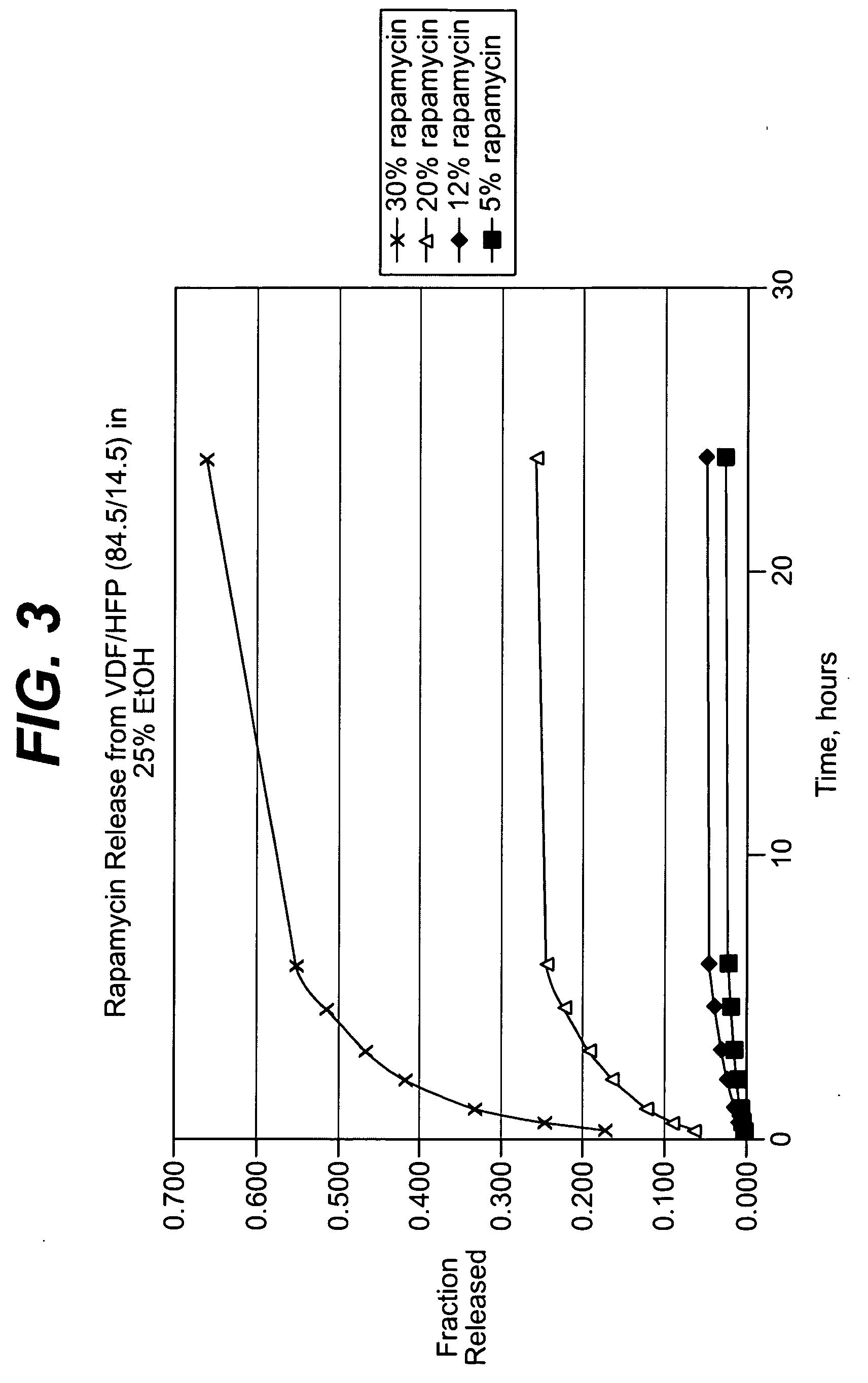
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149 results about "Elution rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
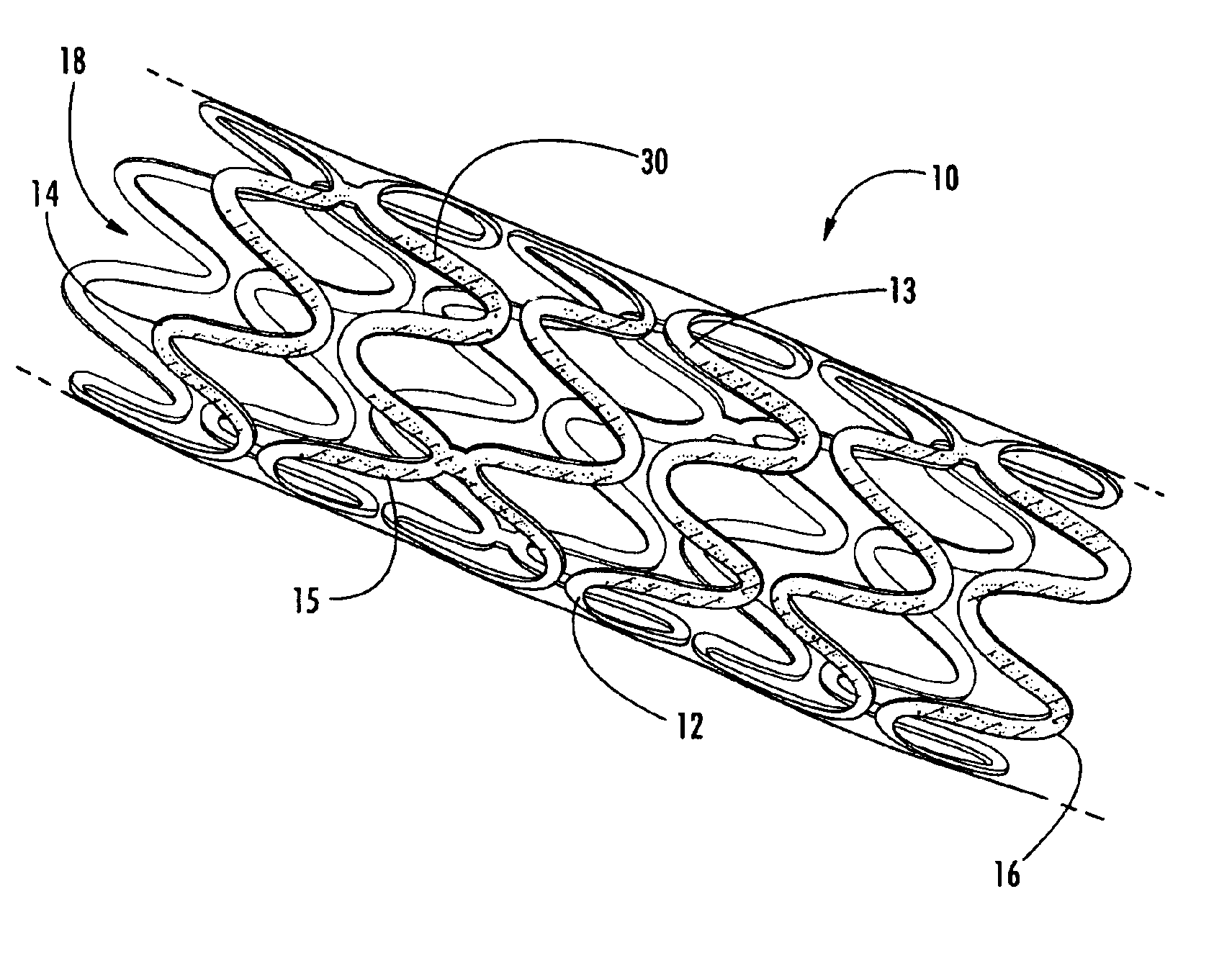
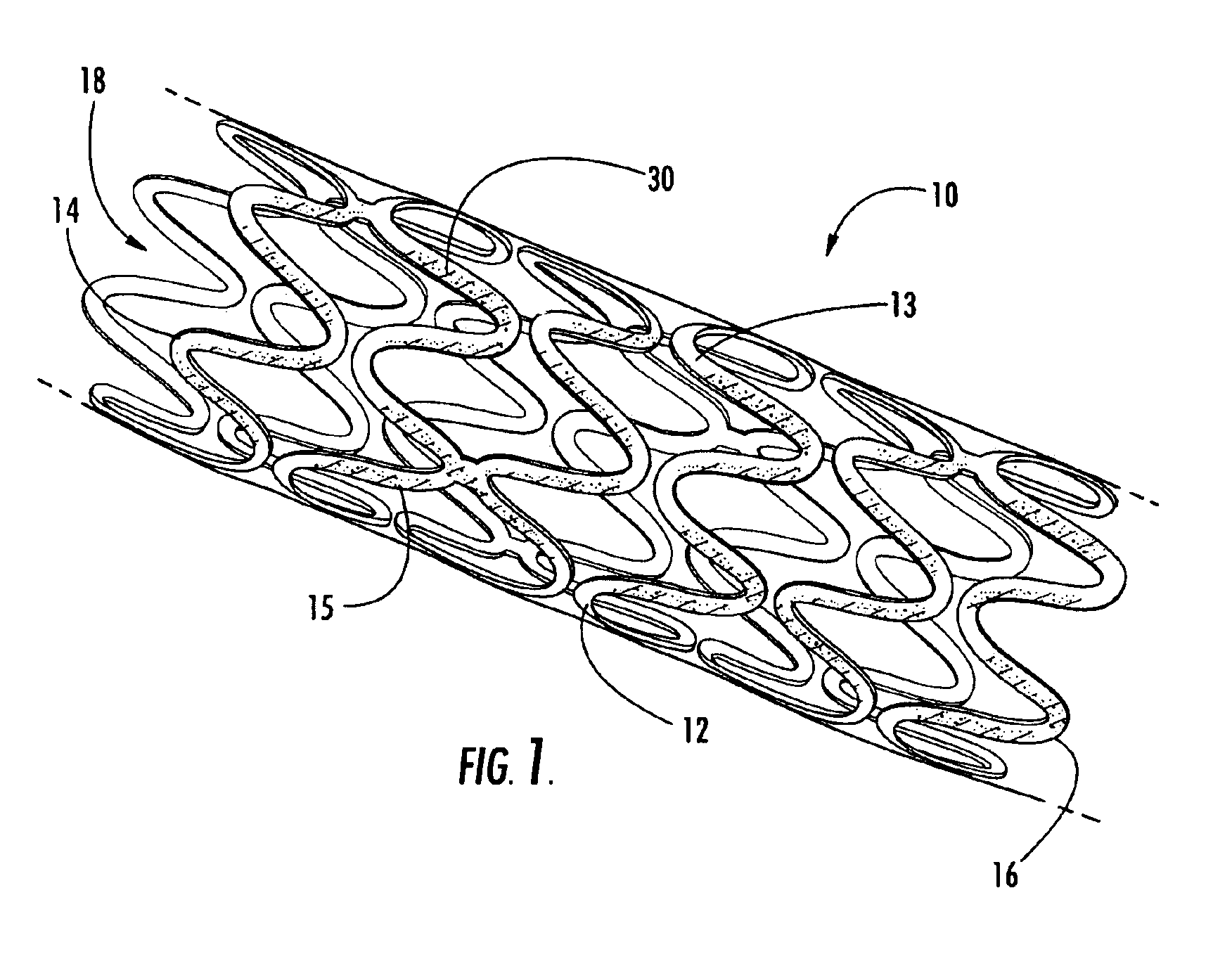
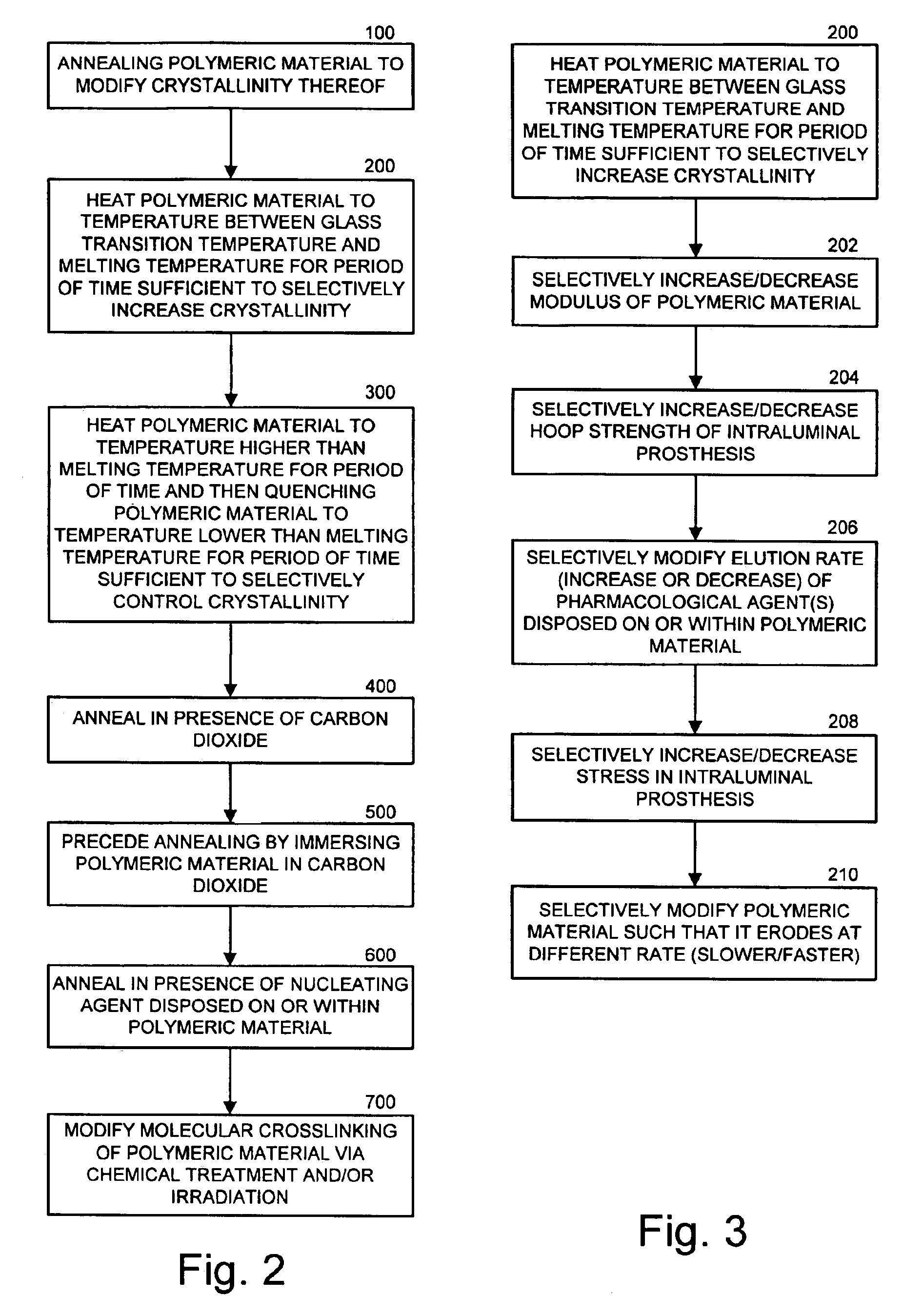
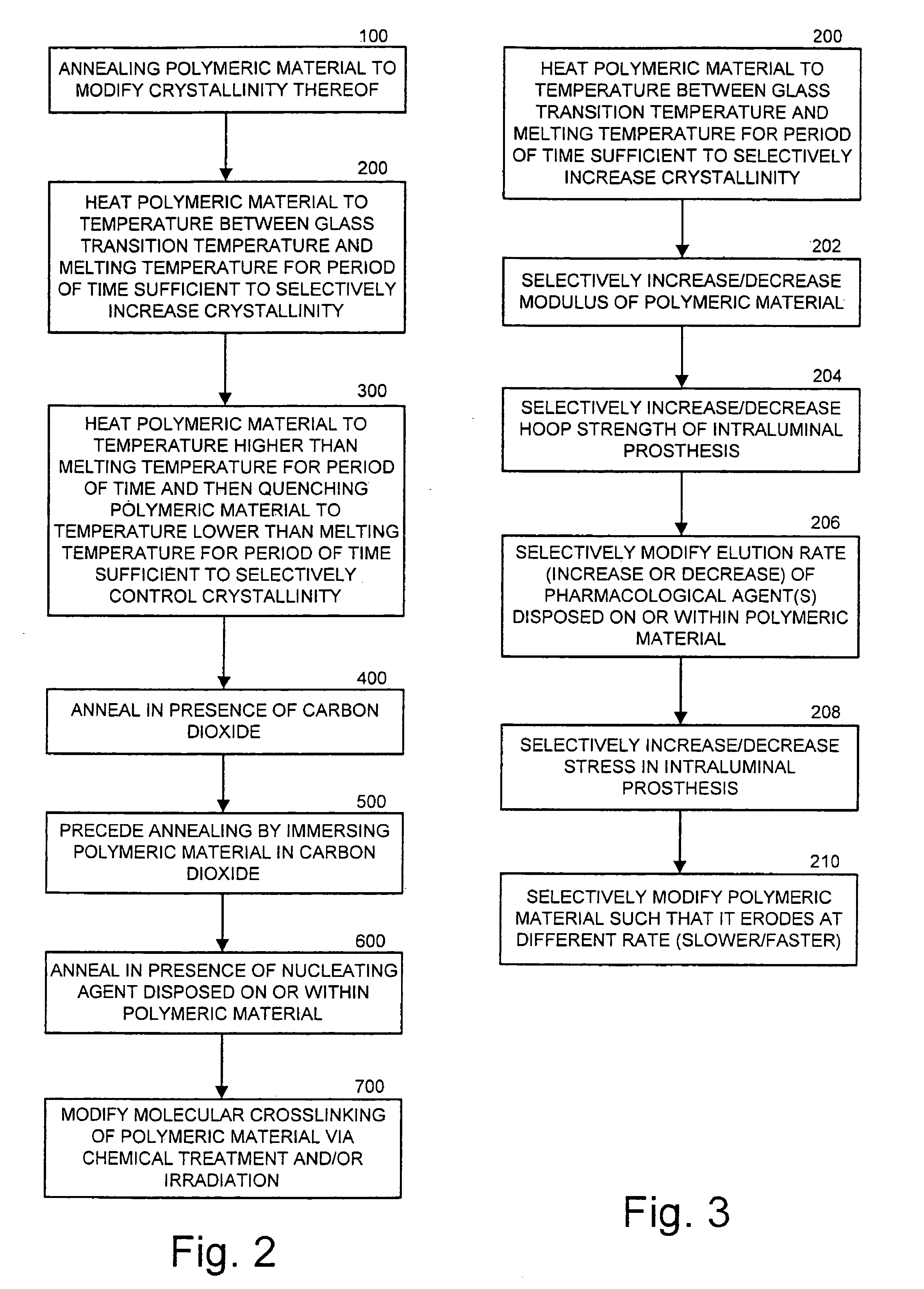
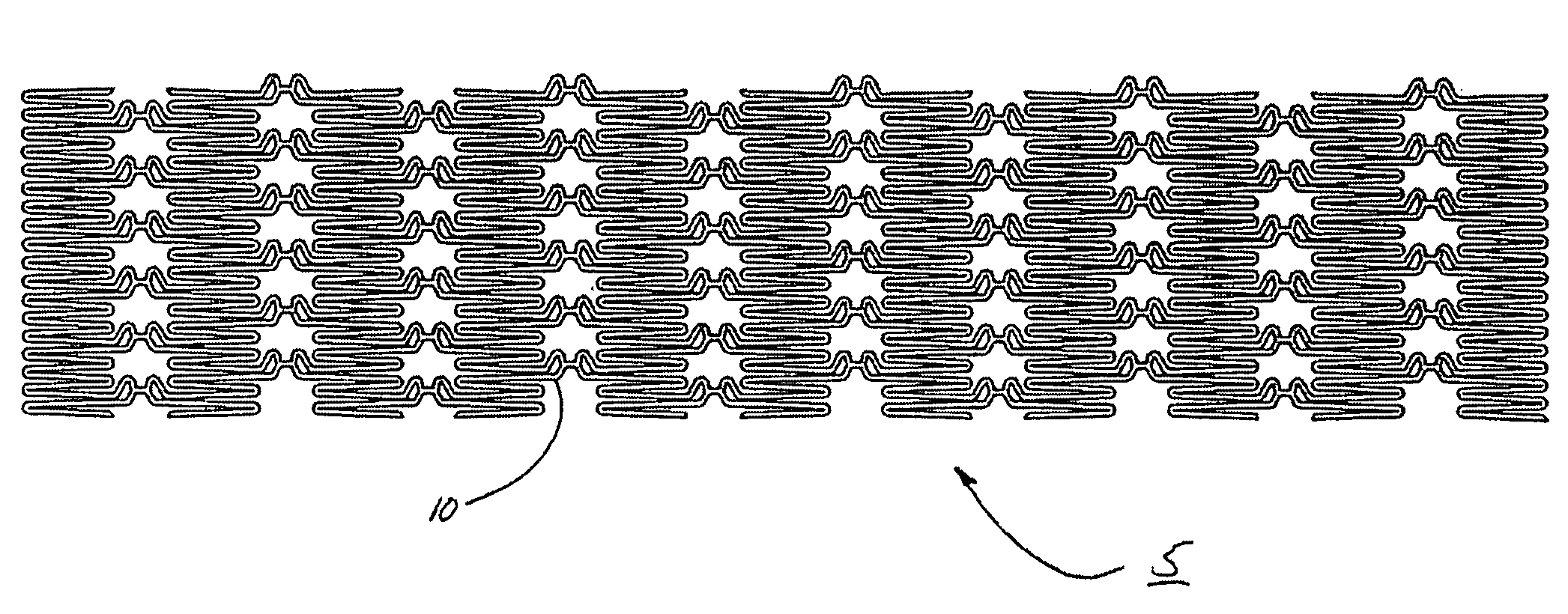
