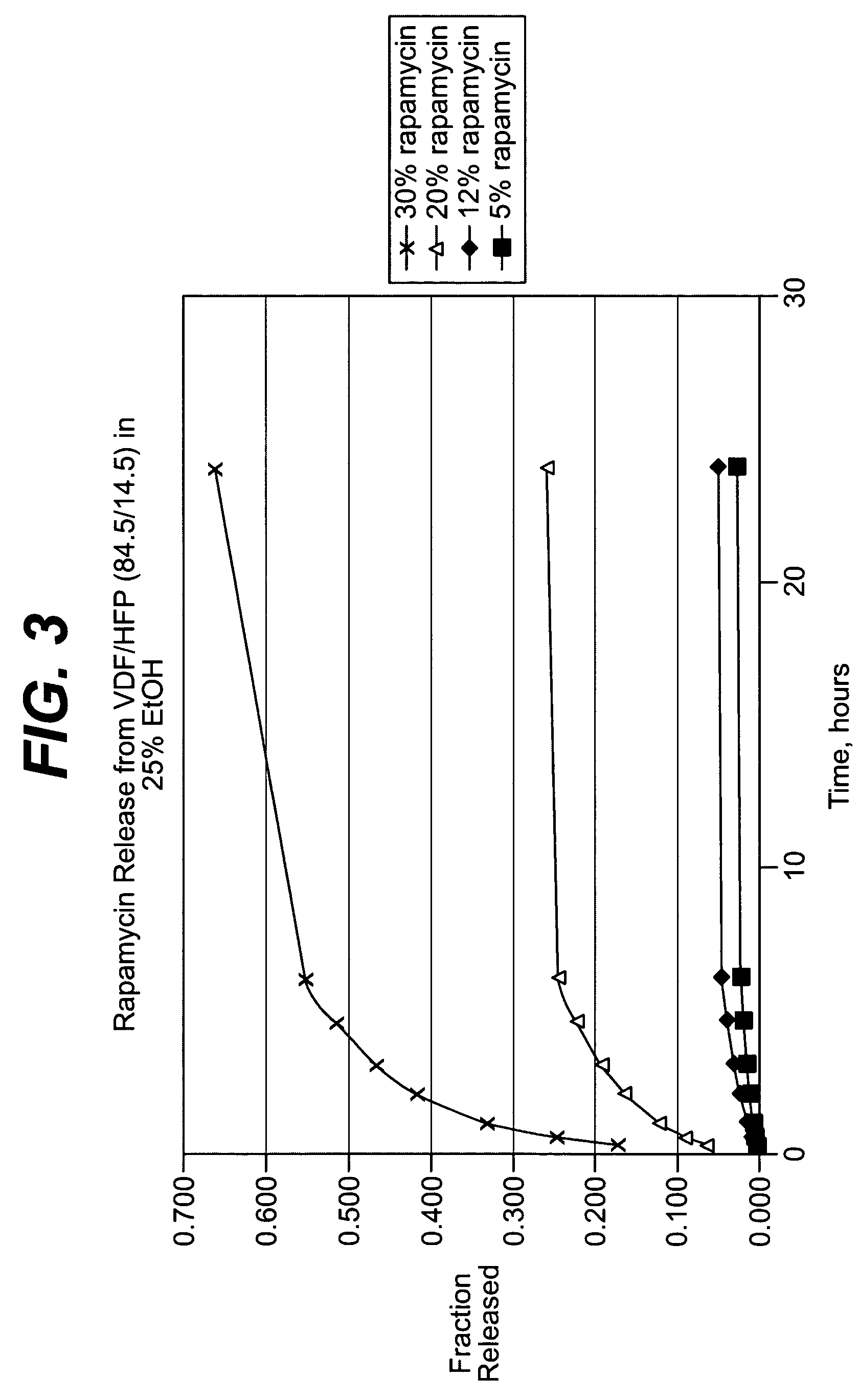
Local vascular delivery of cladribine in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050182485A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
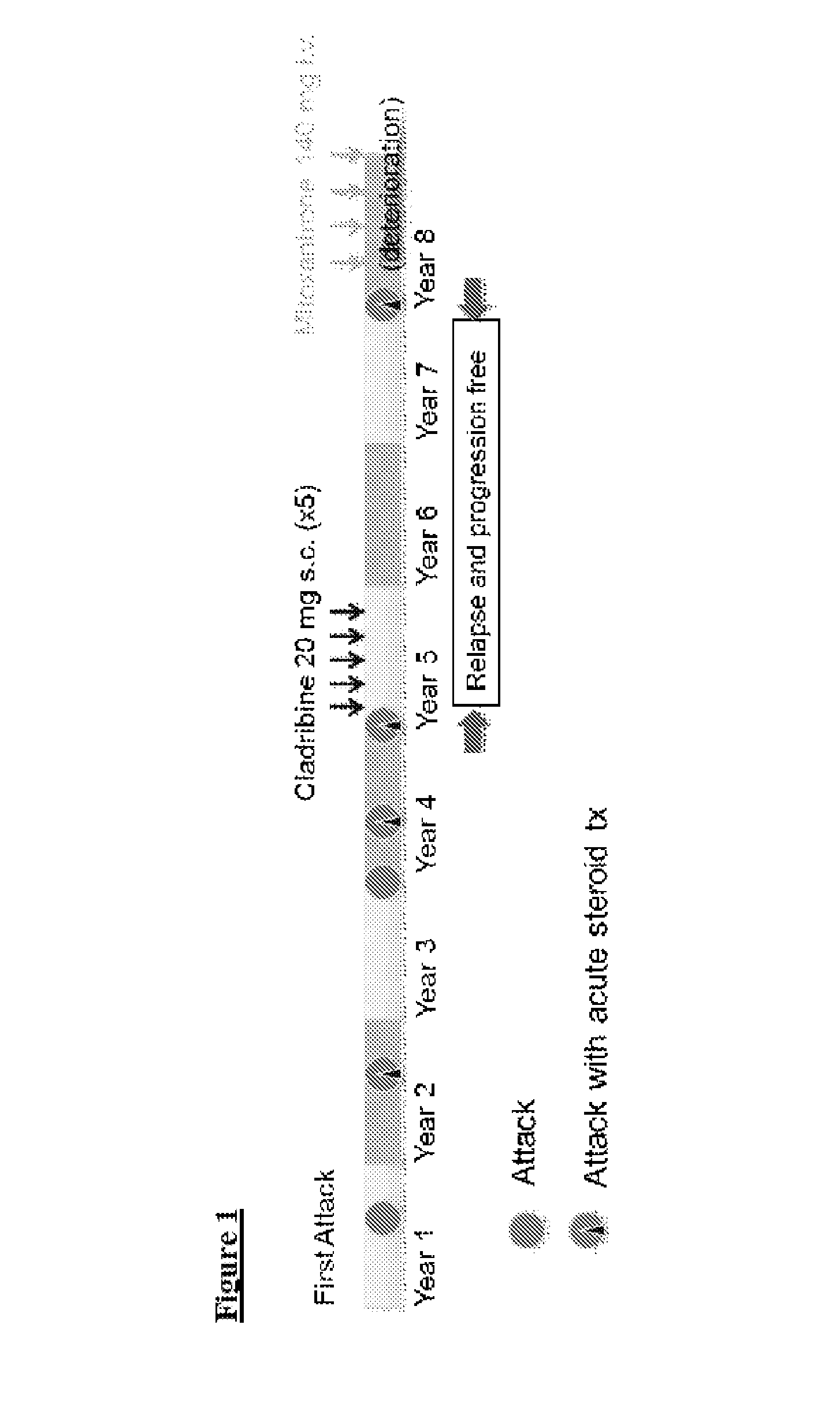
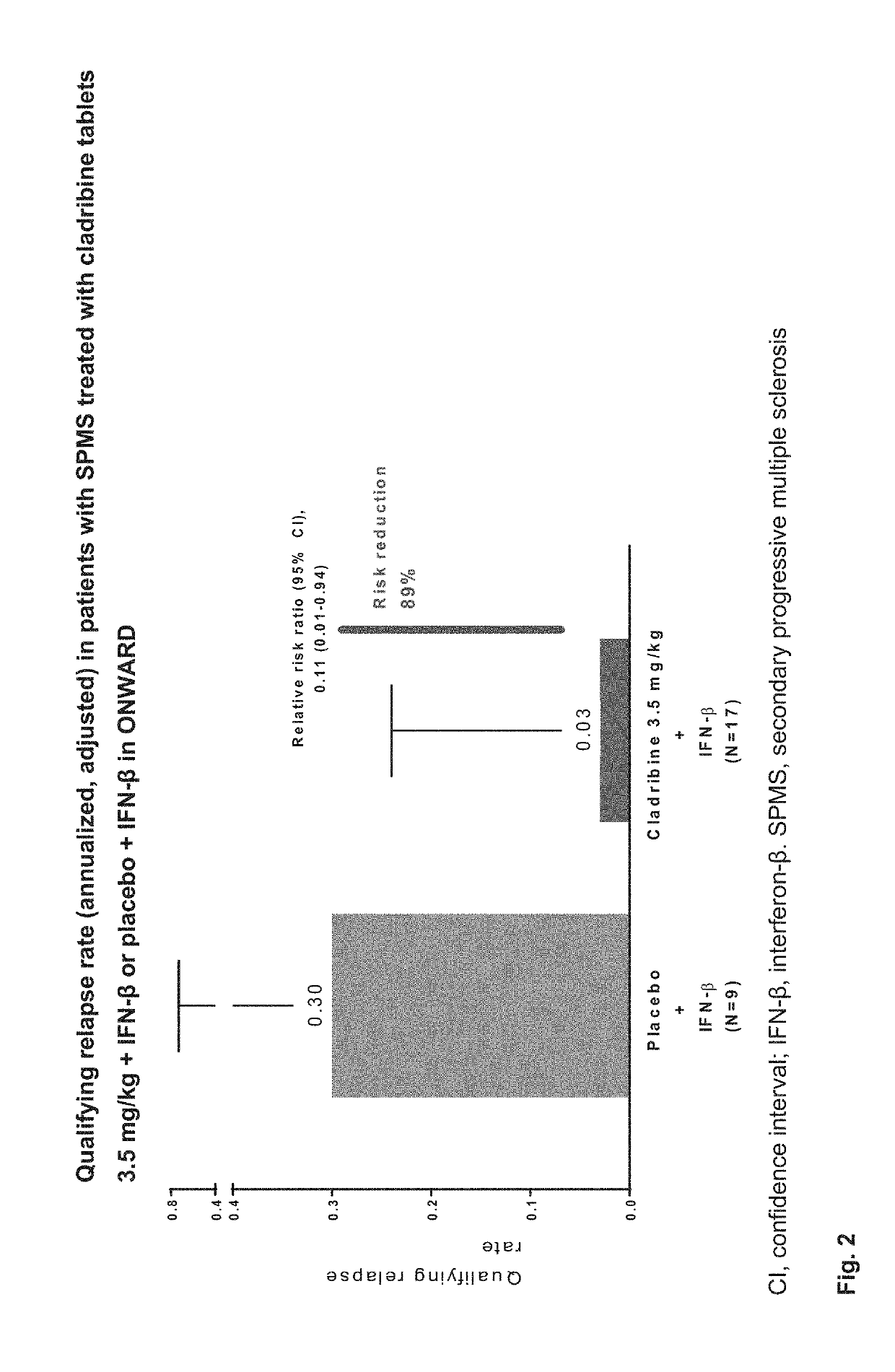
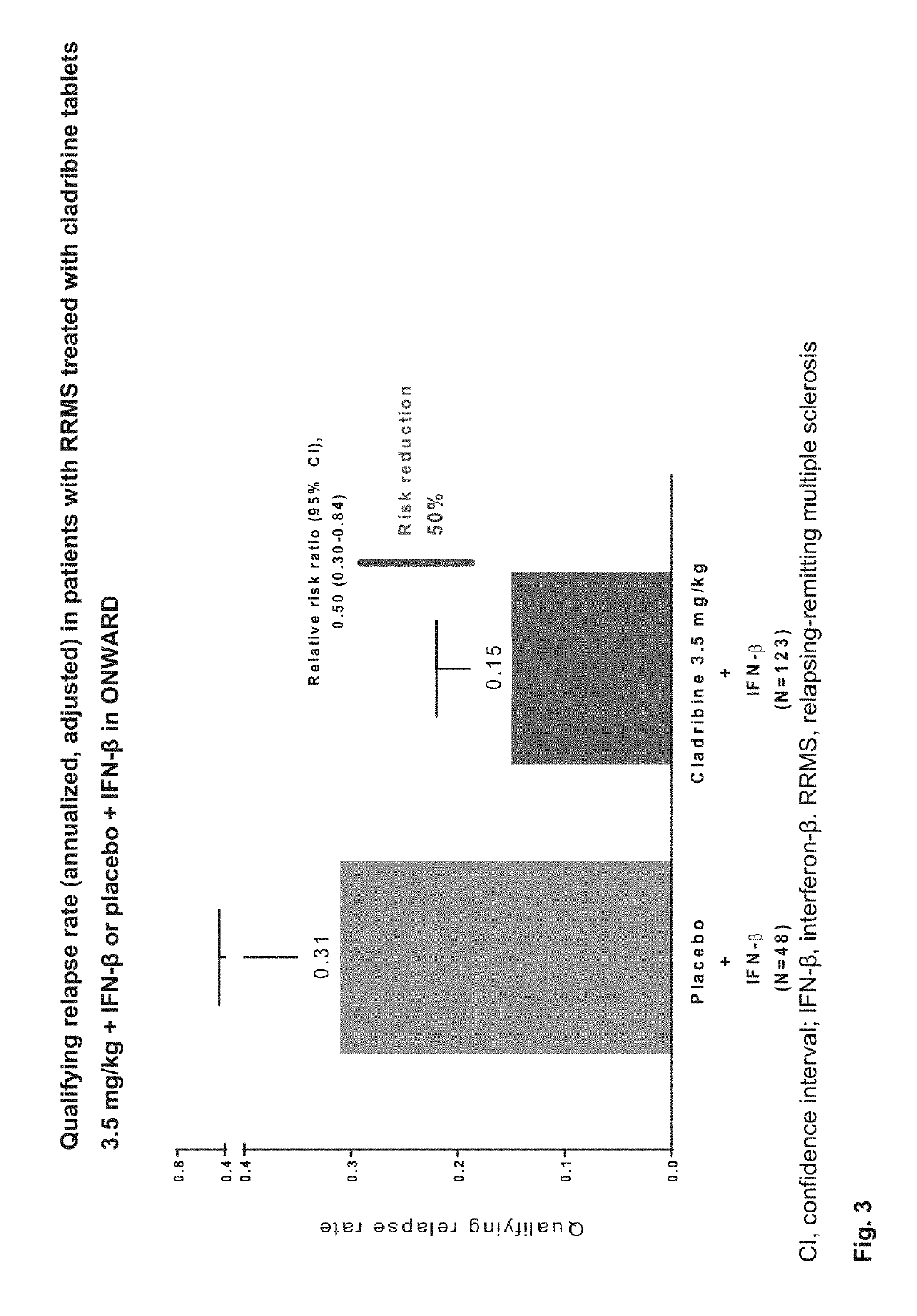
Cladribine regimen for treating multiple sclerosis
ActiveUS20100021429A1Reduce adverse effectsOrganic active ingredientsNervous disorderRegimenBeta interferons
The present invention relates to the use of multiple doses of Cladribine combined with beta interferon for the treatment of multiple sclerosis in patients who are refractory to at least one conventional therapy.
Owner:MERCK SERONO SA