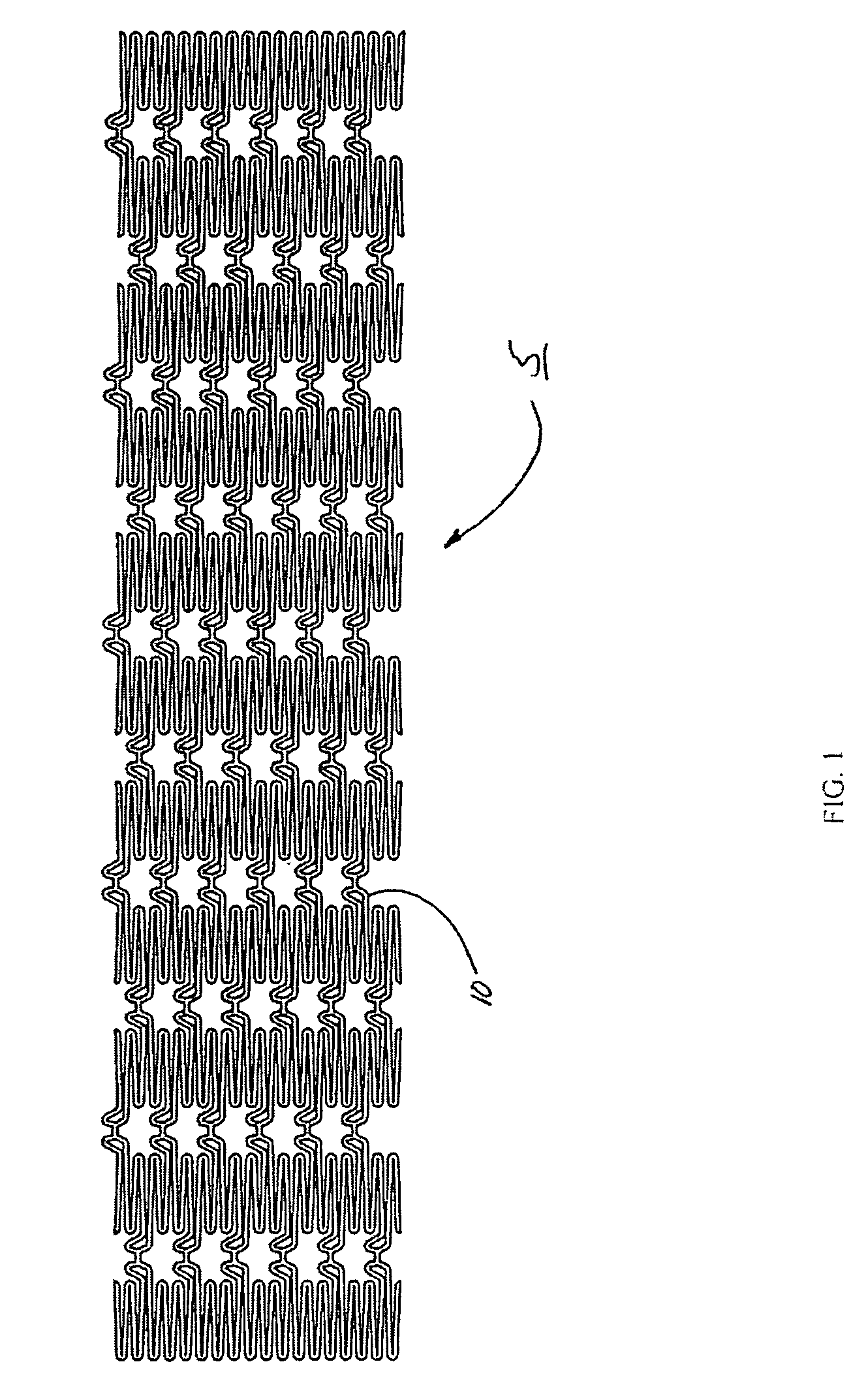
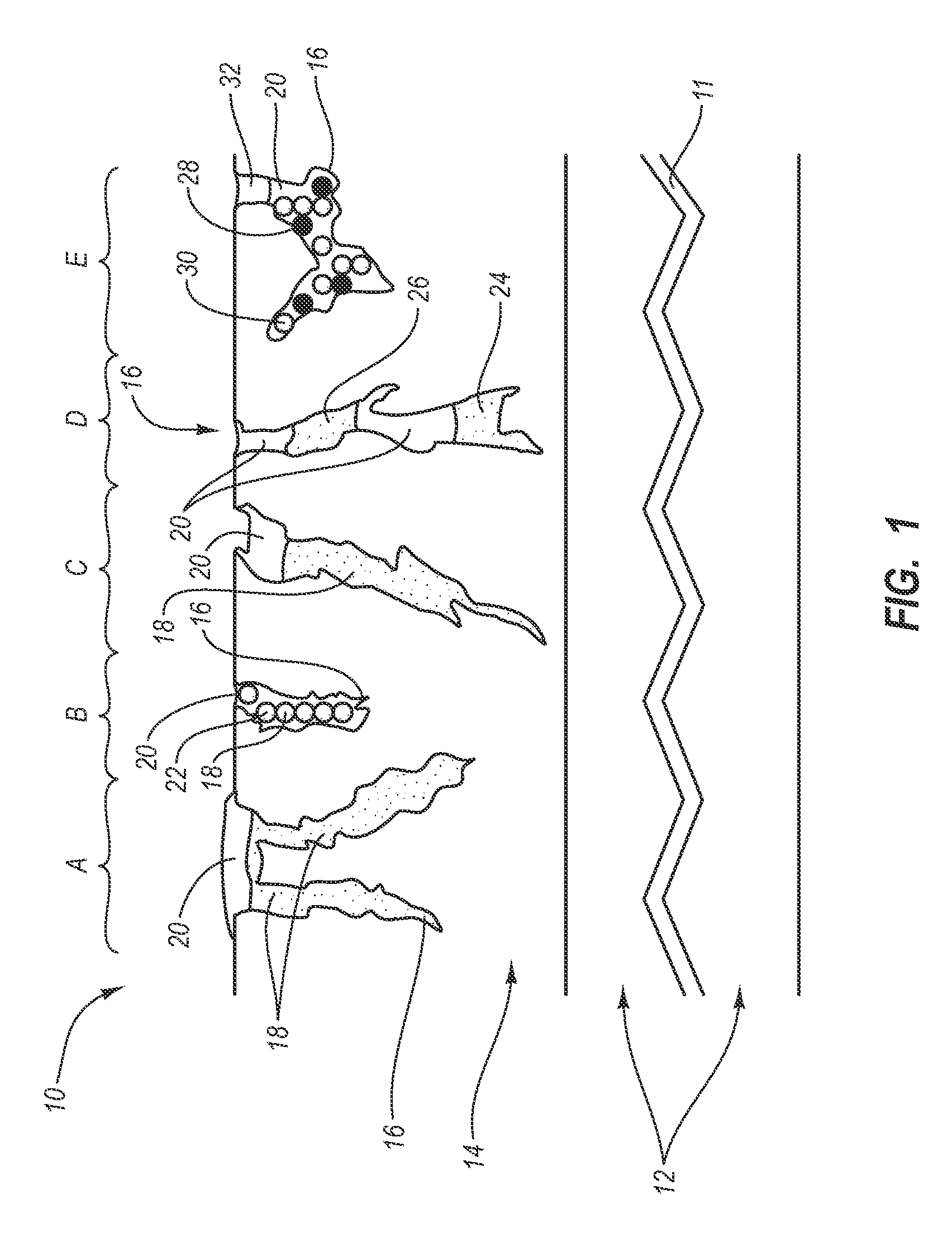
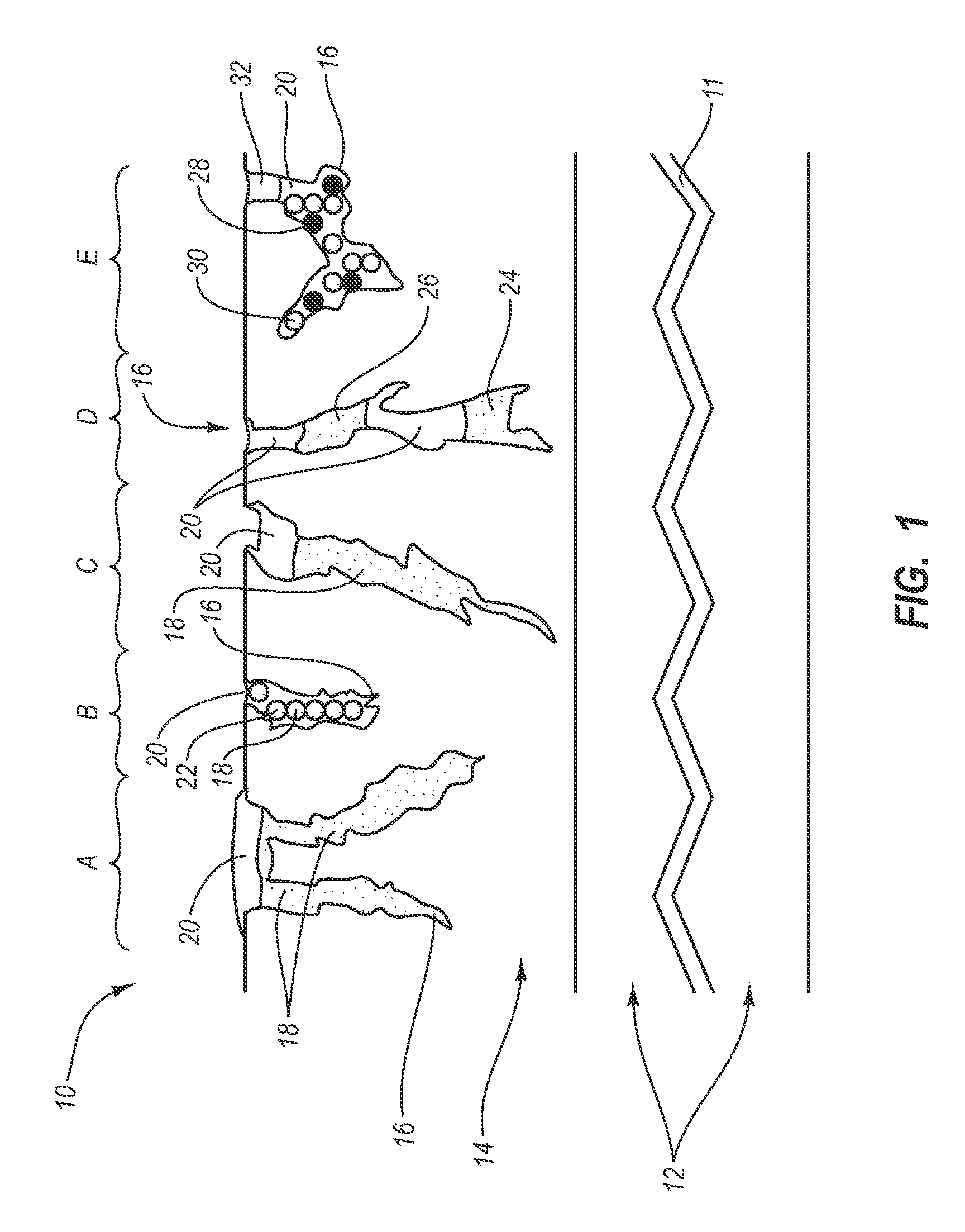
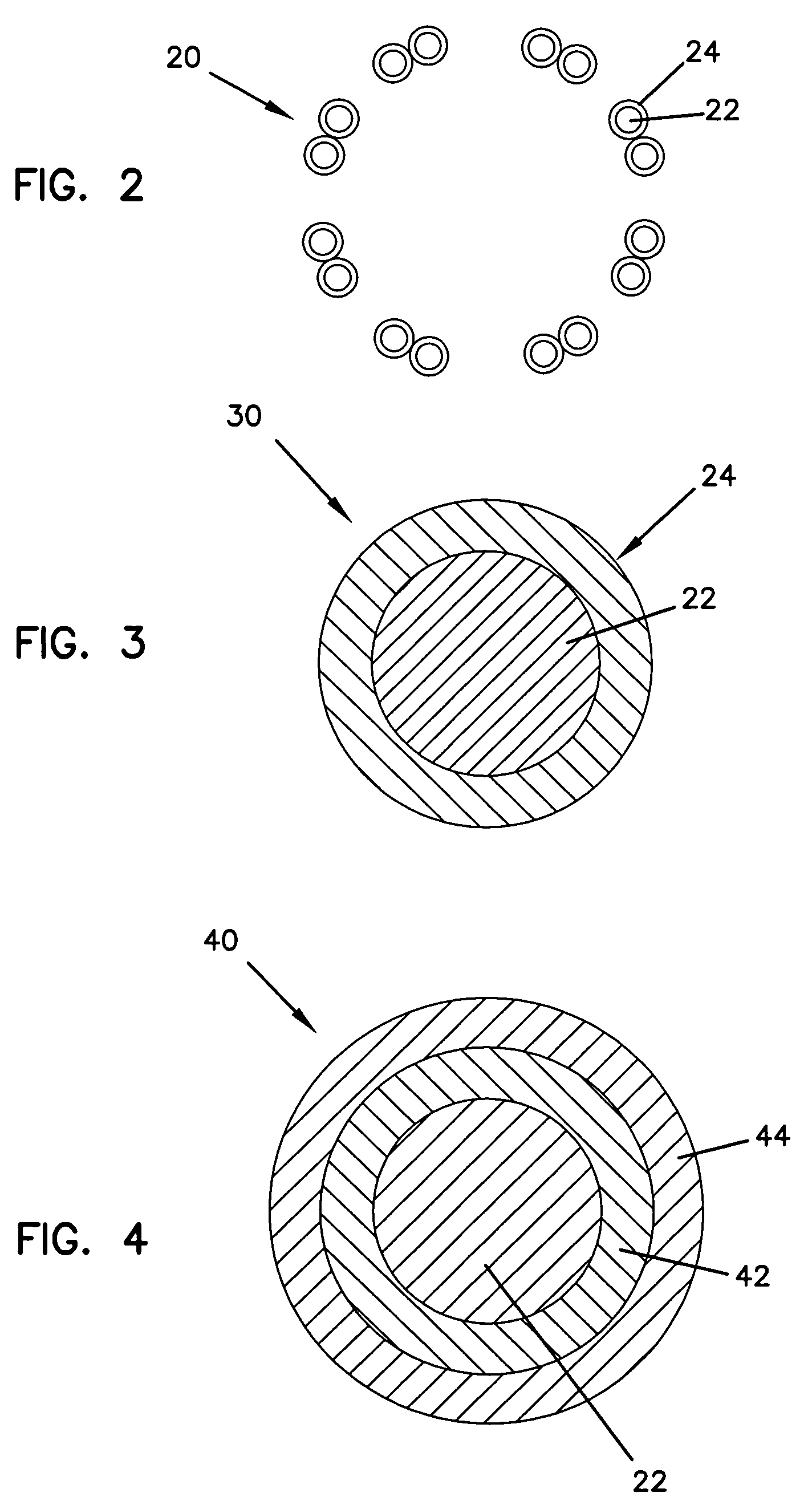
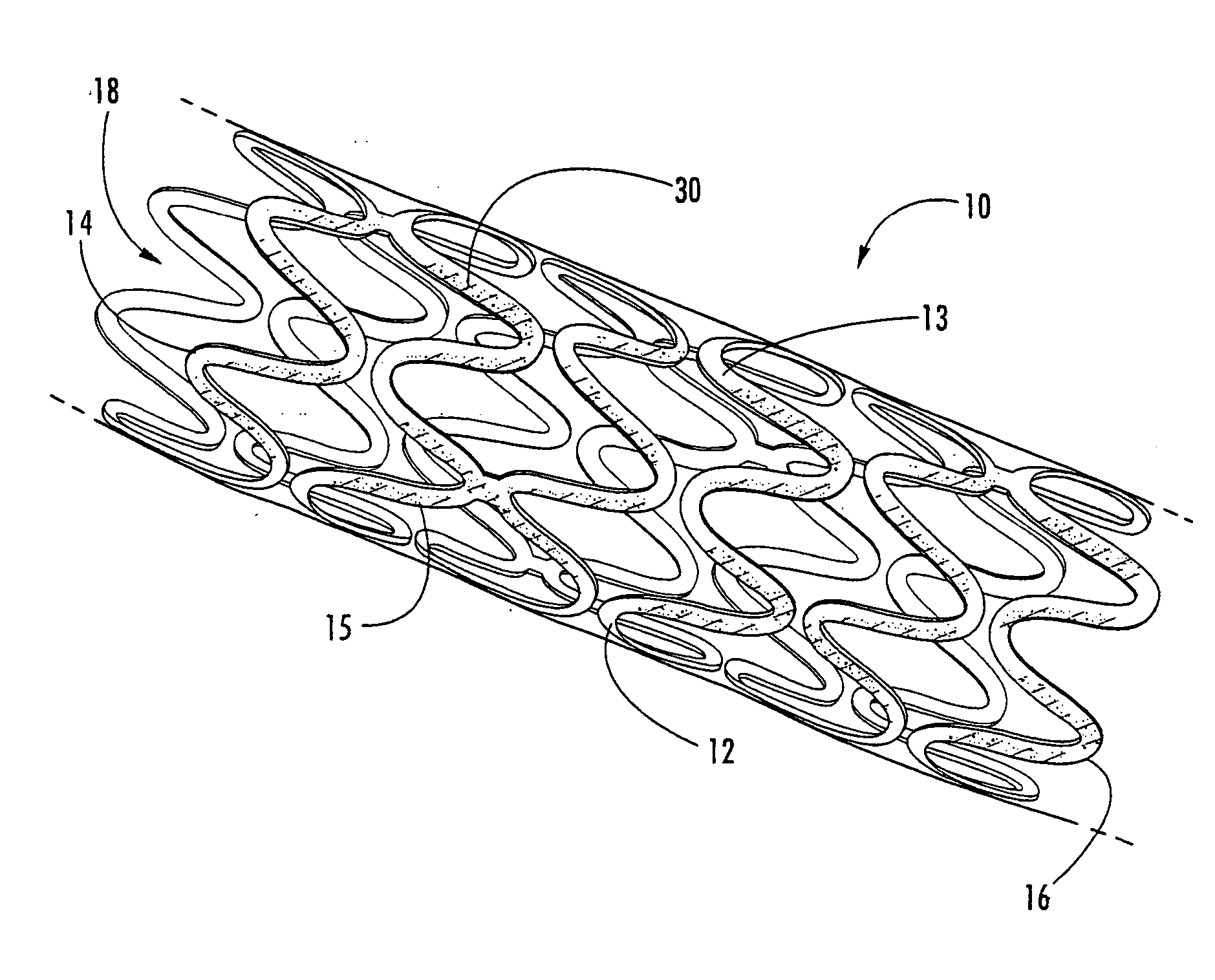
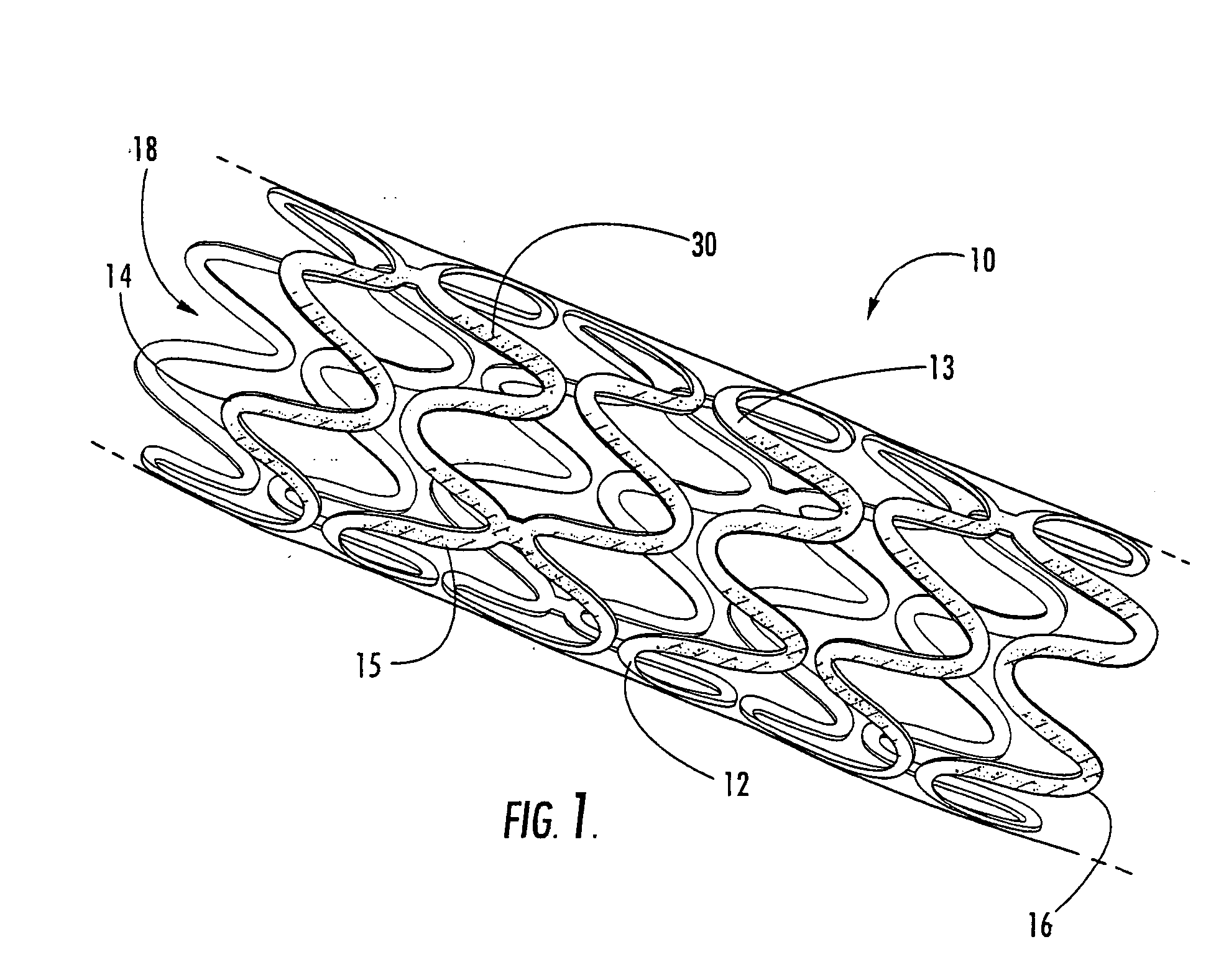
Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same
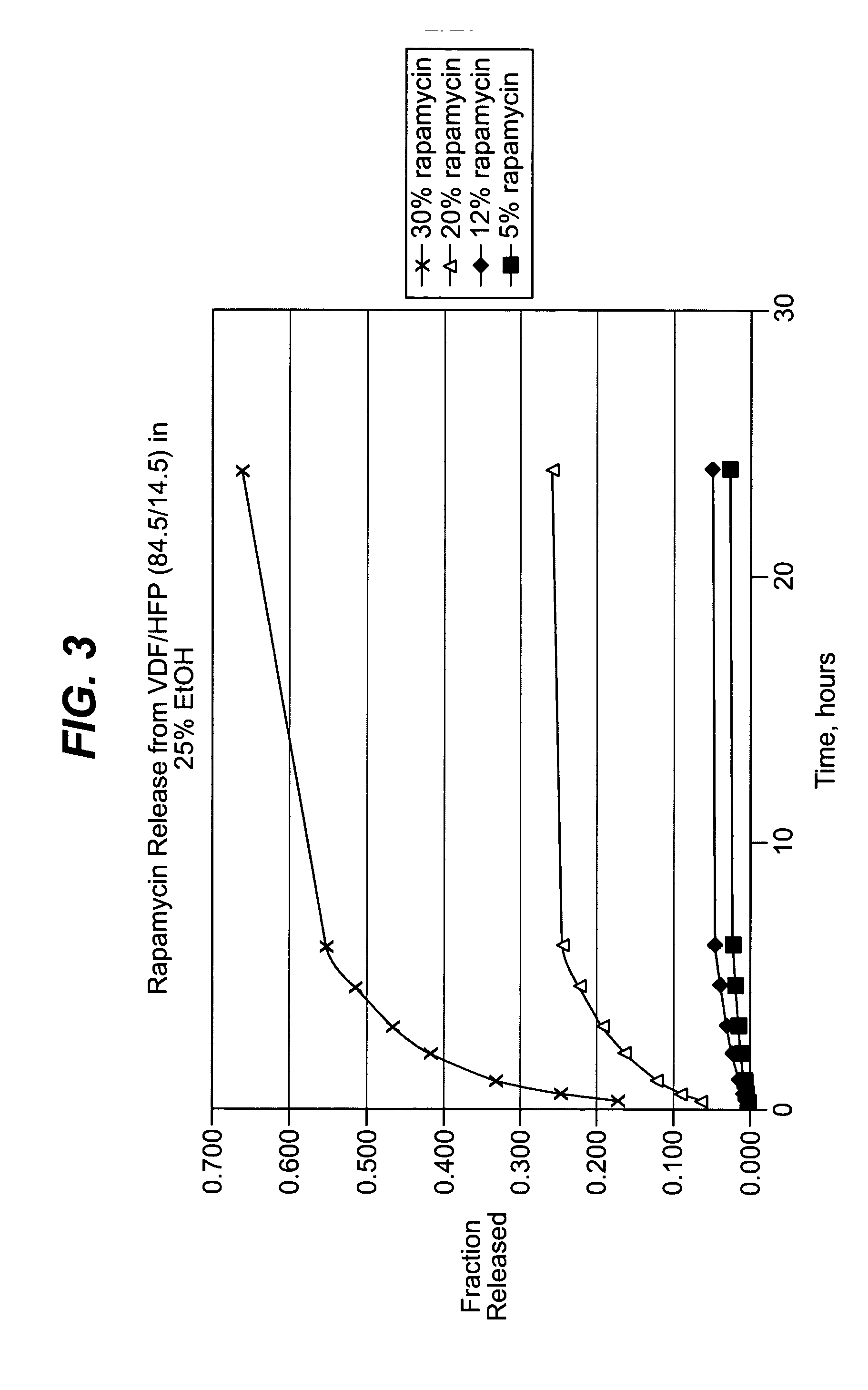
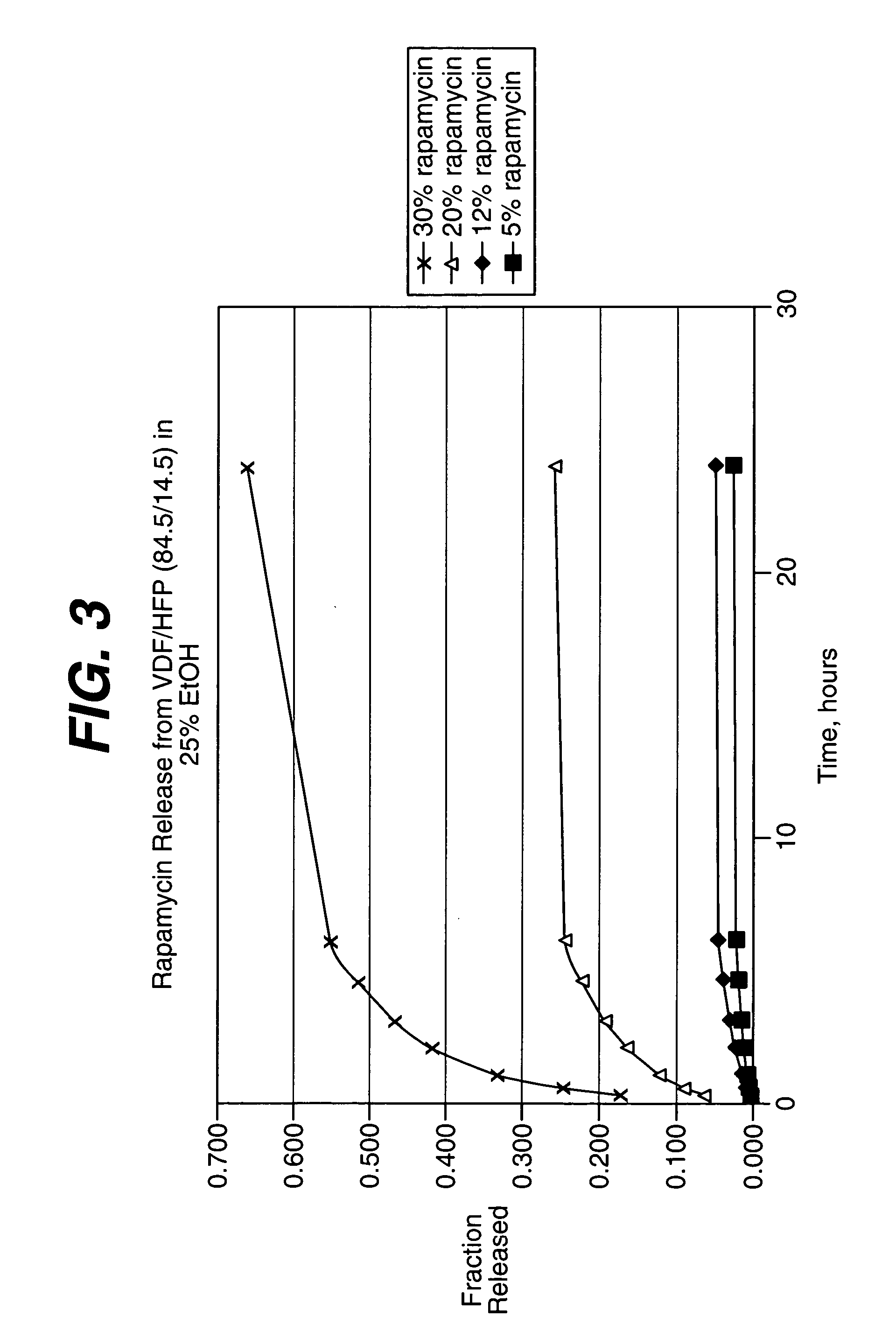
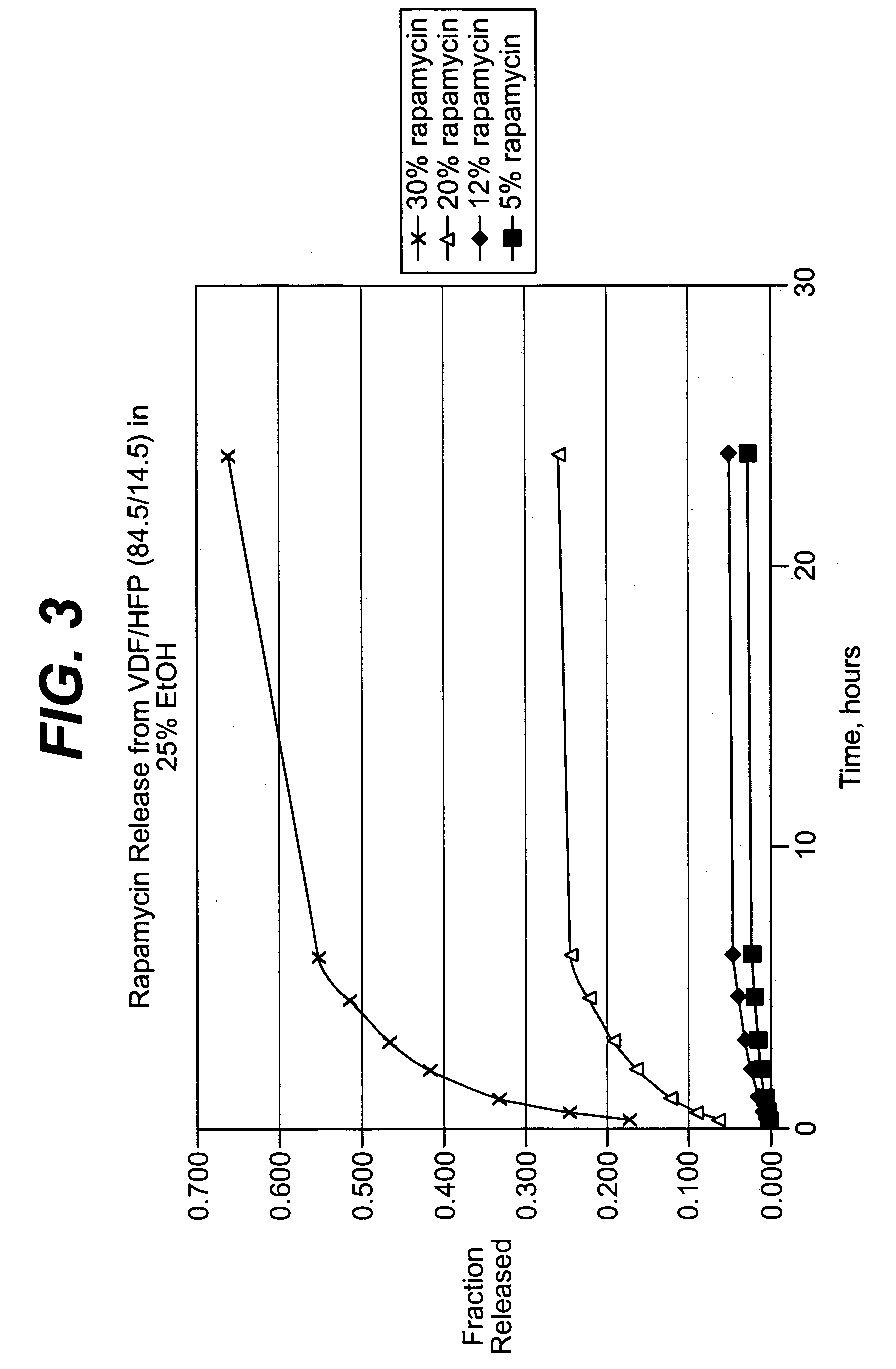
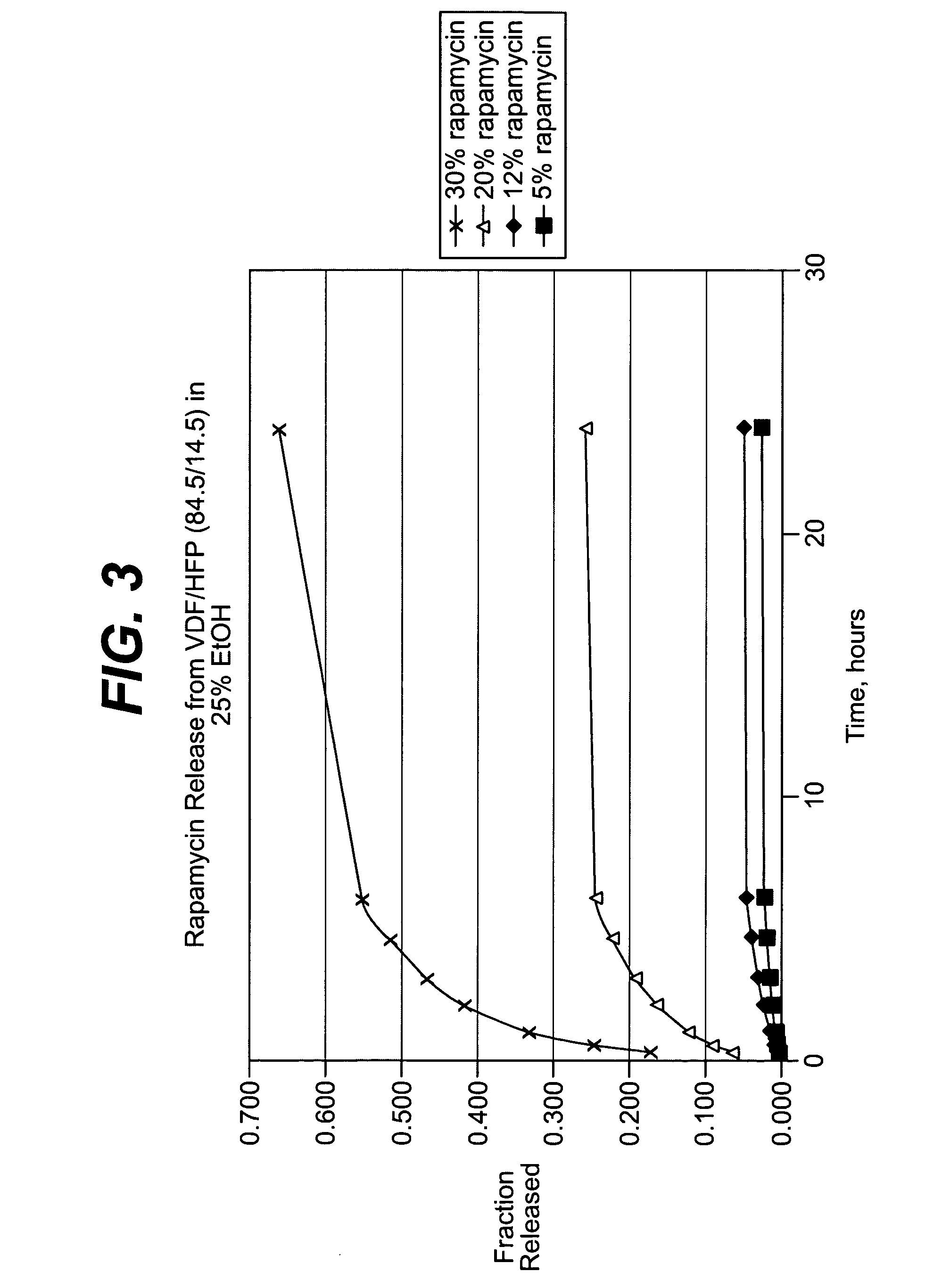
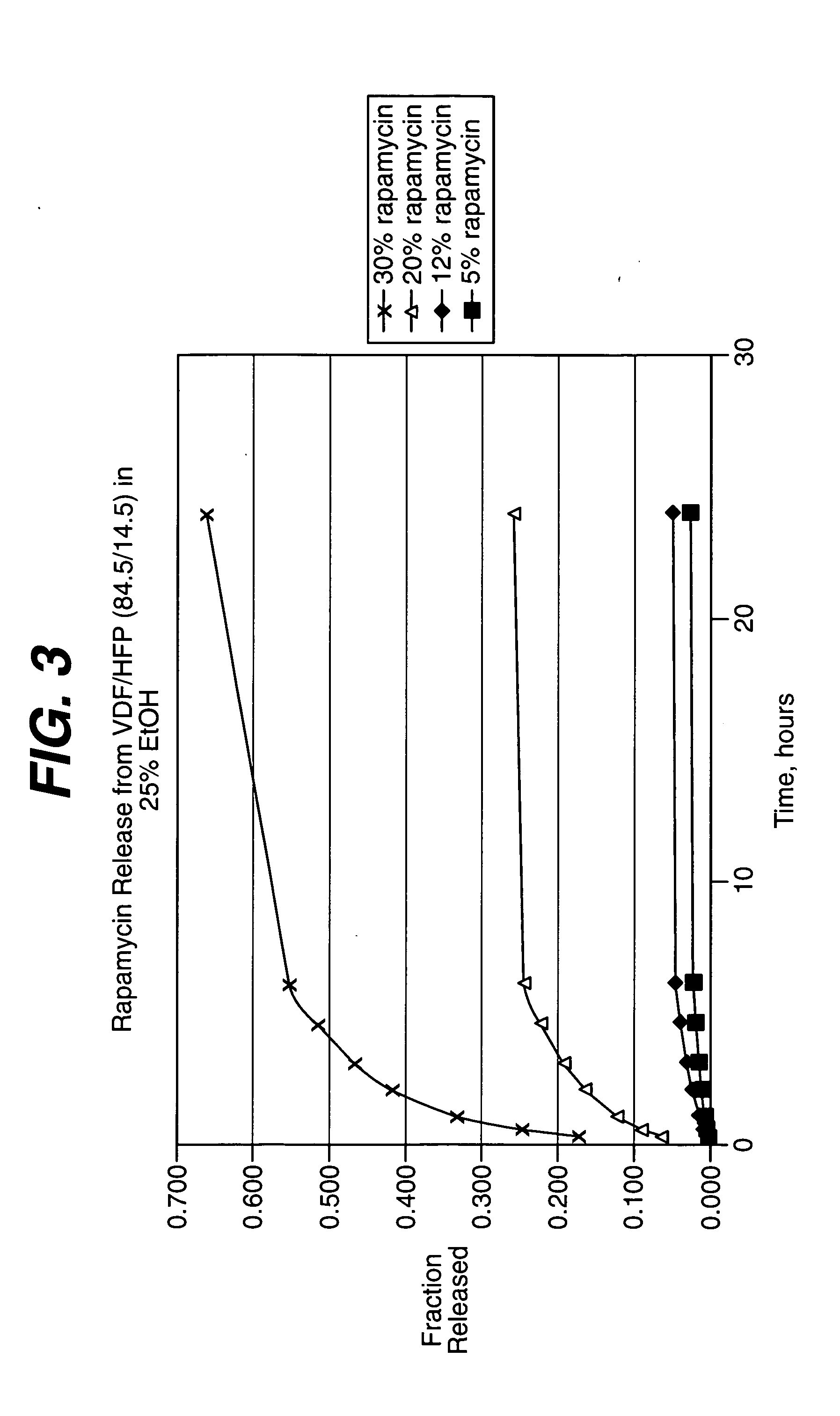
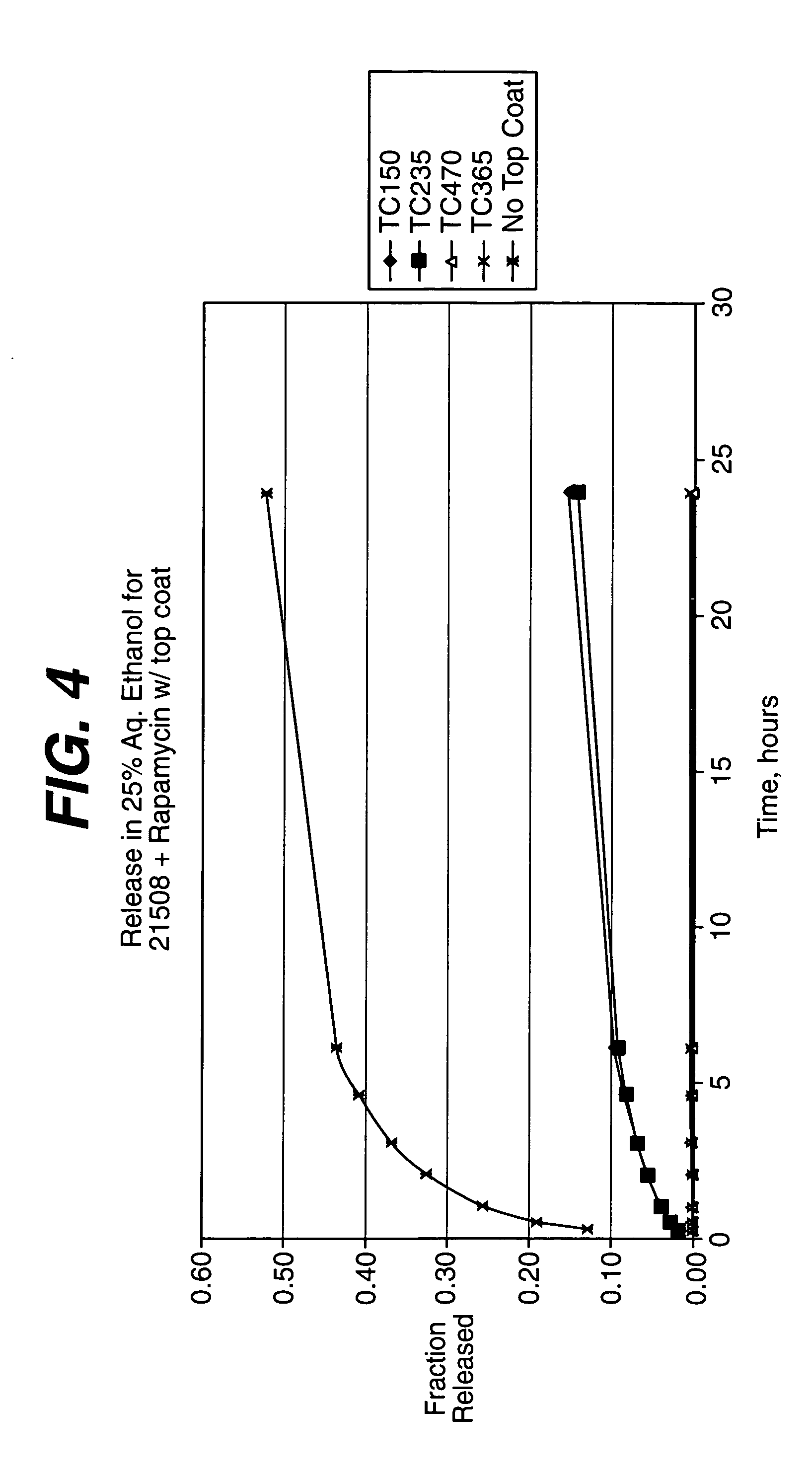
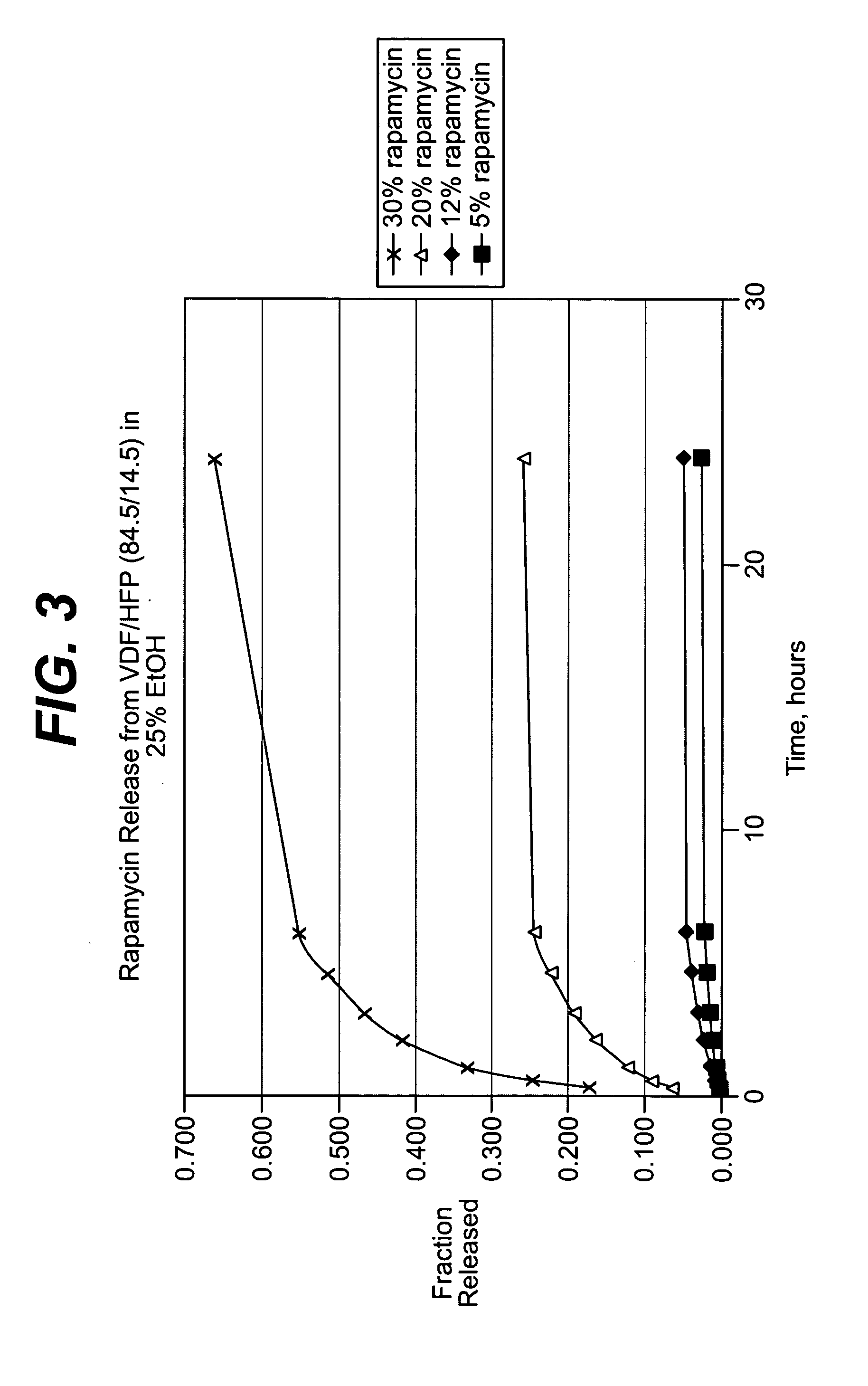
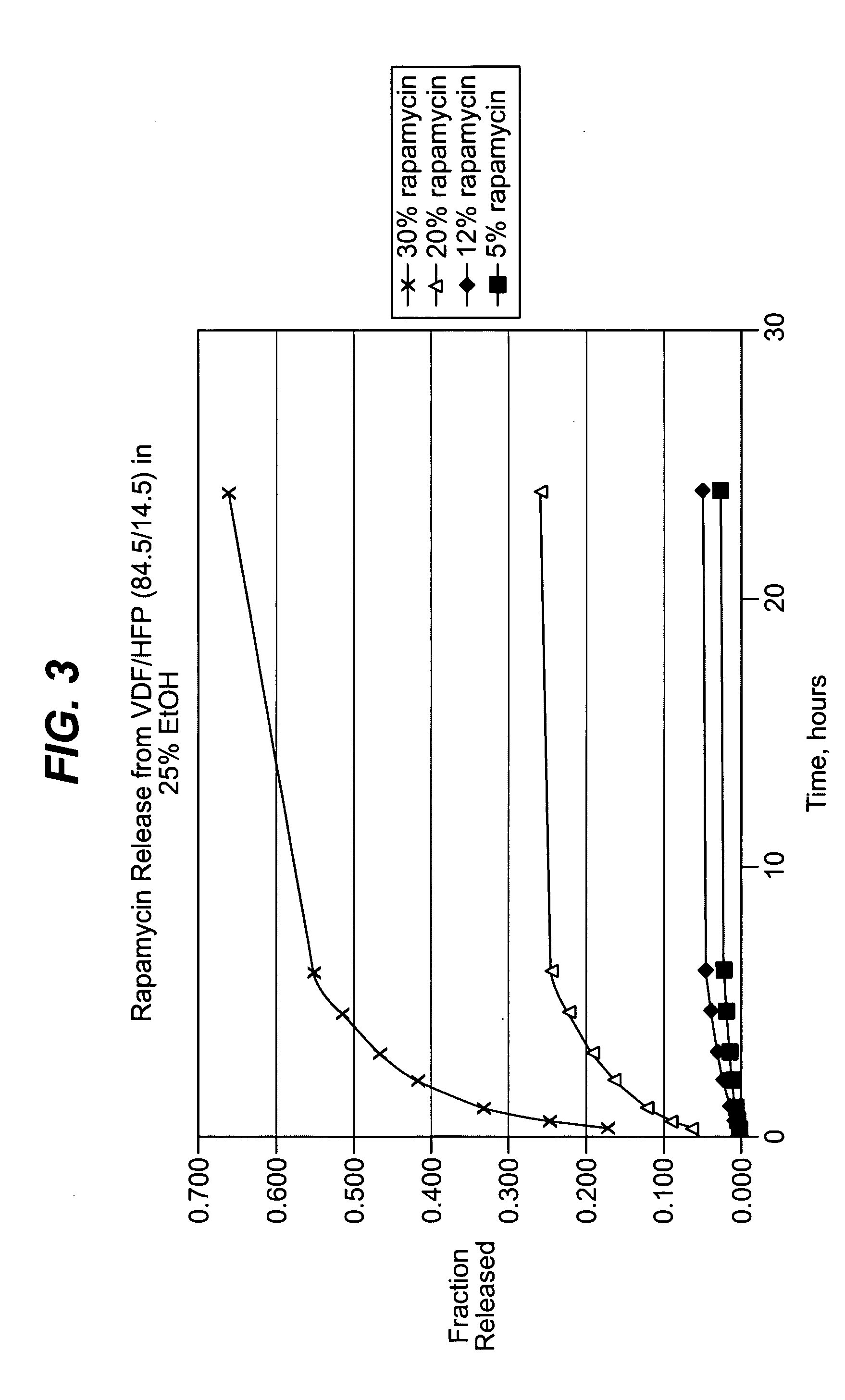
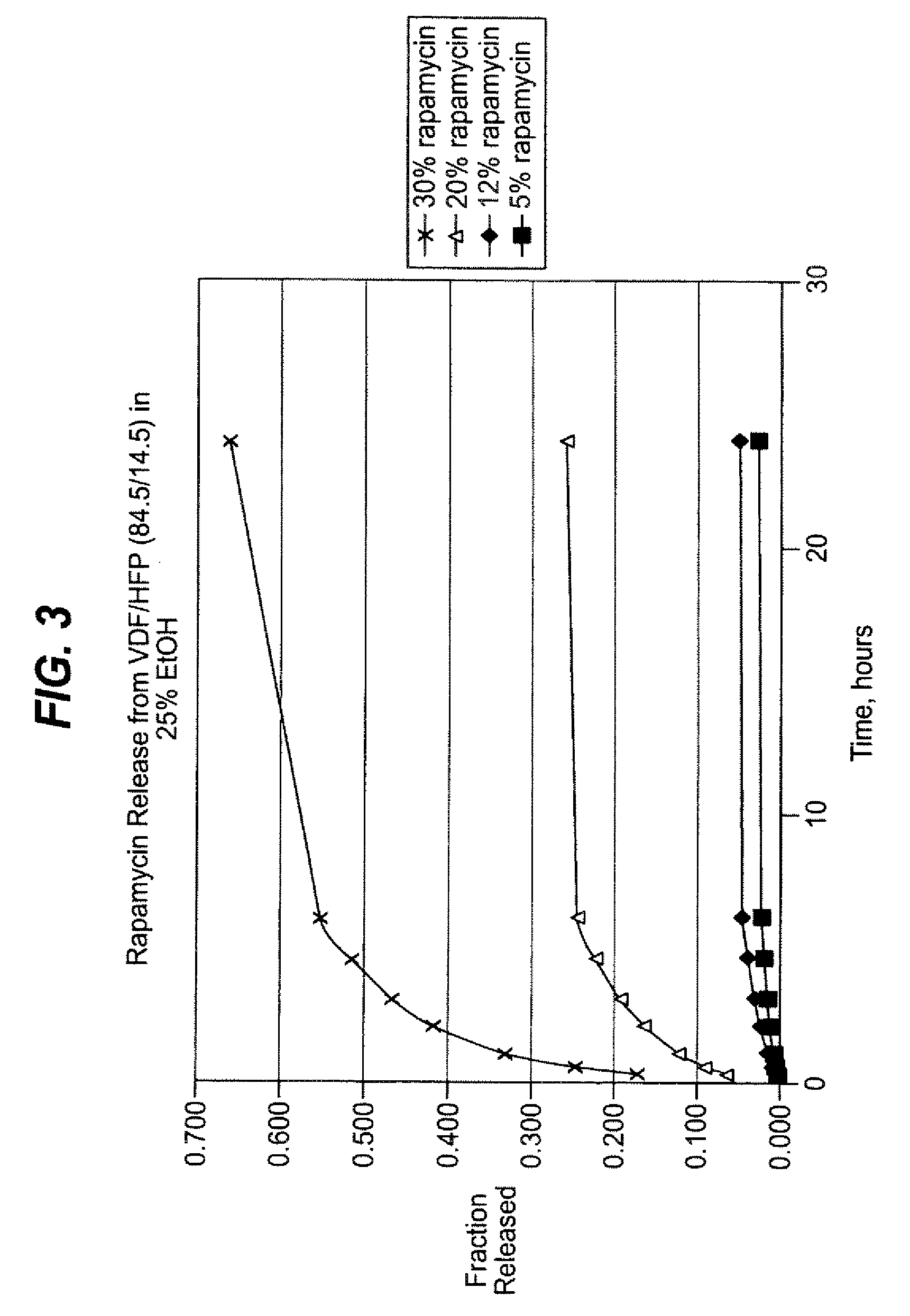
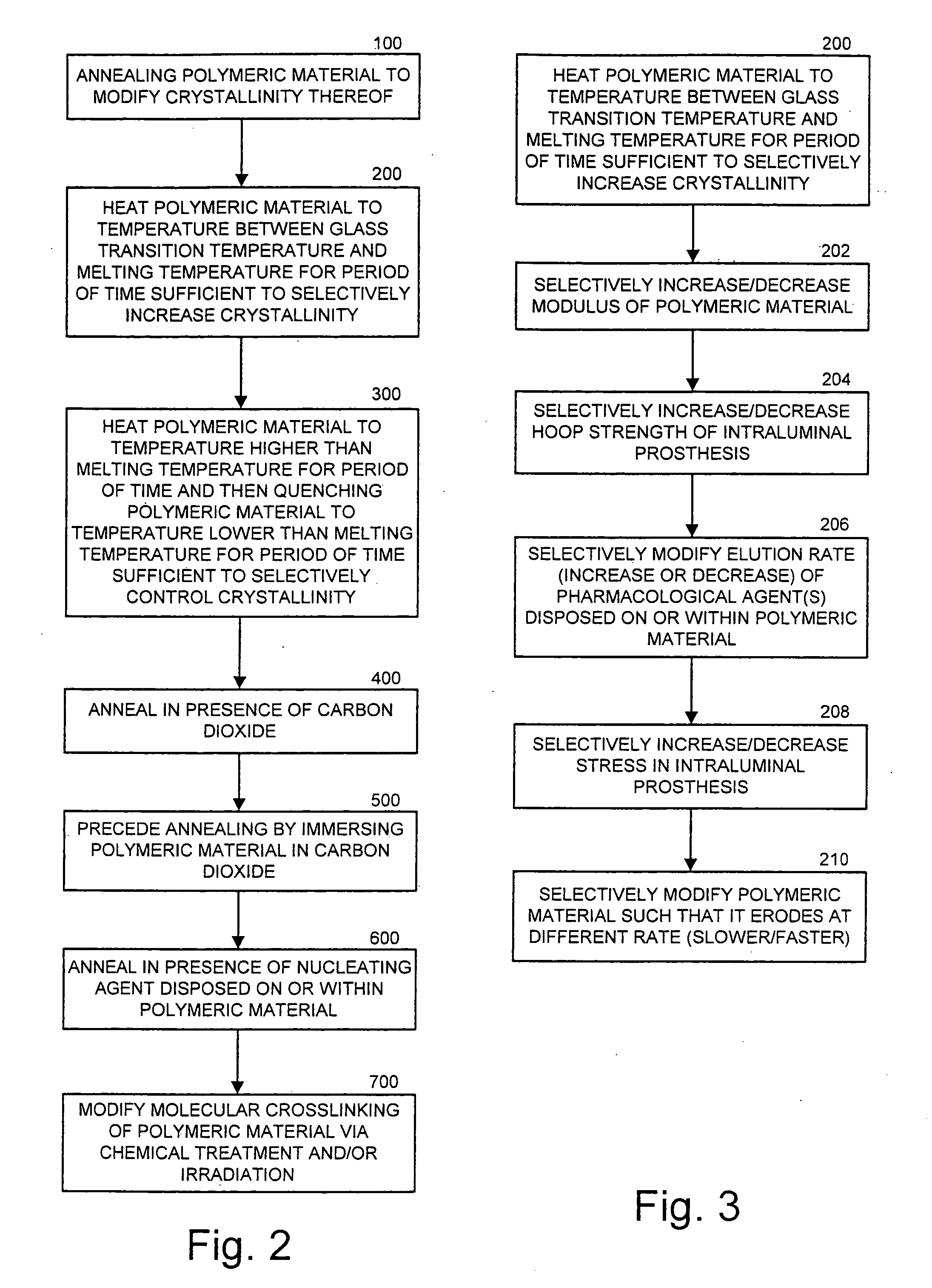
Methods of manufacturing polymeric intraluminal prostheses include annealing the polymeric material to selectively modify the crystallinity thereof. Annealing may be utilized to selectively modify various properties of the polymeric material of an intraluminal prosthesis, including: selectively increasing the modulus of the polymeric material; selectively increasing the hoop strength of the intraluminal prosthesis; selectively modifying the elution rate (increase or decrease) of a pharmacological agent subsequently disposed on or within the annealed polymeric material; selectively increasing / decreasing stress in the intraluminal prosthesis; and selectively modifying the polymeric material such that it erodes at a different rate.