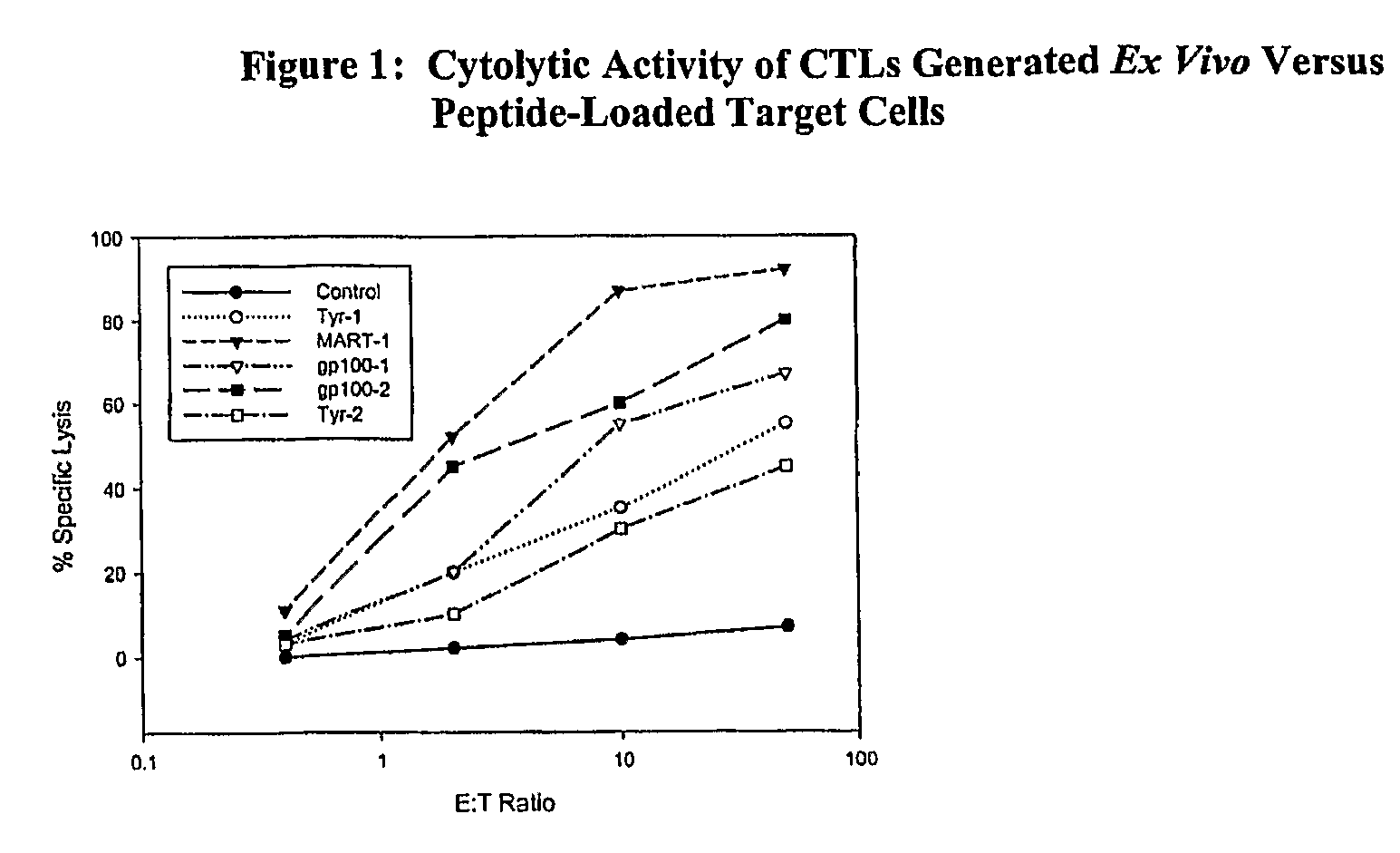
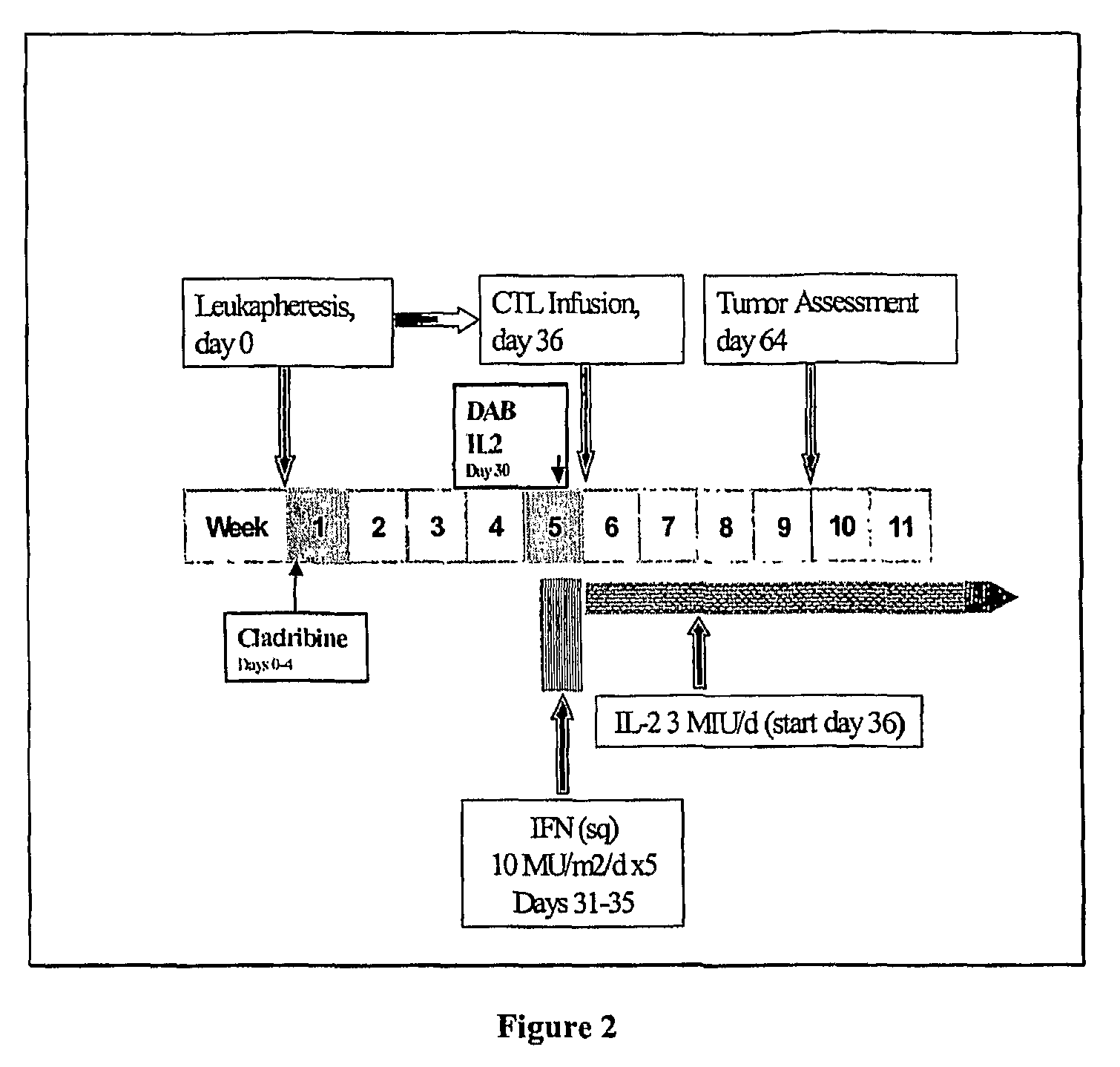
Cancer treatment combining lymphodepleting agent with CTLs and cytokines
In a cancer treatment combining cell therapy with chemotherapy, autologous CD8+ T cells are obtained from a patient, activated ex vivo by contacting them with xenogenic antigen presenting cells loaded with selected peptide antigen, thereby generating antigen-specific activated cytotoxic T lymphocytes. Such activated CTLs are administered to the patient in conjunction with a lymphodepletion and CTL maintenance regimen comprising a non-myeloblative but lymphdepleting agent, such as cladribine or denileukin diftitox, and interleukin-2 and interferon-α-2b stimulatory cytokines.