Owner:SYNECOR LLC
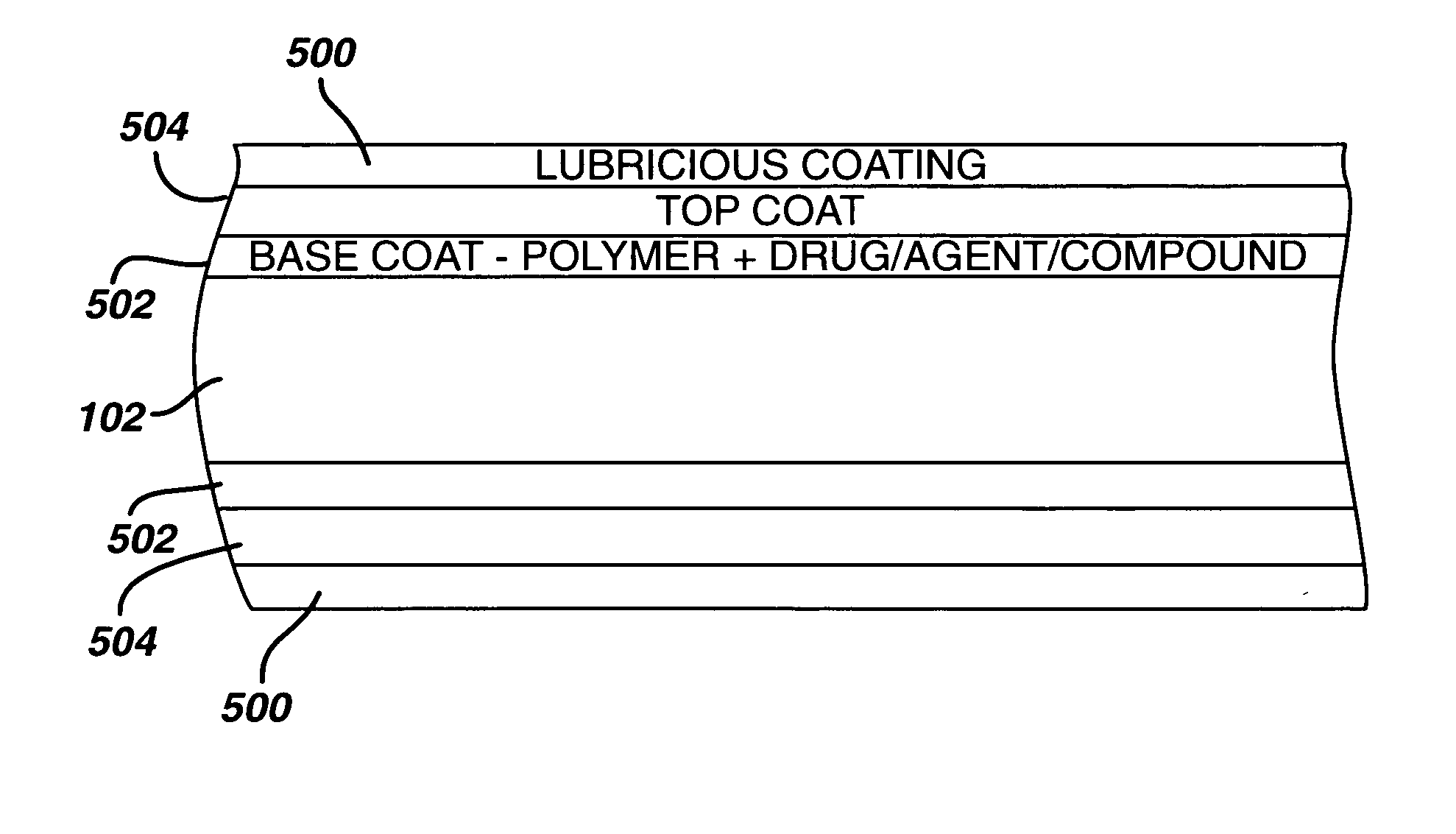
Coating for controlled release of a therapeutic agent
InactiveUS20050033417A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsBlood vesselPolymer
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same
InactiveUS7919162B2High modulusEnhanced hoop strengthStentsSynthetic resin layered productsProsthesisCrystallinity
Methods of manufacturing polymeric intraluminal prostheses include annealing the polymeric material to selectively modify the crystallinity thereof. Annealing may be utilized to selectively modify various properties of the polymeric material of an intraluminal prosthesis, including: selectively increasing the modulus of the polymeric material; selectively increasing the hoop strength of the intraluminal prosthesis; selectively modifying the elution rate (increase or decrease) of a pharmacological agent subsequently disposed on or within the annealed polymeric material; selectively increasing / decreasing stress in the intraluminal prosthesis; and selectively modifying the polymeric material such that it erodes at a different rate.
Owner:SYNECOR LLC
Local vascular delivery of trichostatin a alone or in combination with sirolimus to prevent restenosis following vascular injury
ActiveUS20050136090A1Minimize potential risk of damageReduce frictionBiocideSurgeryPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Medical device having a hydration inhibitor
A medical device having a hydration inhibitor for controlled delivery of a beneficial agent and methods of manufacturing of the same. The medical device includes an interventional component loaded with a beneficial agent having a first Log P value, the beneficial agent being associated with a hydration inhibitor to control the elution rate of at least part of the beneficial agent, the hydration inhibitor having a second Log P value which is greater than the first Log P value.
Owner:ABBOTT LAB INC
Methods of manufacturing medical devices for controlled drug release
The present invention is a medical device for controlling the release of an active agent. The medical device has a supporting structure having a porous body disposed therein. At least one elution rate controlling matrix containing an effective amount of at least one active agent is disposed within the pores of the porous body in a manner that protects the matrix from mechanical damage. The medical device may therefore be used for controlled drug release applications. Additionally, the present invention discloses a method for using the medical device for the treatment and prevention of diseases in mammals. This invention further relates to a method for using the medical device for treating and preventing vascular diseases.
Owner:ABBOTT LAB INC
Device for the delivery of a cardioprotective agent to ischemic reperfused myocardium
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Implantable medical devices may be coated or otherwise have affixed thereto agents for healing ischemic tissue.
Owner:WYETH LLC
Solution formulations of sirolimus and its analogs for CAD treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
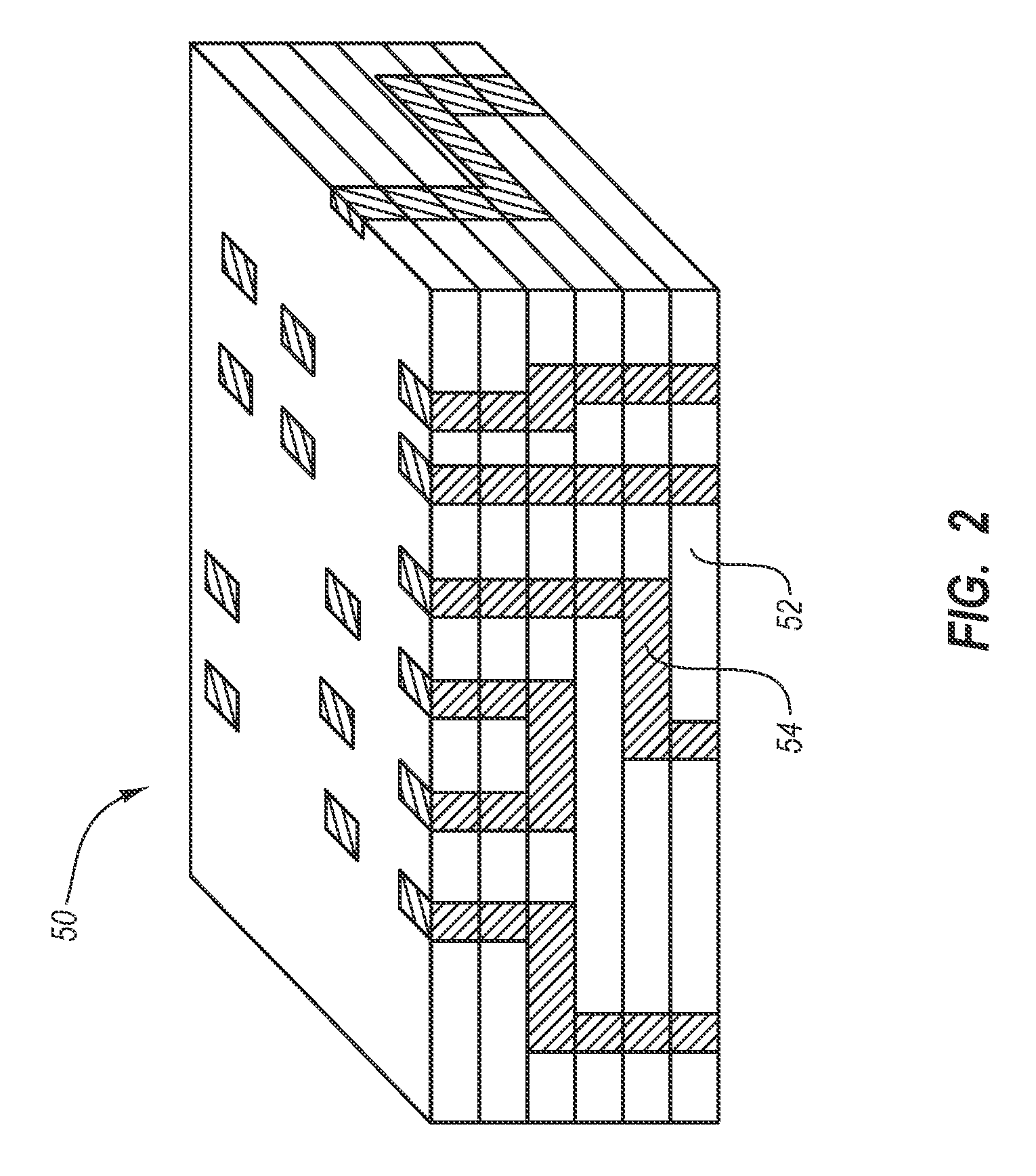
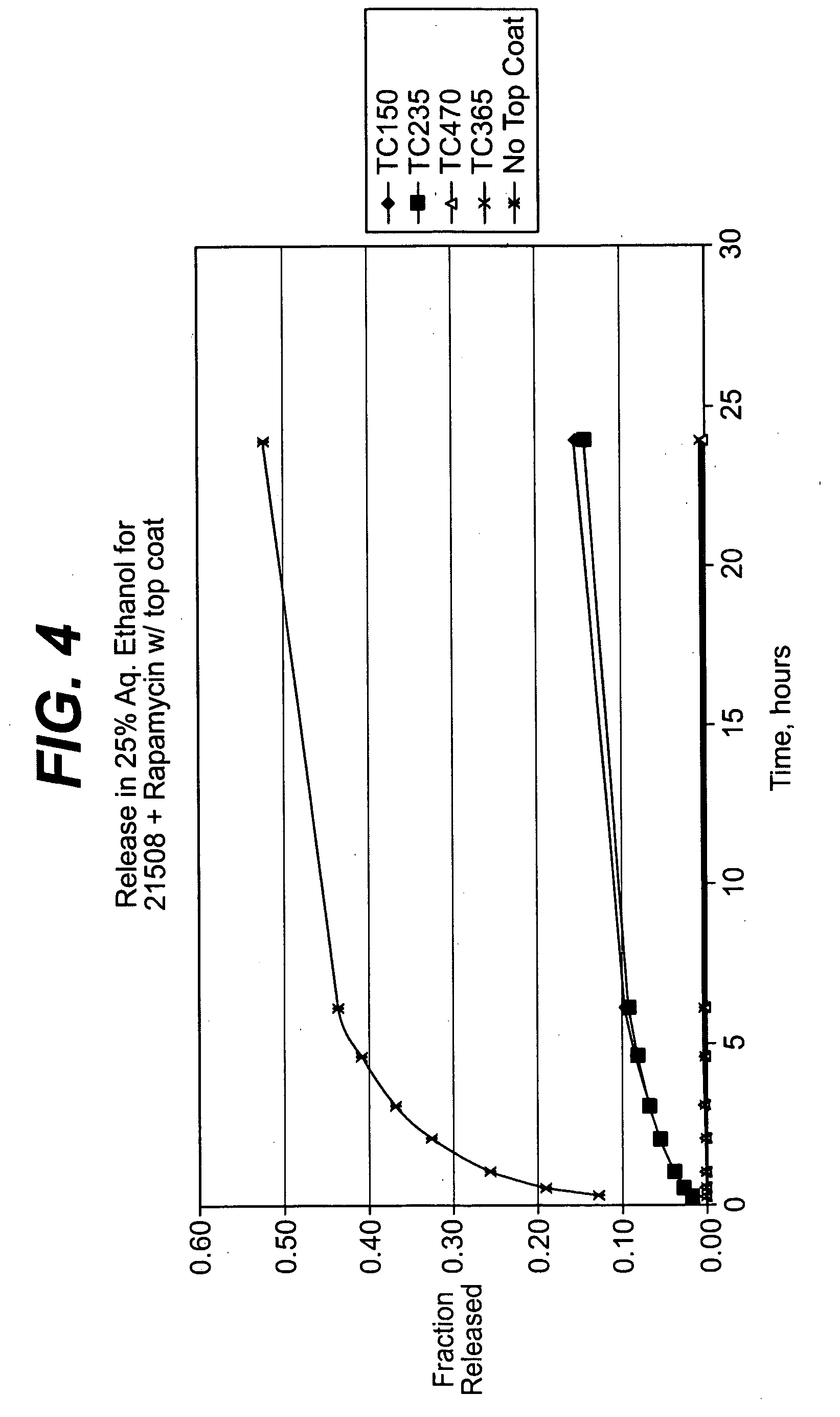
Multi-layered coatings and methods for controlling elution of active agents
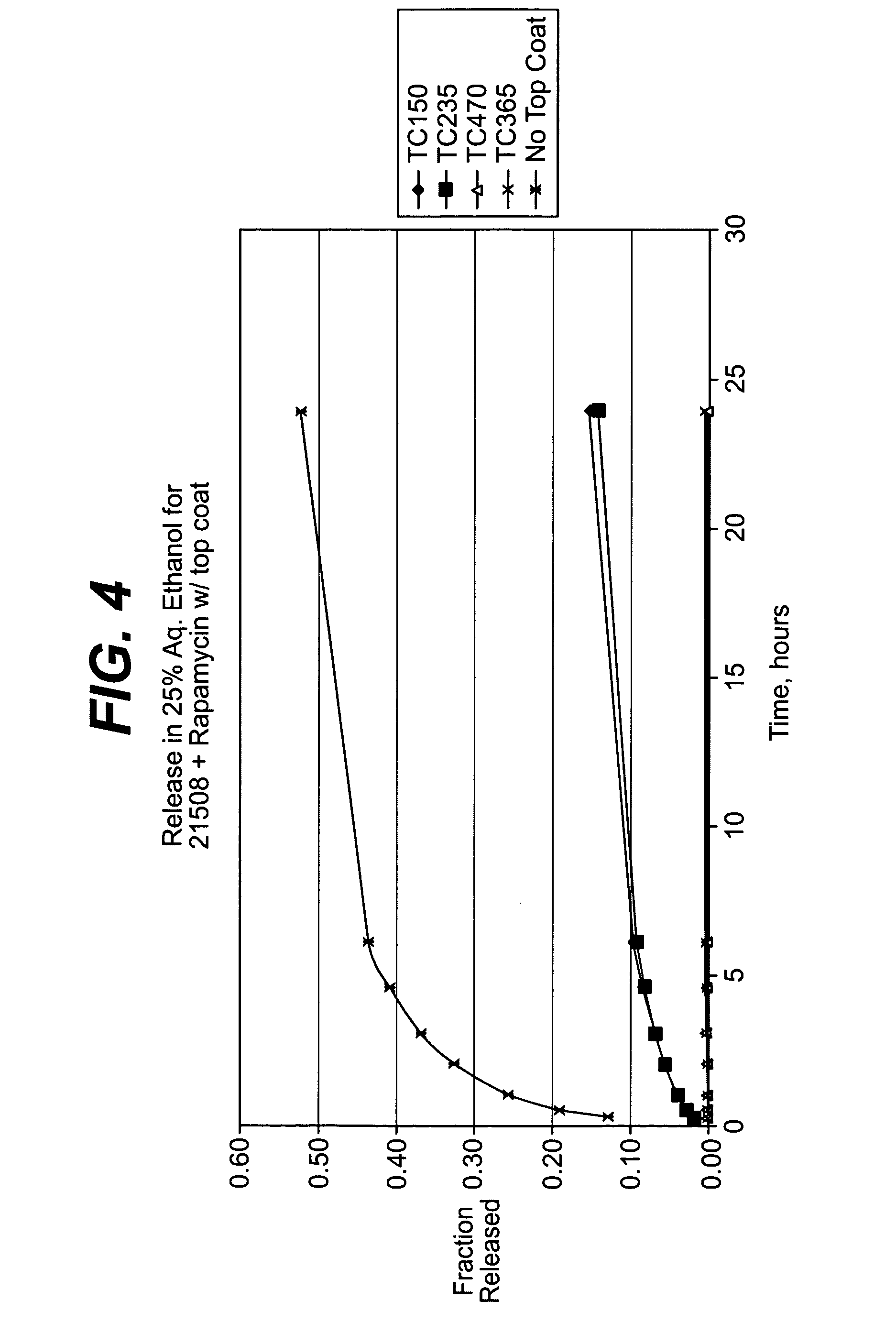
Embodiments of the invention include multi-layered coatings for controlling the elution rates of active agents and methods. In an embodiment, the invention includes a method of applying an elution control coating to a substrate. The method can include depositing a coating solution onto the substrate to form a base layer. The method can also include selecting a desired concentration of the solvent based on a desired elution rate. The method can further include removing solvent from the base layer to reach a desired concentration of the solvent and depositing a layer of parylene on the base layer. In an embodiment, the invention can include a medical device including a substrate, a base layer, and a porous layer. The base layer can include a polymeric matrix and an active agent dispersed within the polymeric matrix. The porous layer can include parylene. Other embodiments are also included herein.