Owner:JANSSEN PHARMA NV
Novel therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
InactiveUS20050233987A1Improve toleranceImprove therapeutic potentialBiocideSugar derivativesDiseaseApoptosis
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
Anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
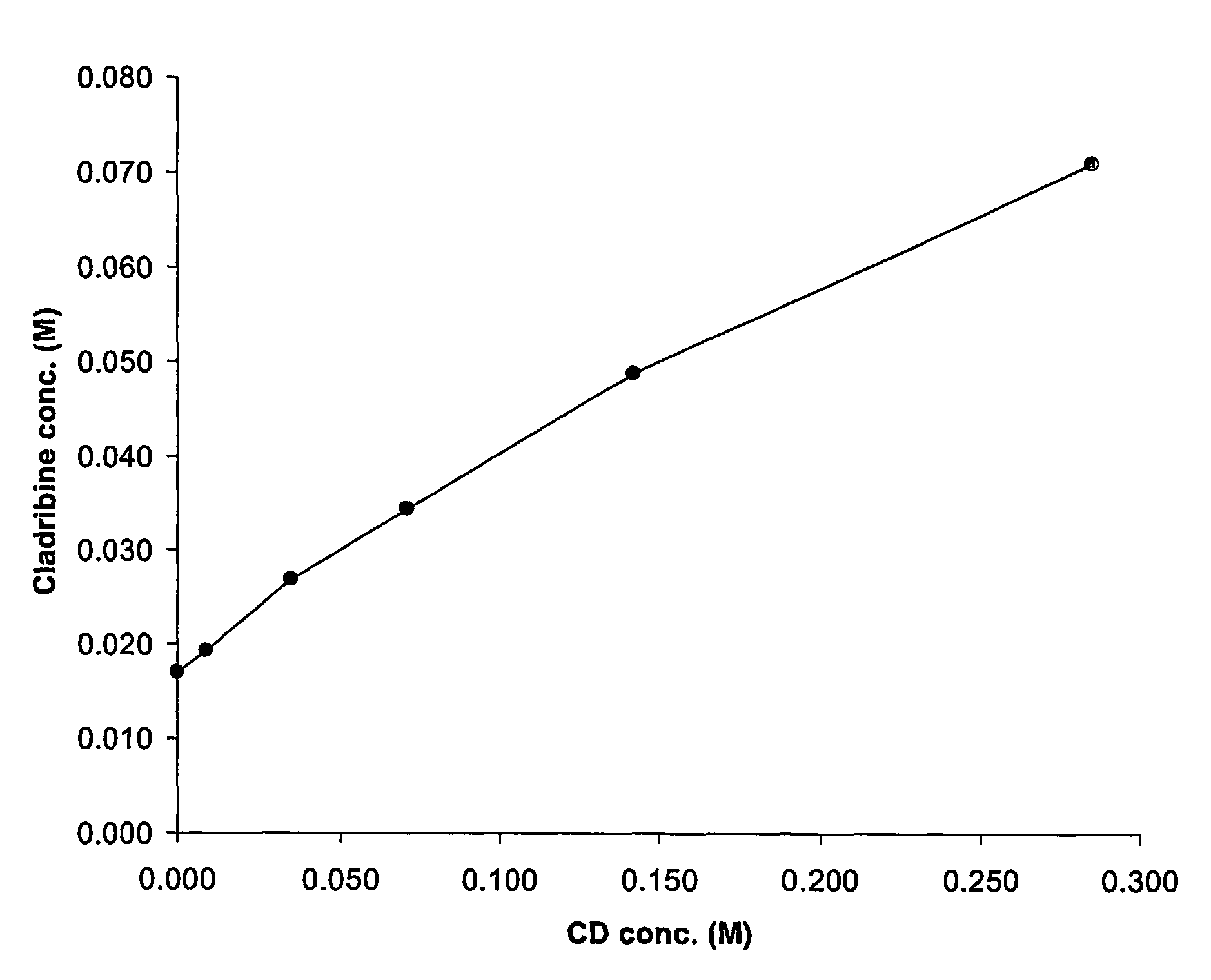
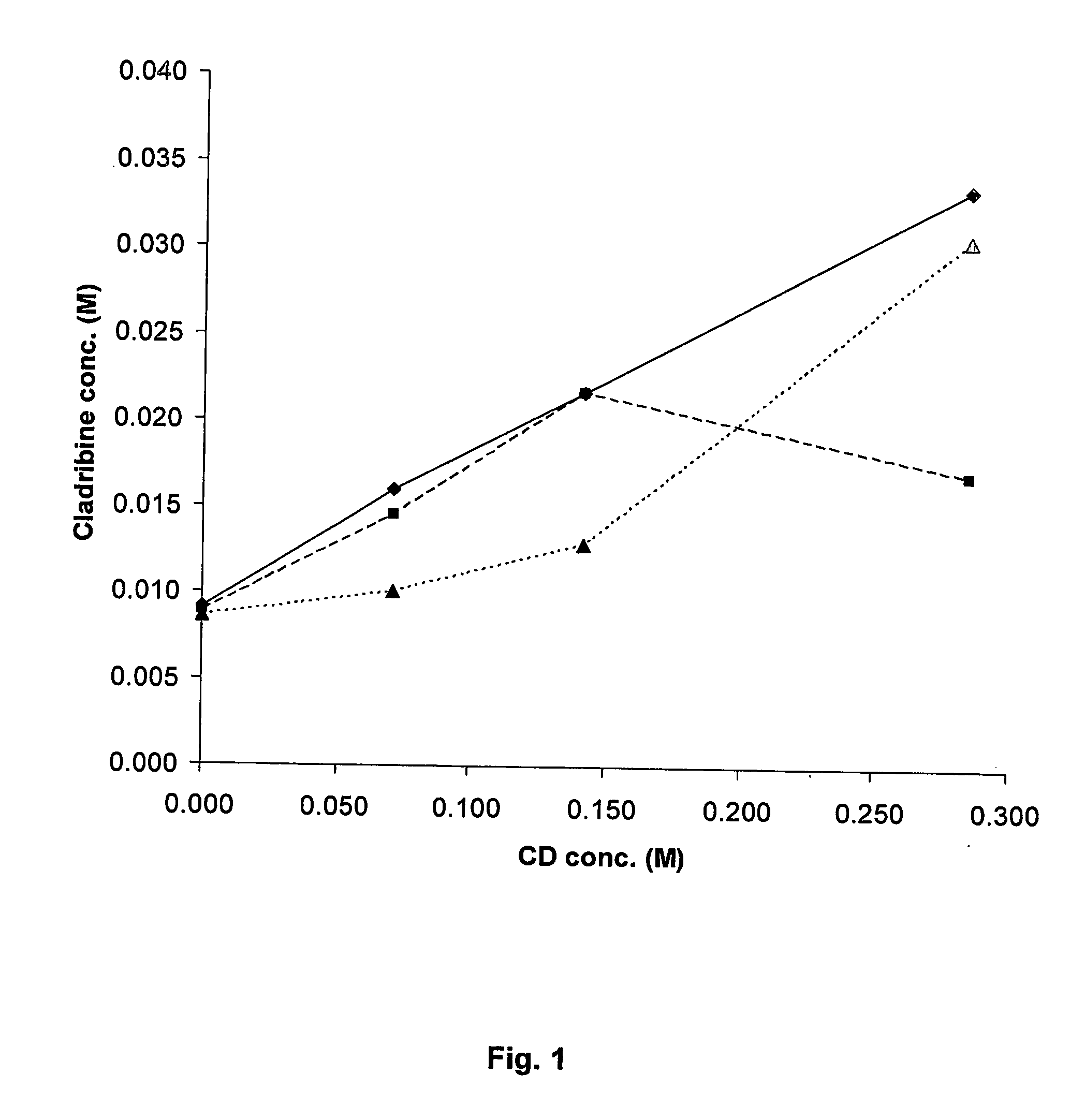
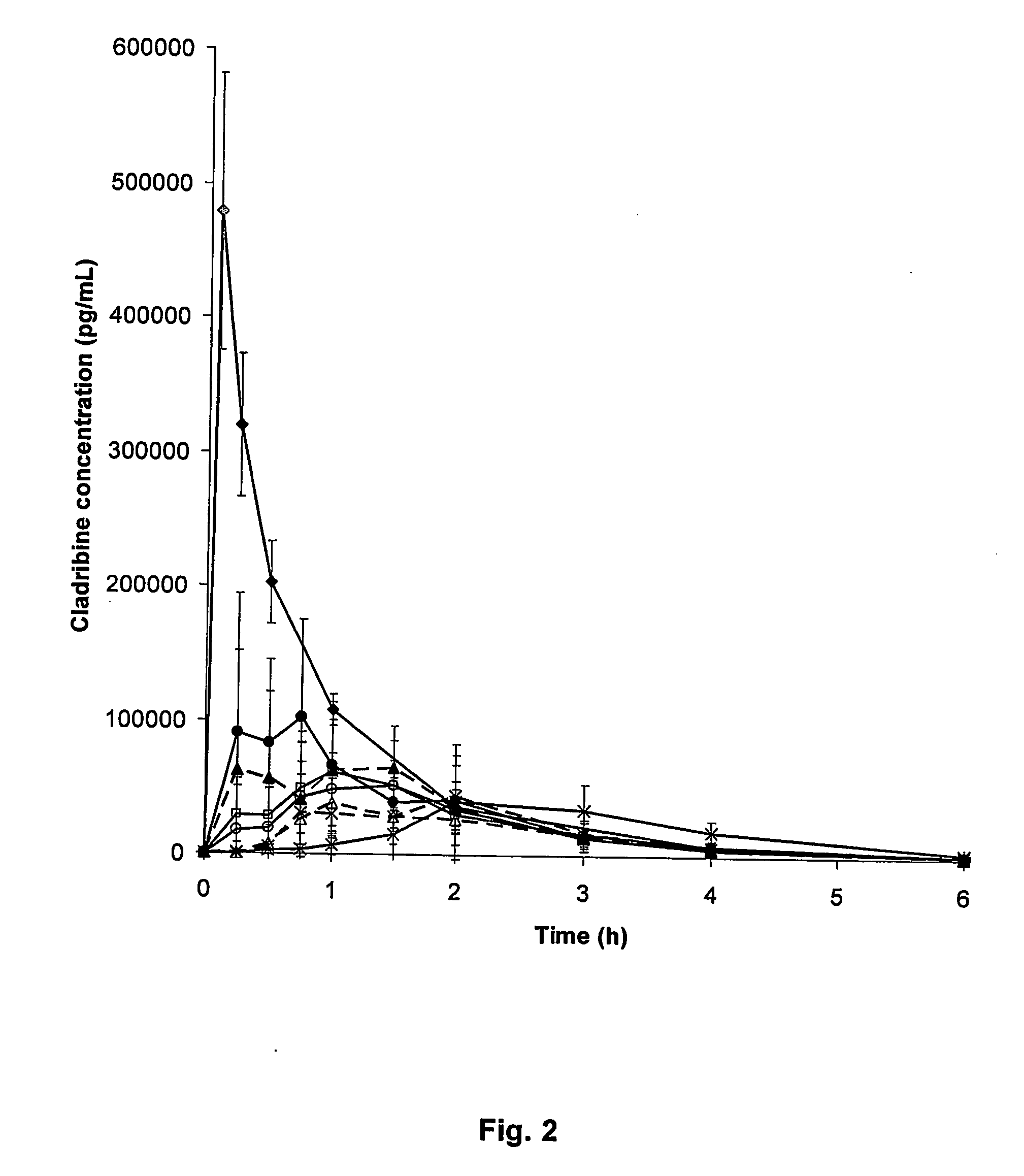
Cladribine formulations for improved oral and transmucosal delivery
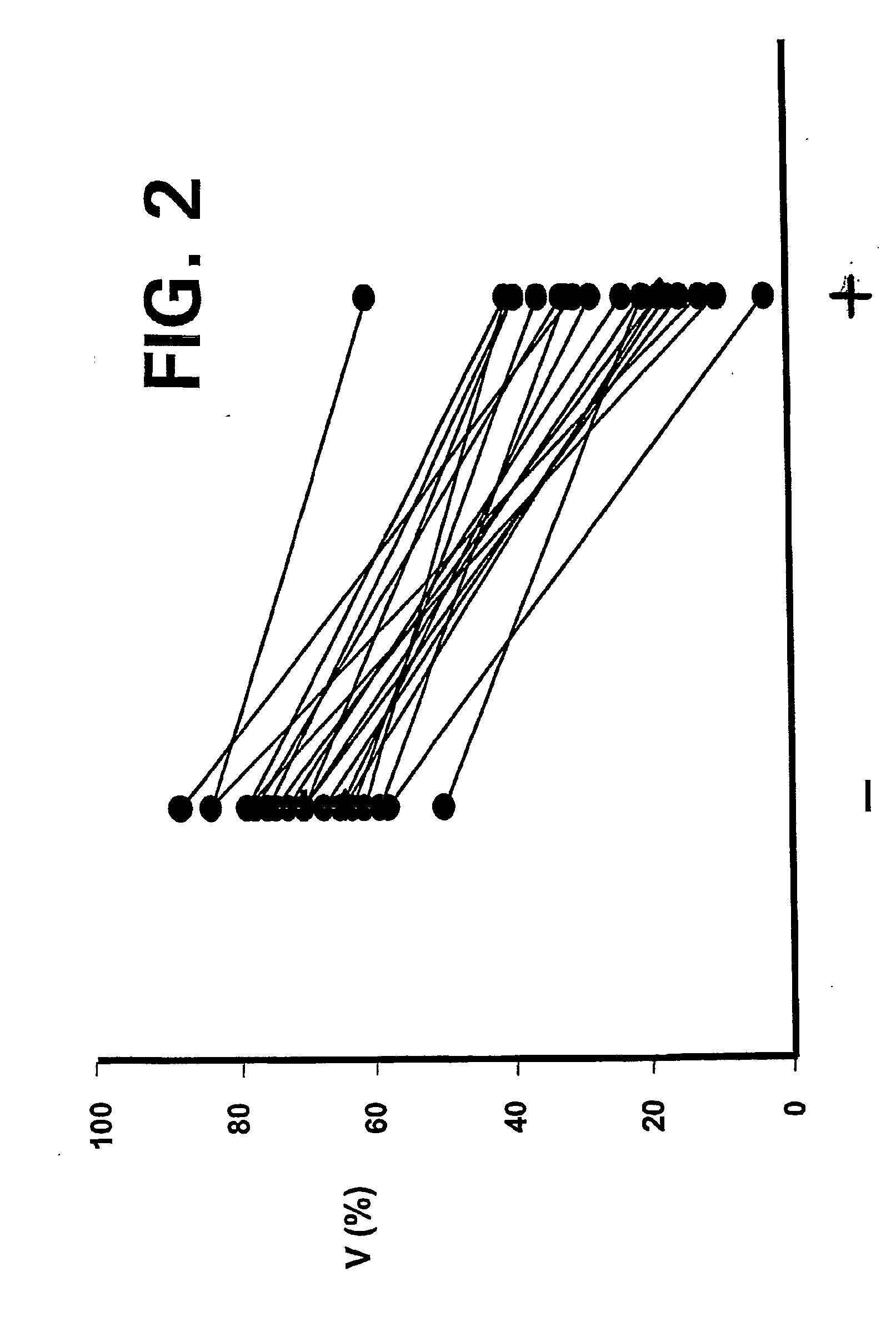
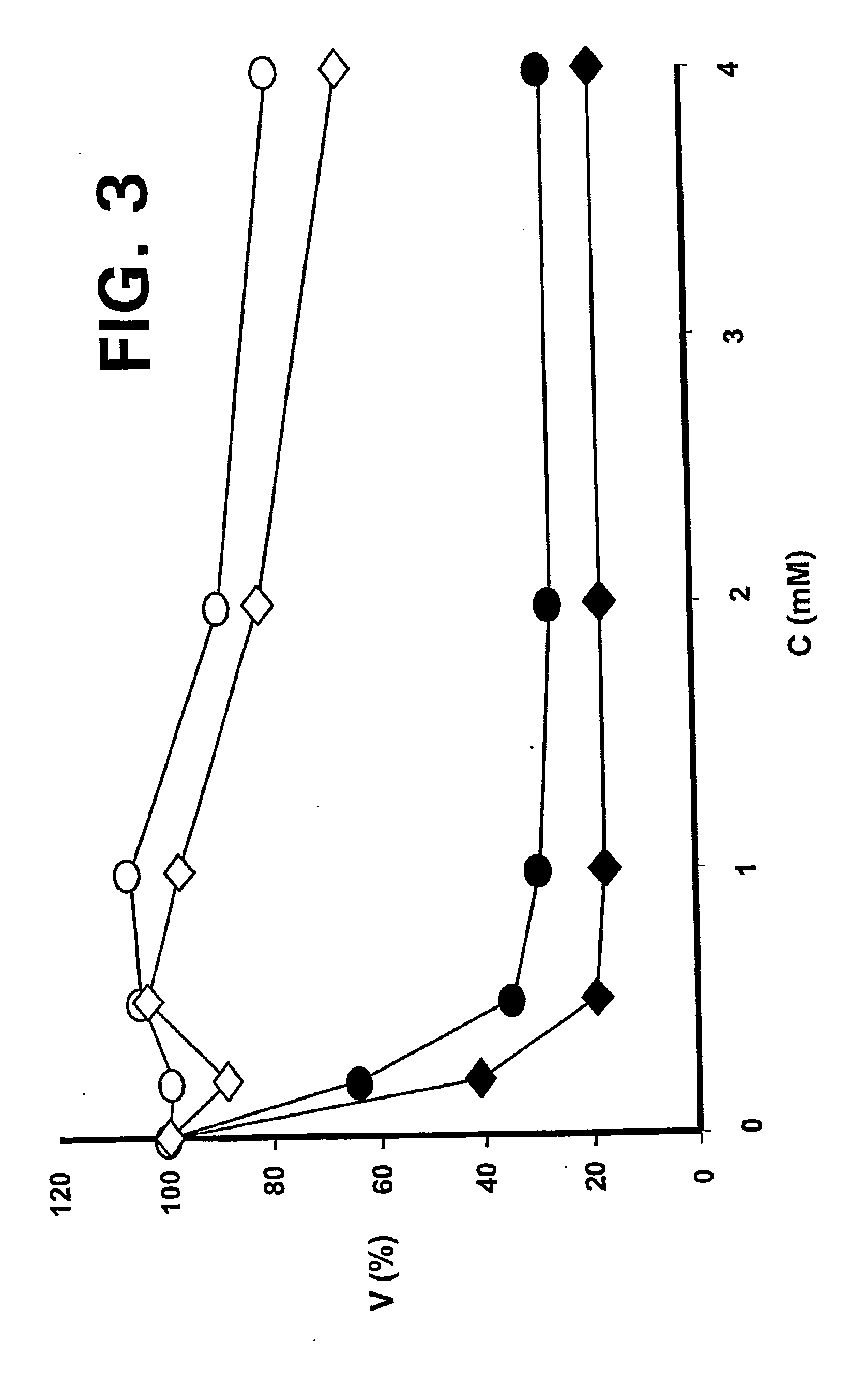
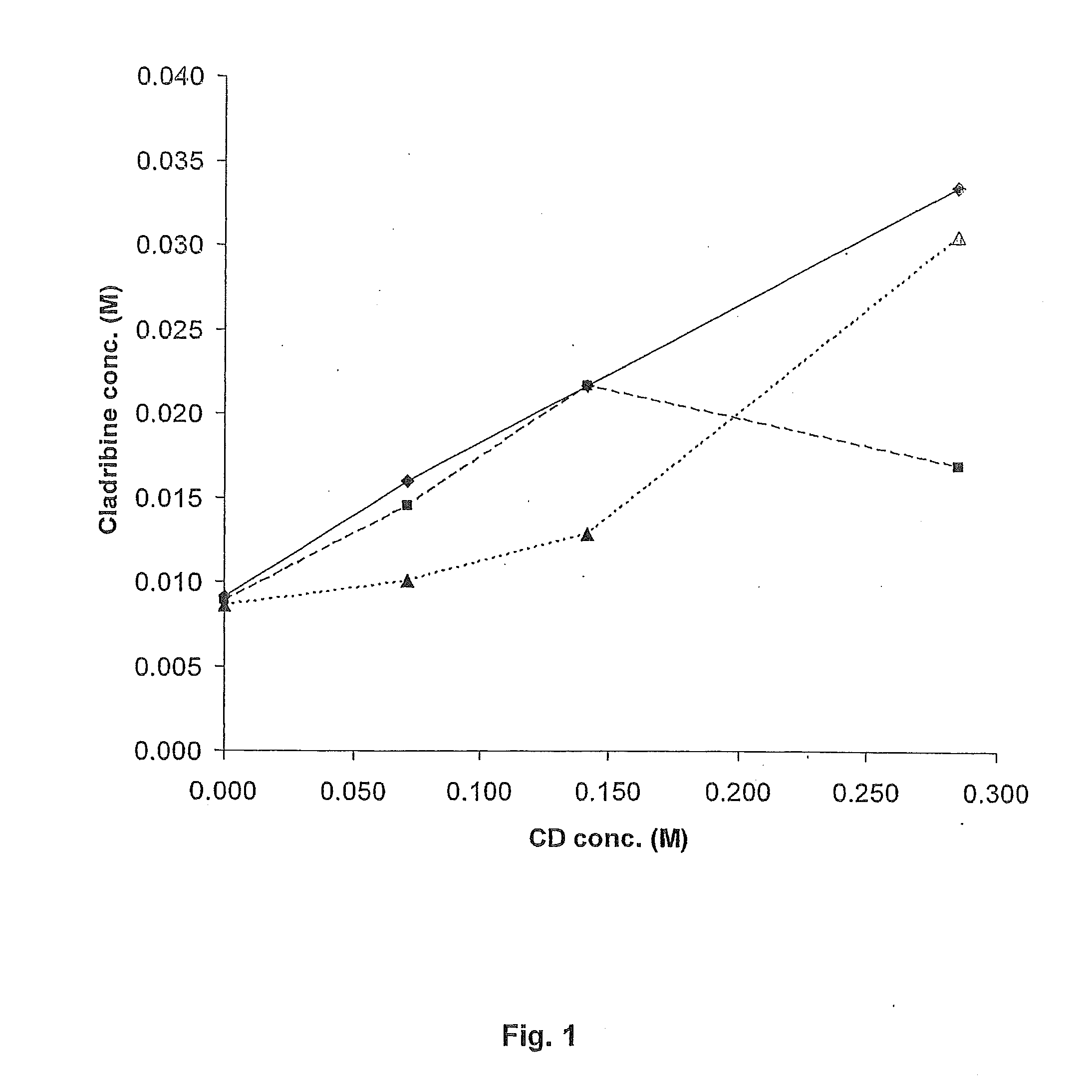
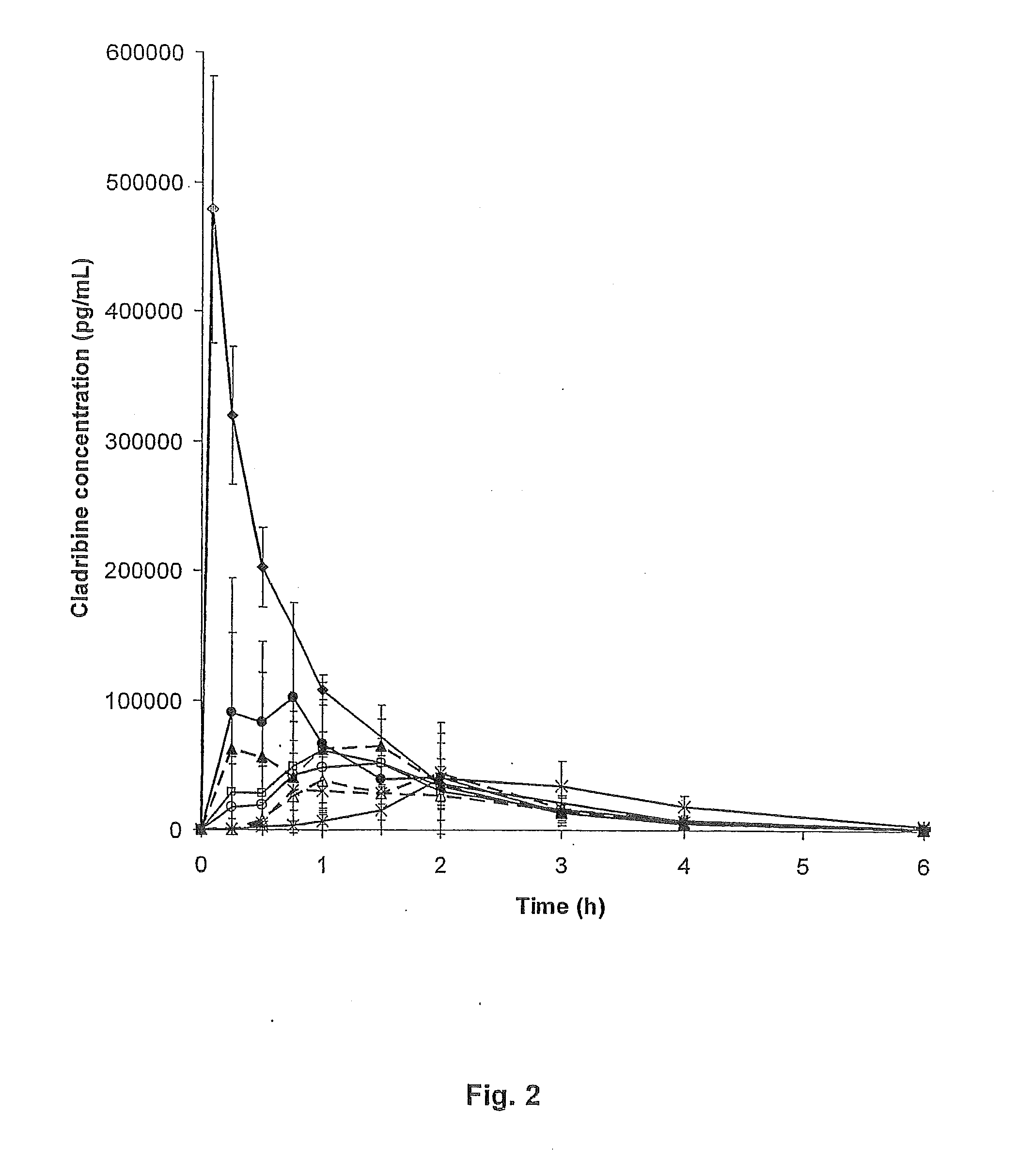
ActiveUS20110015145A1Low interpatientImprove bioavailabilityAntibacterial agentsBiocideBuccal useCyclodextrin
Provided are compositions of cladribine and cyclodextrin which are especially suited for the oral and buccal administration of cladribine.
Owner:ARES TRADING SA
Pharmaceutical composition containing a hypomethylating agent and a histone deacetylase inhibitor
InactiveUS20110129521A1Good curative effectBiocideCarbohydrate active ingredientsTransdermal patchHistone methylation
A pharmaceutical composition for induction therapy which has a hypomethylating agent and a histone deacetylase inhibitor (“HDAC inhibitor”); wherein the hypomethylating agent is a DNA and histone methylation inhibitor such as cladribine and the HDAC inhibitor is, for example, entinostat, panobinostat, vorinostat, and / or romedepsin; further wherein the hypomethylating agent and the HDAC inhibitor are combined in formulations for various administrations including e.g., a continuous delivery system such as a transdermal patch of at least one reservoir or a plurality of reservoirs, oral, a fixed-dose oral combination, intravenous, and combinations thereof. This pharmaceutical composition for induction therapy is used with a monoclonal antibody in the treatment of various cancers, sarcomas, and other malignancies.
Owner:NIMBLE EPITECH
Slow released anticancer medicine preparation with both amrubicin and its synergist
The slow released anticancer medicine injection containing both amrubicin and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 25 deg.c. The effective anticancer component is amrubicin, idarubicin, etc and / or antimetabolite composition selected from carmofur, tegafur, zalcitabine, etc. The slow releasing supplementary material is selected from EVAc, sebacic acid copolymer, lactic acid polymer, etc. The slow released microsphere may be also prepared into slow released implanting agent set around or inside the tumor to strengthen the chemotherapy or radiotherapy effect.
Owner:JINAN KANGQUAN PHARMA TECH
Implantable structures for local vascular delivery of cladribine in combination with rapamycin for restenosis
ActiveUS7806924B2Provide controlSuture equipmentsOrganic active ingredientsBiological bodyPercent Diameter Stenosis
Medical devices, and in particular implantable structures, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization.
Owner:WYETH LLC
Therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
The present disclosure relates to a method of treatment of a human patient suffering from a B-cell lymphoproliferative disorders such as B-cell chronic lymphocytic leukemia (B-CLL), splenic marginal zone lymphoma (SMZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), lymphoplasmacytic lymphoma (LPL), and Waldenström syndrome (WS), by the administration of a therapeutically effective amount of 5-aminoimidazole-4-carboxamide riboside (acadesine) or its precursors (eg. its mono-, di- and tri-5′-phosphates). This makes acadesine and its bioprecursors (eg. its mono-, di- and tri-5′-phosphates) useful as therapeutic agents for B-cell lymphoproliferative disorders in humans. The surprising feature that T cells are virtually not affected means that the side effect (immunosuppression) is minor, what represents a therapeutical advantage of acadesine over cladribine, fludarabine and other nucleosides known in the art.
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
Anti-cancer drug slow release injection containing gemcitabine
The invention relates to an anti-cancer drug slow release injection containing gemcitabine, which comprises slow release microspheres and a menstruum, wherein the microsphere comprises anti-cancer active ingredients and slow release auxiliary materials, and the menstruum is a special menstruum containing a suspending agent; the anti-cancer active ingredients are gemcitabine, or antimetabolites selected from zalcitabine, emtricitabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazole gemcitabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite potentiating agents selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogue and / or DNA repair enzyme inhibitor; the slow release auxiliary materials are polifeprosan, dienoic fatty acid, decanedioic acid copolymer, polylactic acid copolymer, EVAc and the like; the suspending agent has a viscosity of 100cp-3000cp (20-30 DEG C), and is selected from sodium carboxymethylcellulose and the like. The slow release microspheres can be prepared into slow release implants. When injected or placed into tumors or at peripheries of tumors, the slow release microspheres can enhance the treatment effect of non-operative treatment methods, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Use of cladribine for treating autoimmune inflammatory disease
2-Chloro-2′-deoxyadenosine, hereinafter referred to as cladribine, or a pharmaceutically acceptable salt thereof may be used in the treatment or amelioration of neuromyelitis optica, hereinafter referred to as NMO e.g. in patients known to have NMO-IgG seropositivity or in patients optic neuritis, myelitis and at least two of MRI evidence of contiguous spinal cord lesion 3 or more segments in length, onset brain MRI nondiagnostic for multiple sclerosis or NMO-IgG seropositivity.
Owner:CHORD THERAPEUTICS S A R L
Oral formulations of cladribine
ActiveUS7888328B2Interpatient and intrapatient variationImprove oral bioavailabilityBiocideNervous disorderOral medicationCyclodextrin
Provided are compositions of cladribine and cyclodextrin which are especially suited for the oral administration of cladribine.
Owner:ARES TRADING SA
Cladribine formulations for improved oral and transmucosal delivery
InactiveUS20070065492A1Improves oralImproves transmucosal bioavailabilityAntibacterial agentsBiocideBuccal useCyclodextrin
Provided are compositions of cladribine and cyclodextrin which are especially suited for the oral and buccal administration of cladribine.
Owner:ARES TRADING SA
Cladribine regimen for treating multiple sclerosis
The present invention is related to the use of Cladribine for the preparation of a pharmaceutical formulation for the treatment of multiple sclerosis, especially relapsing-remitting multiple sclerosis or early secondary progressive multiple sclerosis, wherein the preparation is to be orally administered and wherein re-treatments are possible.