Owner:SURMODICS INC
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Intraluminal medical devices in combination with therapeutic agents
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Thin-film nitinol based drug eluting stent
InactiveUS20070173787A1Reduce drug toxicityGood curative effectStentsPharmaceutical delivery mechanismDiseaseBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. These devices may also comprise thin films that perform a number of functions. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Coated aneurysmal repair device
InactiveUS20050249776A1Reduce drug toxicityGood curative effectBiocideStentsBlood vesselDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Methods of using medical devices for controlled drug release
InactiveUS20080057103A1Treating and preventing diseaseStentsOrganic active ingredientsDiseaseVascular disease
The present invention is a medical device for controlling the release of an active agent. The medical device has a supporting structure having a porous body disposed therein. At least one elution rate controlling matrix containing an effective amount of at least one active agent is disposed within the pores of the porous body in a manner that protects the matrix from mechanical damage. The medical device may therefore be used for controlled drug release applications. Additionally, the present invention discloses a method for using the medical device for the treatment and prevention of diseases in mammals. This invention further relates to a method for using the medical device for treating and preventing vascular diseases.
Owner:ABBOTT LAB INC
Methods, devices, and coatings for controlled active agent release
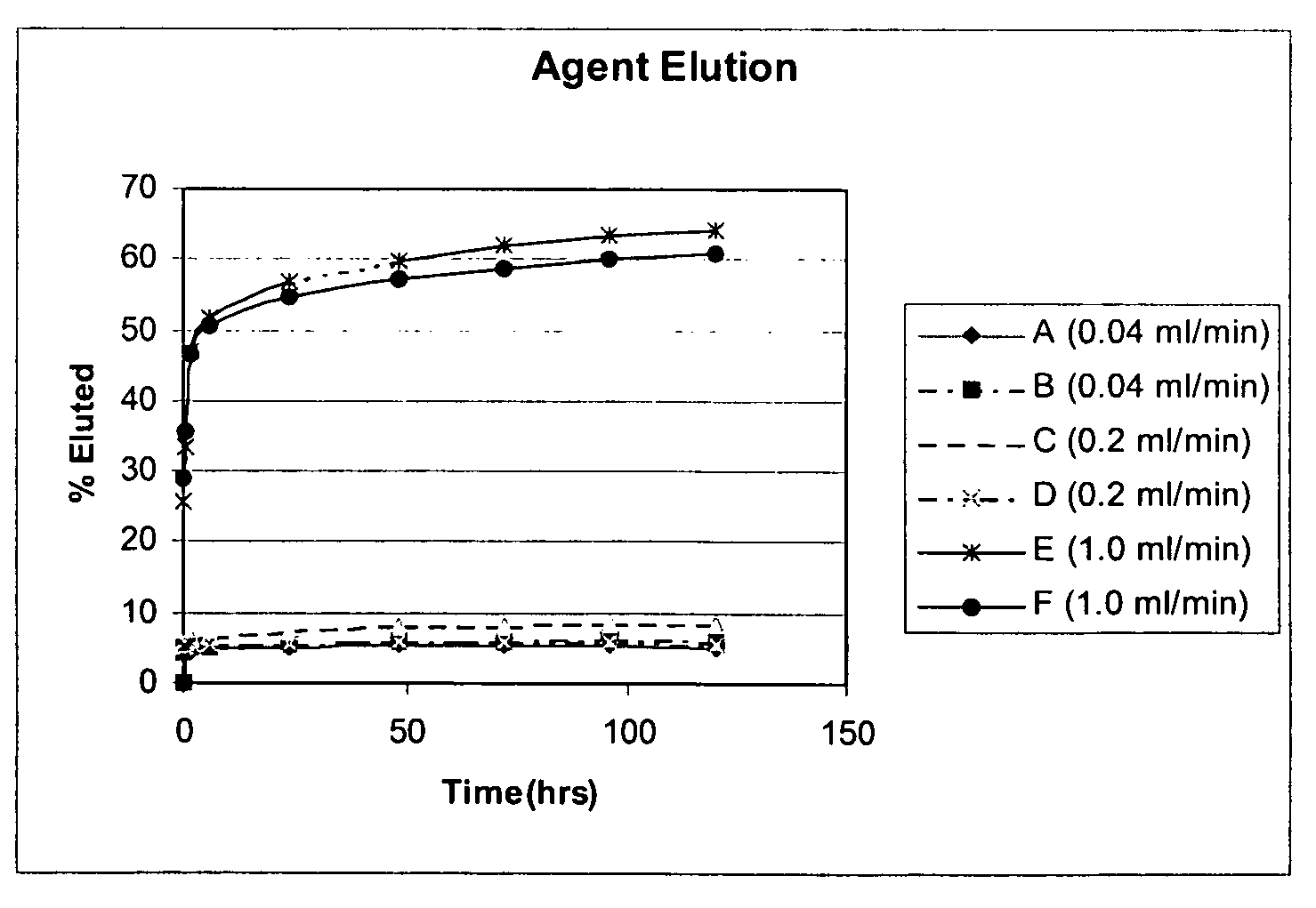
The present invention relates to methods, devices, and coatings, wherein active agent release is determined by deposition rate of a coating or material. In an embodiment, the invention includes a method for coating a medical device, including identifying active agent elution rates for a coating composition applied to substrates at a plurality of coating deposition rates, selecting one of the coating deposition rates, and applying the coating composition to the medical device at the selected deposition rate. In an embodiment, the invention includes a combination including a medical device and a composition for coating the surface of a medical device with an active agent in a manner that permits the coated surface to release the active agent over time when implanted in vivo.
Owner:SURMODICS INC
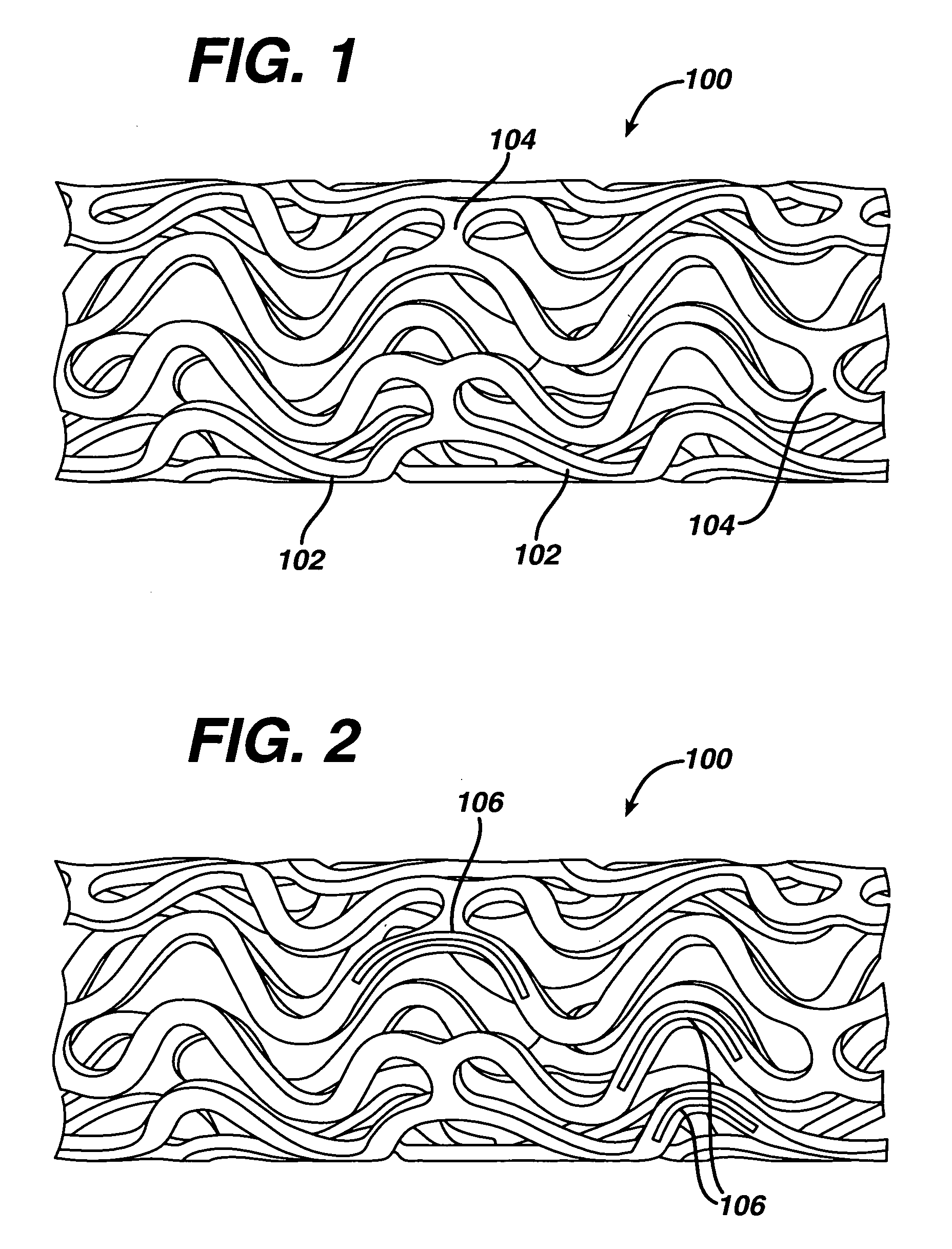
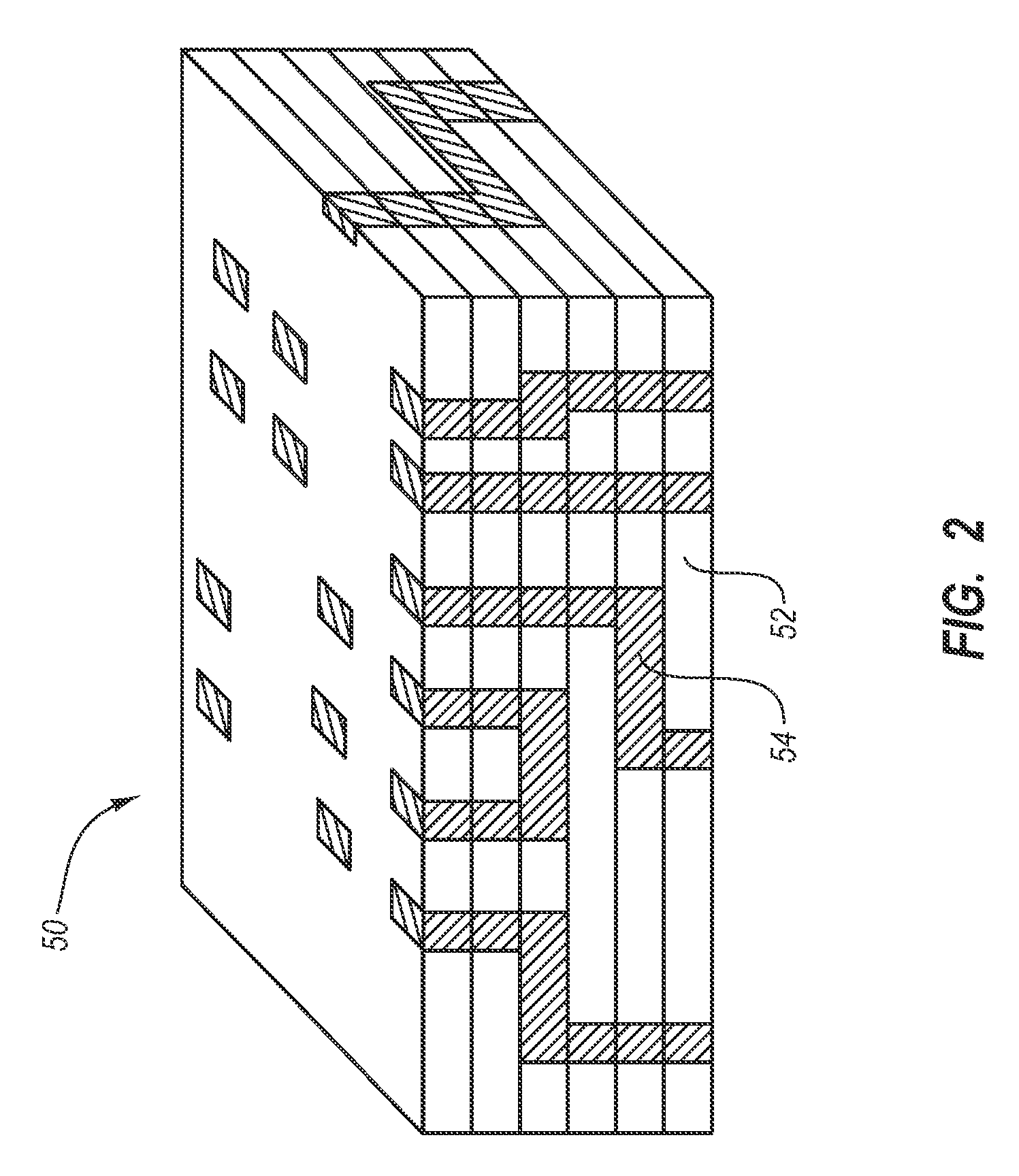
Stent with eccentric coating
InactiveUS20060149365A1Avoid wastingReduce drug deliveryStentsLiquid surface applicatorsInsertion stentDrug carrier
The stent (120) with an eccentric coating (130) of the present invention provides a coating having a different coating thickness on the stent outer diameter and stent inner diameter, i.e., an eccentric coating. The eccentric coating can be the primary carrier for a drug or other therapeutic agent. The eccentric coating can be thicker on the stent outer diameter to supply more drug to the vessel wall in which the coated stent is deployed and less drug to the vessel lumen. In one embodiment, a cap coating (125) can be disposed on the eccentric coating to protect the eccentric coating, control the elution rate from the eccentric coating, provide an additional drug carrier, or provide combinations thereof. The eccentric coating can be applied by spraying a coating liquid on the stent outer diameter with a fixture mandrel interior to the stent to regulate the spray to the stent inner diameter.