Owner:MERCK SERONO SA
Pharmaceutical composition containing a hypomethylating agent and a histone deacetylase inhibitor
InactiveUS20120310183A1Organic active ingredientsGenetic material ingredientsTransdermal patchHistone methylation
A pharmaceutical composition for induction therapy which has a hypomethylating agent and a histone deacetylase inhibitor (“HDAC inhibitor”); wherein the hypomethylating agent is a DNA and histone methylation inhibitor such as cladribine and the HDAC inhibitor is, for example, entinostat, panobinostat, vorinostat, and / or romedepsin; further wherein the hypomethylating agent and the HDAC inhibitor are combined in formulations for various administrations including e.g., a continuous delivery system such as a transdermal patch of at least one reservoir or a plurality of reservoirs, oral, a fixed-dose oral combination, intravenous, and combinations thereof. This pharmaceutical composition for induction therapy is used with a monoclonal antibody in the treatment of various cancers, sarcomas, and other malignancies.
Owner:NIMBLE EPITECH
Cladribine preparation administrated by injection and preparation method thereof
InactiveCN101756891AOrganic active ingredientsPharmaceutical delivery mechanismWater solubleChronic granulocytic leukemia
The invention relates to a cladribine preparation administrated by injection and a preparation method thereof. The preparation is characterized by consisting of cladribine, a water-soluble filler, a pH regulator, water for injection and an osmotic pressure regulator, wherein the preparation contains 0.01 to 50 weight percent of cladribine and the balance of pharmaceutical adjuvant or water. The cladribine preparation administrated by the injection of the invention has the advantages of good stability and high bioavailability and satisfies need for injection medicaments required by the treatment of clinical acute and chronic granulocytic leukemia.
Owner:TIANJIN PACIFIC PHARMA
Method for the production of cladribine
InactiveUS20100291632A1Improve isolationHigh yieldOrganic chemistryFermentationPurine nucleoside phosphorylaseSolvent
A method for producing cladribine (2-chloro-2′-deoxyadenosine) comprising the steps of:a) reaction of 2-deoxyuridine with 2-chloroadenine, in the presence of uridine phosphorylase (UPase) and purine nucleoside phosphorylase (PNPase) in an aqueous reaction medium possibly containing up to 40% v / v of an aprotic dipolar solvent, to obtain cladribine dissolved in said reaction medium;b) isolation of the cladribine by precipitation by means of concentration and alkalinisation of the reaction medium up to pH 11.5-12.5.
Owner:EXPLORA LAB
Gemcitabine-containing anti-cancer medicine sustained-release injection
The invention relates to cancer therapy drug sustained-release injection containing gemcitabine. The injection comprises sustained-release microspheres and dissolvent, wherein, the sustained-release microspheres comprise active ingredients for cancer therapy and sustained-release auxiliary materials, and the dissolvent is special dissolvent containing suspending agent. The active ingredients for cancer therapy comprise antimetabolite of gemcitabine or antimetabolite selected from zalcitabine, emtritabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite synergist selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogs and / or DNA repair enzyme inhibitor; the sustained-release auxiliary materials comprise polifeprosan, bi-fatty acid, decanedioic copolymer, polylactic copolymer and EVAc; the viscocity of the suspending agent is 100cp to 3000cp (at 20 to 30 DEG C) and the suspending agent is selected from sodium carboxymethyl cellulose. The sustained-release microspheres can also be produced into sustained-release implant, and by injecting or positioning the sustained release agent in tumor or tumor margin, the efficacy of non-operative treatment such as radiation treatment and chemical treatment can be enhanced.
Owner:SHANDONG LANJIN PHARMA +1
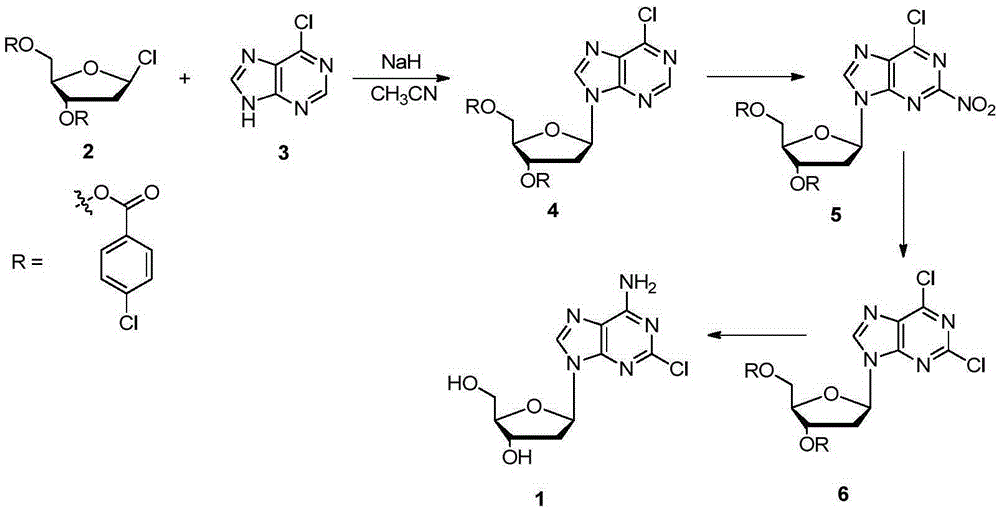
Method for the preparation of 2-halo-2'-deoxyadenosine compounds for 2'-deoxyguanosine
The present invention is a method for preparing 2-halo-6-aminopurines, and more specifically for preparing the clinical agent cladribine (2-chloro-2′-deoxyadenosine, CldAdo, 4), a drug of choice against hairy-cell leukemia and other neoplasms, from 2-amino-6-oxopurines, which are readily obtained from the naturally occurring compound 2′-deoxyguanosine. According to the methods of the present invention, the 6-oxo group of a protected 2′-deoxyguanosine (1) is converted to a 6-(substituted oxy) leaving group, or alternatively to a 6-chloro leaving group, the 2-amino group is replaced with a 2-chloro group, the 6-(substituted oxy) leaving group, or alternatively the 6-chloro leaving group, is replaced with a 6-amino group or, alternatively, a 2,6-dichloro substituted compound is selectively replaced with a 6-amino group, and the protecting groups are removed.
Owner:BRIGHAM YOUNG UNIV
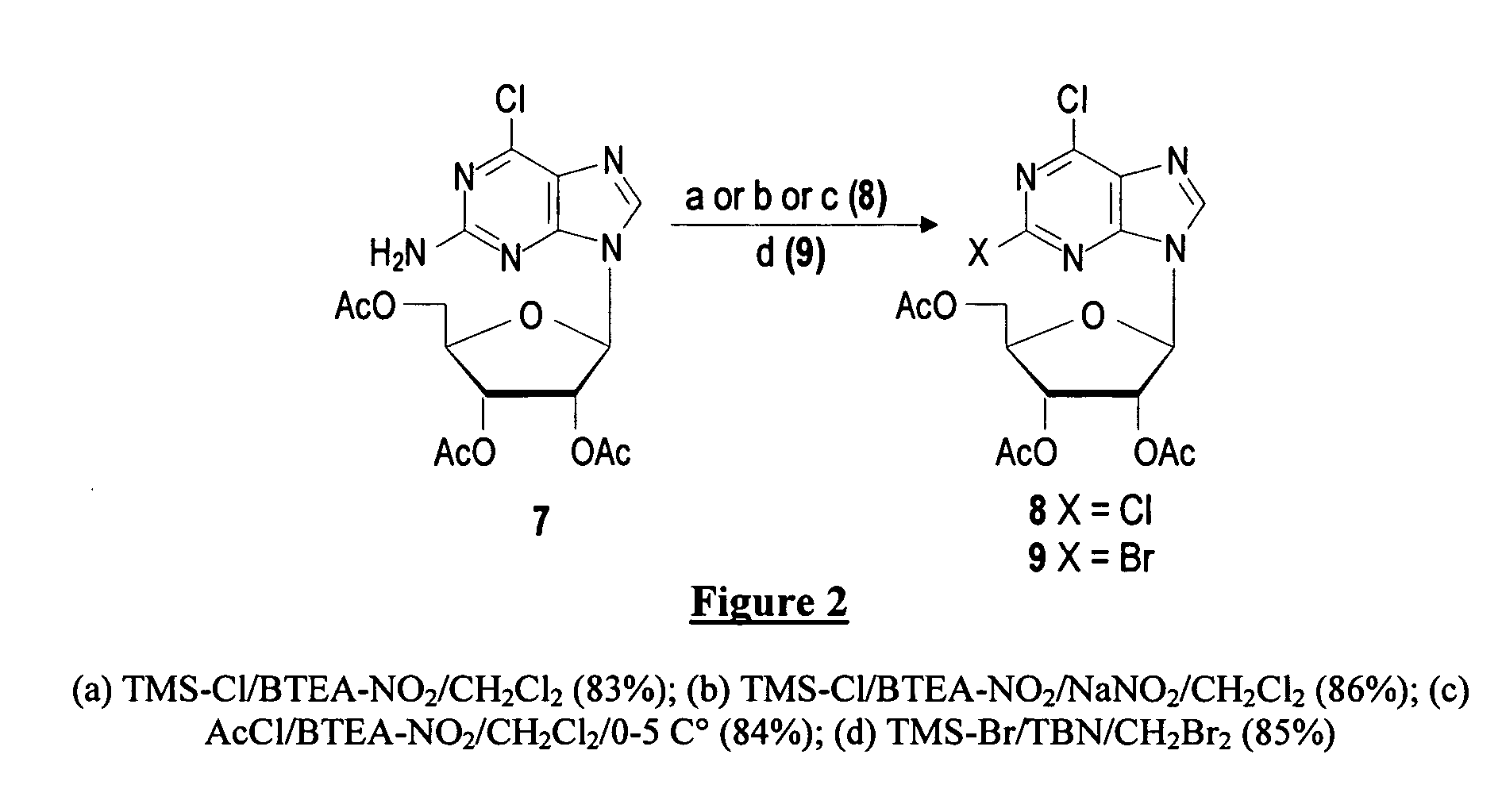
Method for synthesizing cladribine through nitration-chlorination method
InactiveCN105367616ASolve the problem of selectivityHigh yieldSugar derivativesSugar derivatives preparationTrifluoromethanesulfonic anhydrideNitration
The invention discloses a novel method for synthesizing cladribine through a nitration-chlorination method. According to the method, cheap 6-chloropurine and 1-chloro-2,3,5-tris-O-p-chlorobenzoyl-D-ribose are taken as raw materials, sodium hydride is taken as alkali, acetonitrile is taken as reaction solvent, and a beta isomer is obtained through a high-selectivity reaction; the obtained beta isomer does not need to remove protecting groups, the beta isomer reacts with trifluoromethanesulfonic anhydride after being separated, and nitryl is introduced at the second position; the nitryl is transformed into chlorine atoms in an ethanol solution of NH4Cl; two steps of reactions of protecting group removal and chlorine atom ammonolysis are completed in a methanol solution saturated by ammonia gas, and then the cladribine is obtained through four steps at the total yield of 78%. The method has the advantages that the raw materials are low in cost and easy to obtain, the technology is simple and convenient, the total yield is higher, the cost is low, amplification is easy, expensive reagents and poisonous and harmful heavy metal catalysts are prevented from being used, and when the reaction scale is expanded to the 100-g scale, the yield is not decreased obviously. A novel synthesizing path is supplied to synthesizing of the cladribine, and the potential application prospect is achieved.
Owner:XINXIANG UNIV
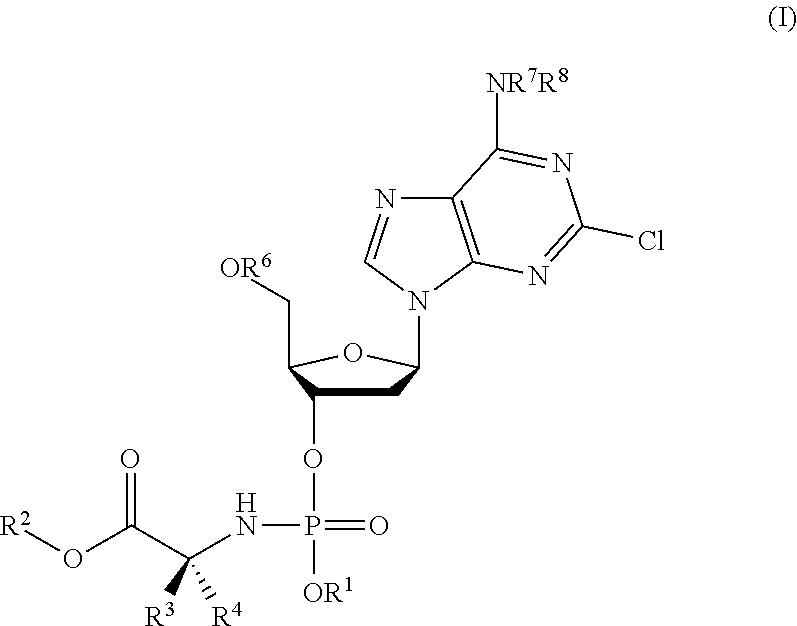
Phosphoramidate nucleoside derivatives as anticancer agents
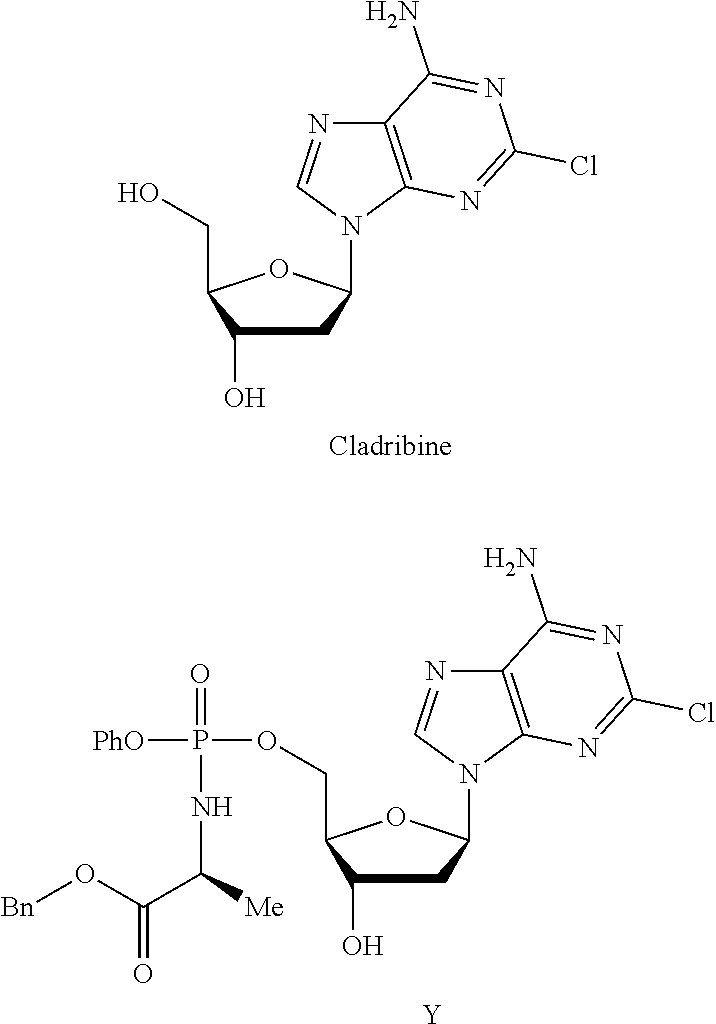
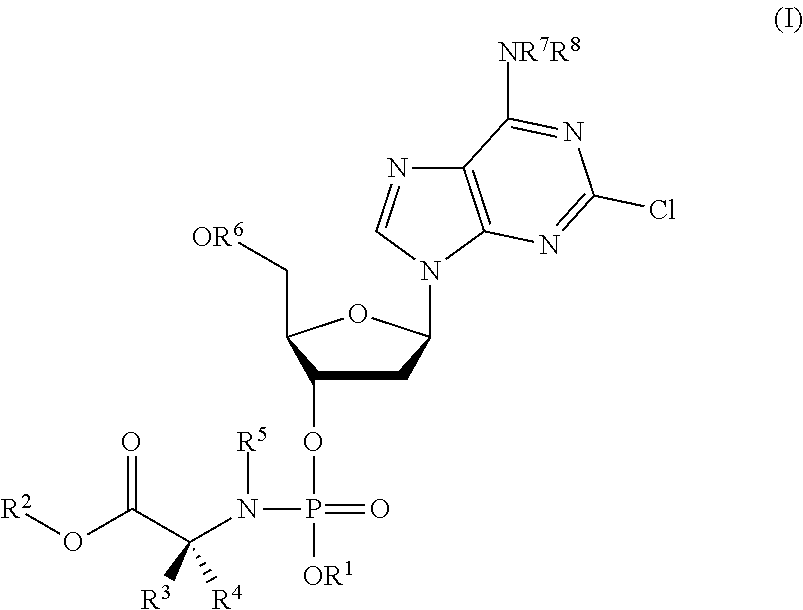
This invention relates to derivatives of cladribine of Formula (I). The compounds are phosphoramidate derivatives in which the phosphoramidate moiety is situated on the 3′-hydroxyl group of cladribine. The invention also relates to pharmaceutical formulations of the cladribine derivatives and their use in methods of treatment. The compounds are useful in the treatment of cancer.
Owner:NUCANA PLC
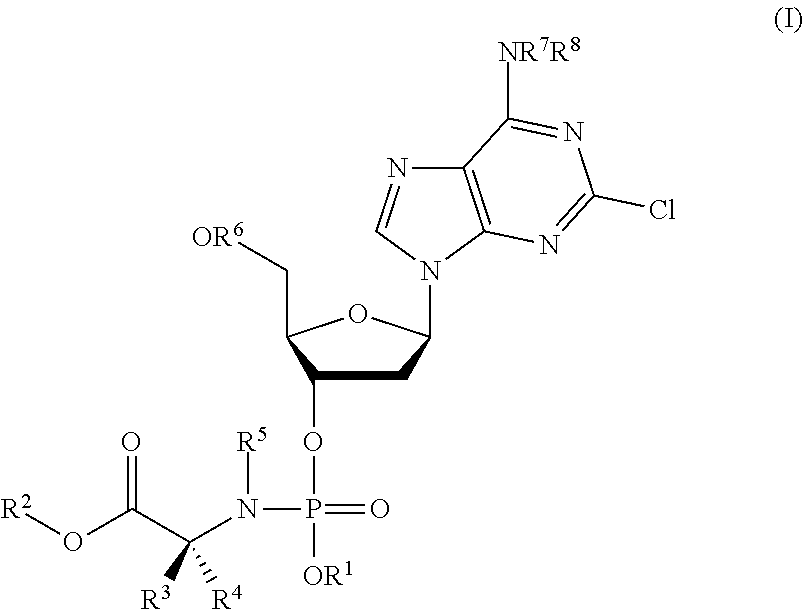
Phosphoramidate nucleoside derivatives as anticancer agents
ActiveUS20190375779A1Induces cancer cell deathInduce deathSugar derivativesAntineoplastic agentsPharmaceutical formulationPhosphoramidate
One aspect of the invention relates to derivatives of cladribine of Formula (I). The compounds are phosphoramidate derivatives in which the phosphoramidate moiety is situated on the 3′-hydroxyl group of cladribine. The invention also relates to pharmaceutical formulations of the cladribine derivatives, and their use in methods of treatment. The compounds are useful in the treatment of cancer.
Owner:NUCANA PLC
Cladribine regimen for treating progressive forms of Multiple Sclerosis
ActiveUS20190167707A1Increase doseEfficacy to patientOrganic active ingredientsNervous disorderProgressive multiple sclerosisRegimen
Specific oral dosings, specific oral dosage forms, and / or specific oral dose regimens including Cladribine can be effective for the treatment of progressive forms of Multiple Sclerosis, especially Primary Progressive Multiple Sclerosis and / or Secondary Progressive Multiple Sclerosis. Methods of treatment can be based on specific oral dosings, specific oral dosage forms, and / or specific oral dose regimens including Cladribine.
Owner:MERCK PATENT GMBH
Synthesis of nucleosides
ActiveUS9109000B2Inhibition formationEasy to removeTin organic compoundsSugar derivativesMizoribinePurine
A process for the preparation of nucleosides, derivatives and analogues thereof by coupling reaction of a protected suitable nitrogeneous purine or pyrimidine base, a derivative or analogue thereof and a protected suitable sugar in the presence of SnCl4 comprising the removal of SnCl4 by adding DMSO directly into the reaction mixture is described. Preferably said process is used for the preparation of antiviral and antitumor agents having a nucleoside or nucleoside-like structure, still more preferably for the preparation of azacytidine, decitabine, chlorfarabine, cladribine, mizoribine. A residual tin content lower than 300 ppm is obtained with said process.