Owner:MEDTRONIC VASCULAR INC
Medical devices for controlled drug release
The present invention is a medical device for controlling the release of an active agent. The medical device has a supporting structure having a porous body disposed therein. At least one elution rate controlling matrix containing an effective amount of at least one active agent is disposed within the pores of the porous body in a manner that protects the matrix from mechanical damage. The medical device may therefore be used for controlled drug release applications. Additionally, the present invention discloses a method for using the medical device for the treatment and prevention of diseases in mammals. This invention further relates to a method for using the medical device for treating and preventing vascular diseases.
Owner:ABBOTT LAB INC
Local vascular delivery of probucol alone or in combination with sirolimus to treat restenosis, vulnerable plaque, aaa and stroke
InactiveUS20080241215A1Minimize potential risk of damageReduce frictionBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Use of antioxidants to prevent oxidation and reduce drug degradation in drug eluting medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:WYETH LLC
Radioprotective compound coating for medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Other compounds may include those that prevent damage from ionizing radiation. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:OHARA MICHAEL D
Local vascular delivery of cladribine in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050182485A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Coating for controlled release of a therapeutic agent
InactiveUS20090082855A1Provide controlSuture equipmentsOrganic active ingredientsTherapeutic effectPolymer
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:BORJOHN +4
Methods, devices, and coatings for controlled active agent release
The present invention relates to methods, devices, and coatings, wherein active agent release is determined by deposition rate of a coating or material. In an embodiment, the invention includes a method for coating a medical device, including identifying active agent elution rates for a coating composition applied to substrates at a plurality of coating deposition rates, selecting one of the coating deposition rates, and applying the coating composition to the medical device at the selected deposition rate. In an embodiment, the invention includes a combination including a medical device and a composition for coating the surface of a medical device with an active agent in a manner that permits the coated surface to release the active agent over time when implanted in vivo.
Owner:SURMODICS INC
Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050158360A1Minimize potential risk of damageReduce frictionStentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Method for preparing ammonium perrhenate from waste liquid containing molybdenum and rhenium
ActiveCN102173457APromote enrichmentSave resourcesRhenium compoundsCalcium hydroxideAmmonium perrhenate
The invention relates to a method for preparing ammonium perrhenate from waste liquid containing molybdenum and rhenium. In the technical scheme of the invention, the method comprises the following steps of: adding hydrogen peroxide into the waste liquid containing molybdenum and rhenium until the solution turns to yellow, then adding a mixed agent until the pH of the solution is 6 to 7, separating by filter pressing, collecting the filtrate, absorbing the filtrate by a resin exchange column, stopping adsorption until the concentration of rhenium in effluent is constant, eluting with NH3.H2O,collecting the eluate, heating to concentrate the eluate at 98-100 DEG C, cooling, and crystallizing to obtain ammonium perrhenate. The mixed agent is a mixture of calcium hydroxide and calcium oxidein a weight ratio of 5:1. According to the invention, the waste liquid containing molybdenum and rhenium, particularly the absorption liquid of the flue gas during molybdenum roasting, is used as theraw material; the enrichment of rhenium is increased by about 20 times; ammonia water is determined as the eluent of rhenium; the elution rate of rhenium is higher than 98%; the recovery rate of rhenium is higher than 93%, the purity of the ammonium perrhenate product is higher than 99.5%, and the economic, social and environmental benefits are significantly improved.
Owner:爱瑞克(大连)安全技术集团有限公司
Drug delivery device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Local vascular delivery of Panzem in combination with rapamycin to prevent restenosis following vascular injury
InactiveUS20050209688A1Reduce frictionLittle strengthStentsSurgeryPercent Diameter StenosisBlood vessel
Owner:WYETH LLC
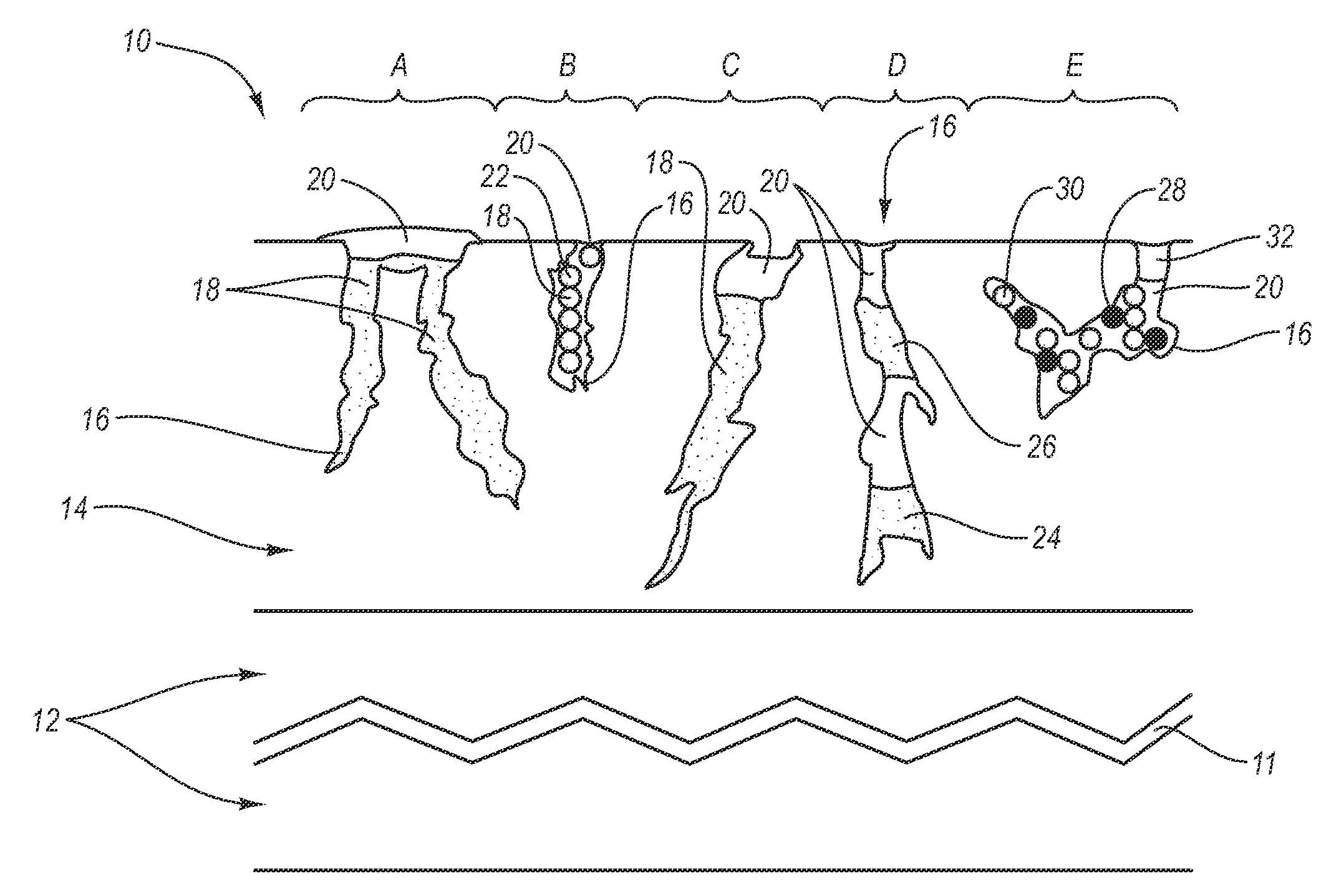
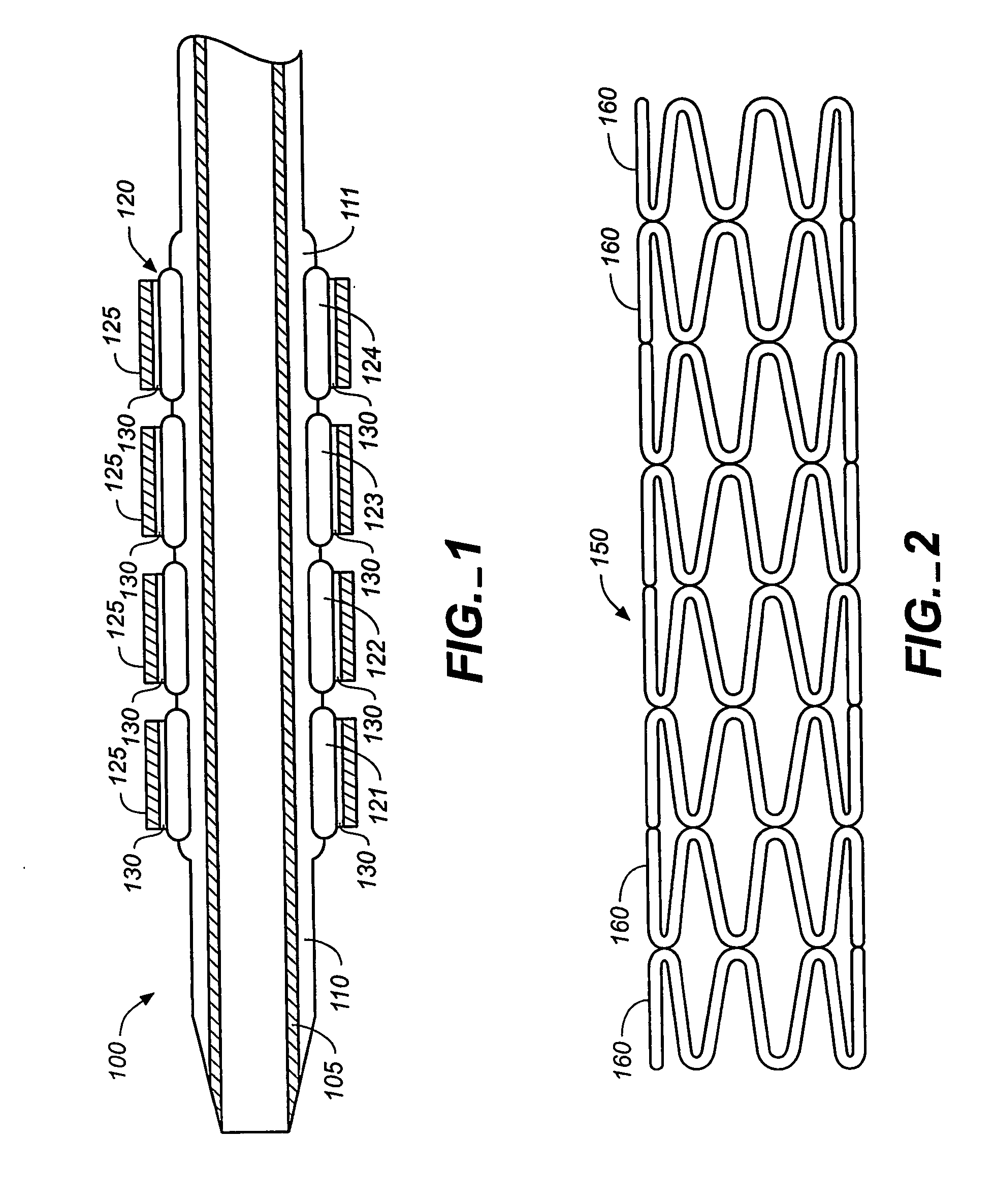
Stent
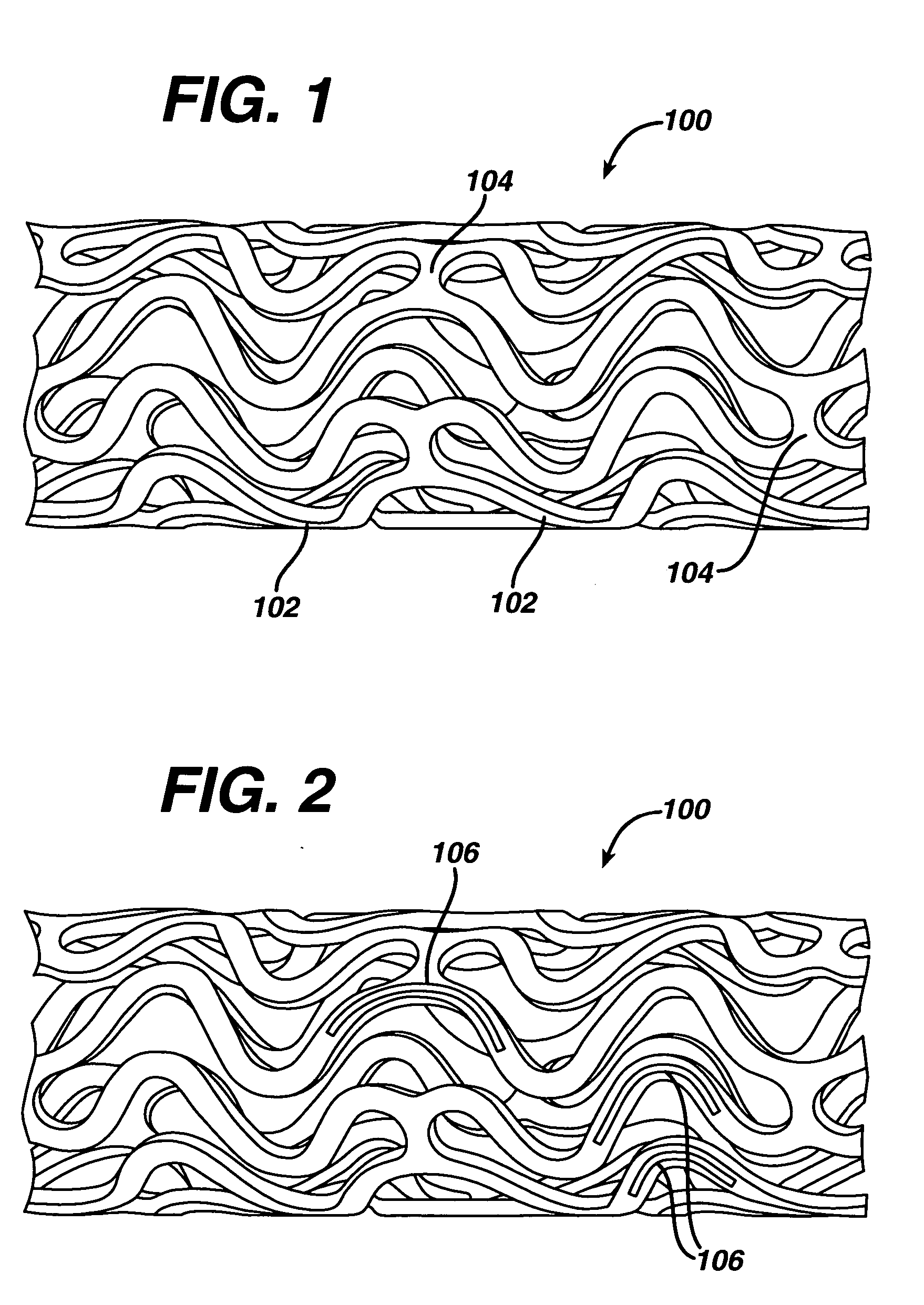
In at least one embodiment, the invention is directed to mechanisms that affect the elution rate of a therapeutic agent that has been deposited on the surface of at least a portion of a stent. Mechanisms include grooves formed in the therapeutic agent that is coating at least a portion of the surface of the stent.In at least one embodiment, the invention is directed to the directional release of a therapeutic agent contained within a reservoir formed in at least one member of a stent.
Owner:BOSTON SCI SCIMED INC
Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS7303758B2Prevent elutionProvide controlStentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Owner:WYETH LLC
Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same
Methods of manufacturing polymeric intraluminal prostheses include annealing the polymeric material to selectively modify the crystallinity thereof. Annealing may be utilized to selectively modify various properties of the polymeric material of an intraluminal prosthesis, including: selectively increasing the modulus of the polymeric material; selectively increasing the hoop strength of the intraluminal prosthesis; selectively modifying the elution rate (increase or decrease) of a pharmacological agent subsequently disposed on or within the annealed polymeric material; selectively increasing / decreasing stress in the intraluminal prosthesis; and selectively modifying the polymeric material such that it erodes at a different rate.
Owner:SYNECOR LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com