Owner:FARMABIOS
Slow-released injection containing nolatrexed dihydrochloride and synergist thereof
The invention relates to a slow-release injection containing nolatrexed and a synergistic agent of the nolatrexed, which consists of a slow-release microballoon sphere and a solution medium, wherein, the slow-release microballoon sphere comprises active anti-cancer ingredients and slow-release accessories; and the solution medium is a special solution medium containing a suspending agent. The active anti-cancer ingredients are antimetabolites, such as pemetrexed, rumitrexed, raltitrexed, nolatrexed, carmofur, dexrazoxane, tegafur, zalcitabine, emtricitabine, ibatabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and the like, and synergistic agents of the antimetabolites selected from topoisomerase inhibitors and / or tetrazine compounds; the slow-release accessories are selected from one of polifeprosan, di-fatty acid and decanedioic acid copolymer, polylactic acid copolymer and EVAC or the combination thereof; the viscosity of the suspending agent is 100cp to 3000cp (at the temperature of 20 DEG C to 30 DEG C) and the suspending agent is selected from carboxymethylcellulose sodium and the like. The slow-release microballoon sphere can also be prepared into a slow-release implant used for being injected or put in tumors or the surrounding of the tumors so as to enhance the effects of radiotherapy and chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Anti-cancer drugs slow release agent comprising anticancer antibiotics and synergist thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow releaseauxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
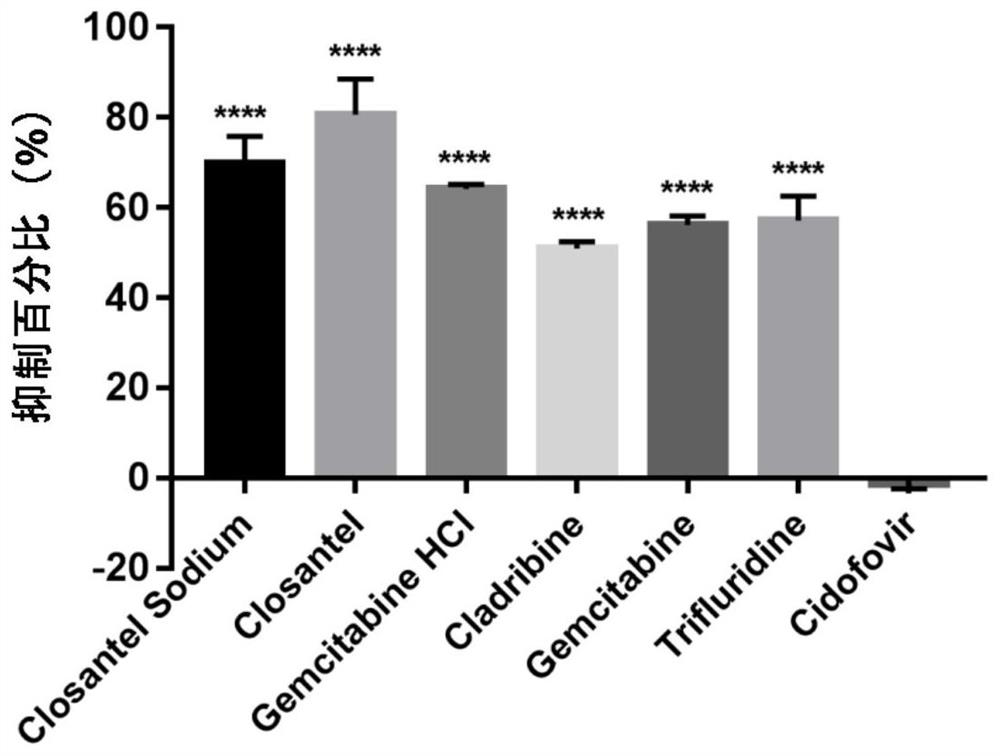
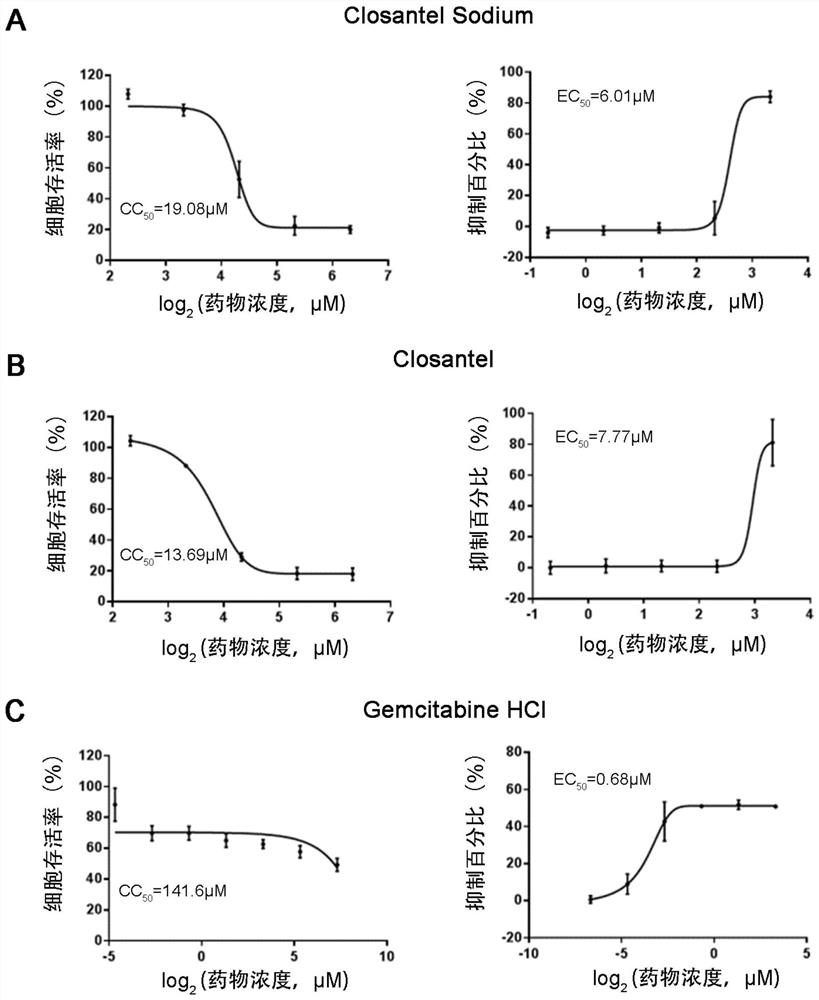
Application of 6 Small Molecule Drugs in Inhibiting Canine Parvovirus
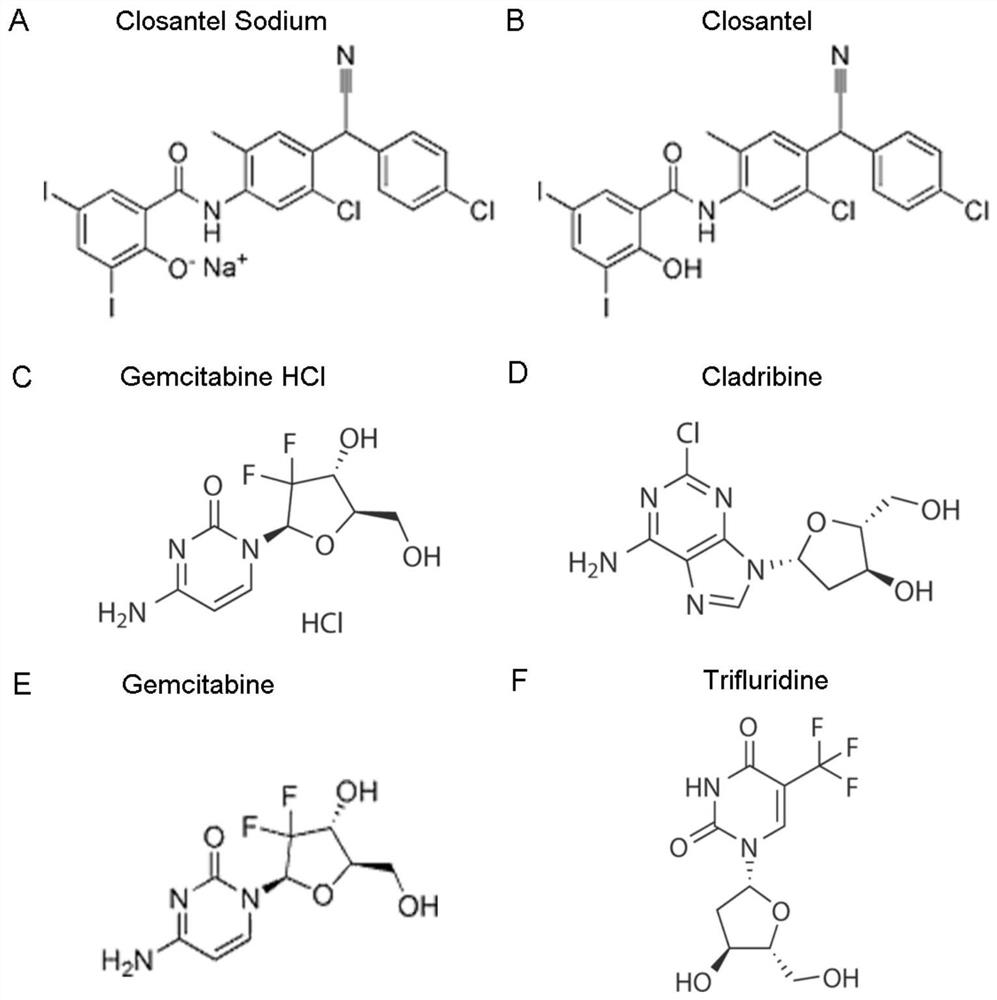
ActiveCN111840308BAntiviralsNitrile/isonitrile active ingredientsCLOSANTEL SODIUMPharmaceutical drug
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Cladribine regimen for treating multiple sclerosis
InactiveCN101090726AWeakening of pathological processesReduced pathological processOrganic active ingredientsNervous disorderRegimenPharmaceutical formulation
The present invention is related to the use of Cladribine for the preparation of a pharmaceutical formulation for the treatment of multiple sclerosis, especially relapsing-remitting multiple sclerosis or early secondary progressive multiple sclerosis, wherein the preparation is to be the orally administered and wherein re-treatments are possible.
Owner:MERCK SERONO SA
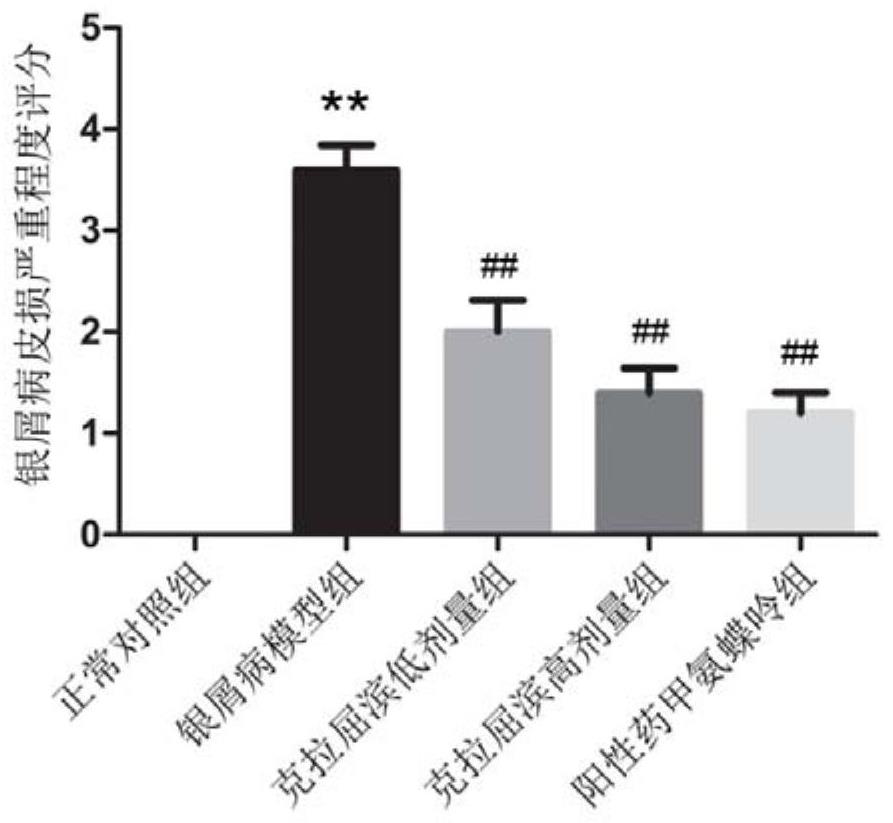
Application of cladabine in preparation of medicine for preventing or treating psoriasis
PendingCN114288314AEffective treatmentOrganic active ingredientsDermatological disorderPharmaceutical drugTraditional medicine
The invention relates to an application of cladribine in preparation of a medicine for preventing or treating psoriasis. The medicine can effectively treat psoriasis, and by using the medicine, the content of Th1 and / or Th17 cells in the body of a subject can be reduced, and the content of Th2 and / or Treg cells in the body of the subject can be increased.
Owner:BEIJING TSINGHUA CHANGGUNG HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com