Methods of using medical devices for controlled drug release
a technology of medical devices and controlled drugs, applied in the direction of drugs, antinoxious agents, prostheses, etc., can solve the problems of affecting the treatment effect of animals, cracking, delamination, and material biocompatibility, and achieve the effect of treating and/or preventing diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
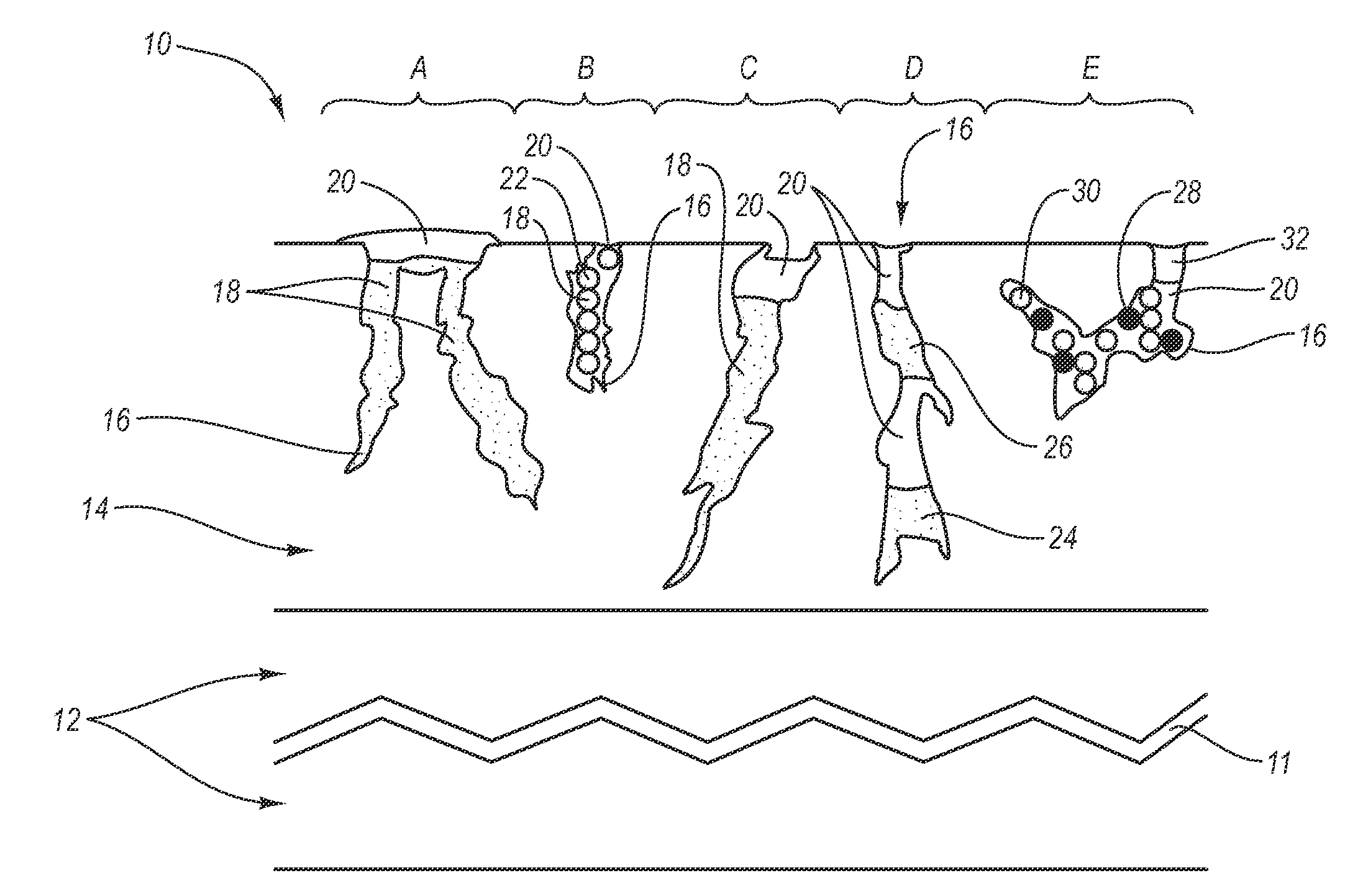
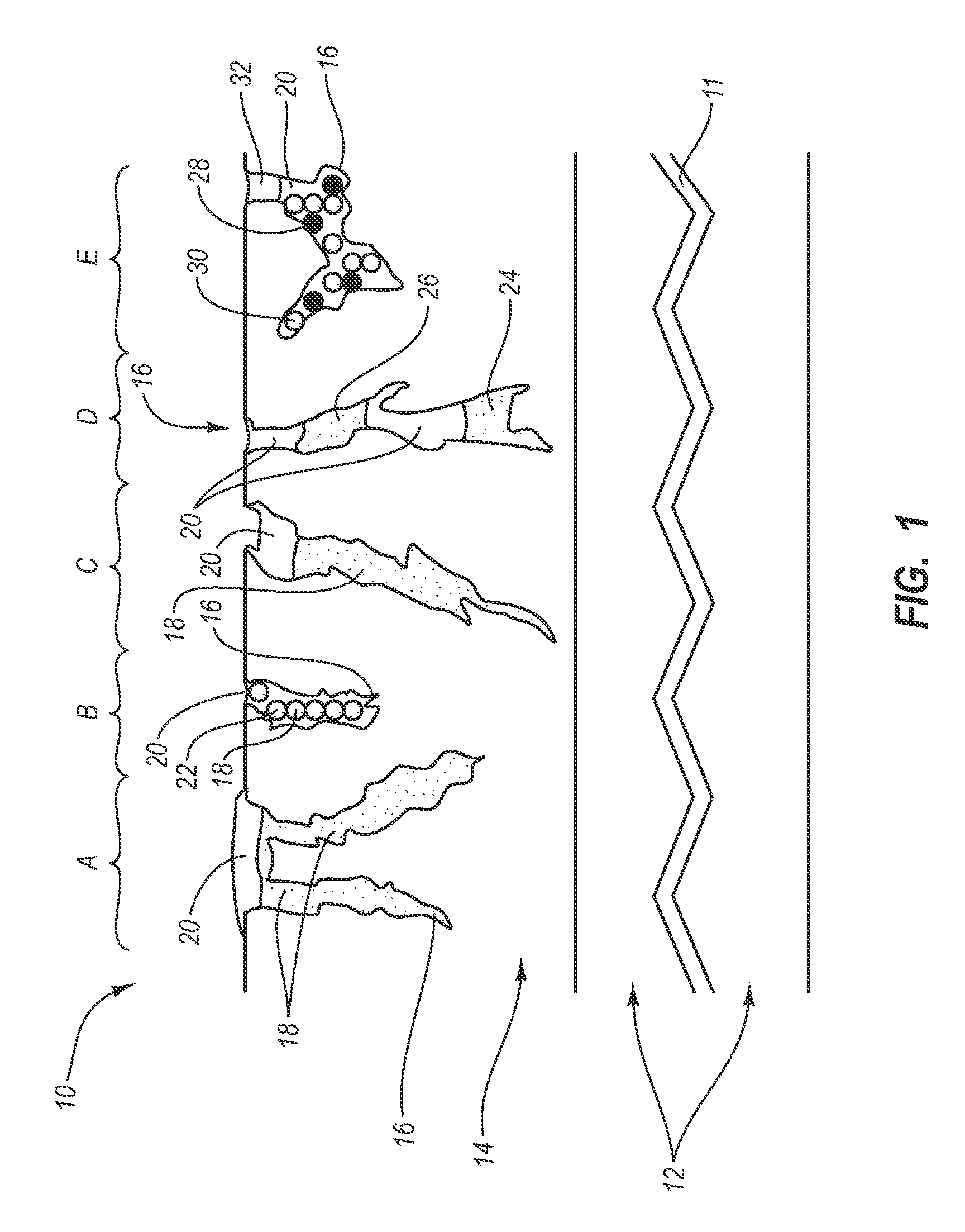
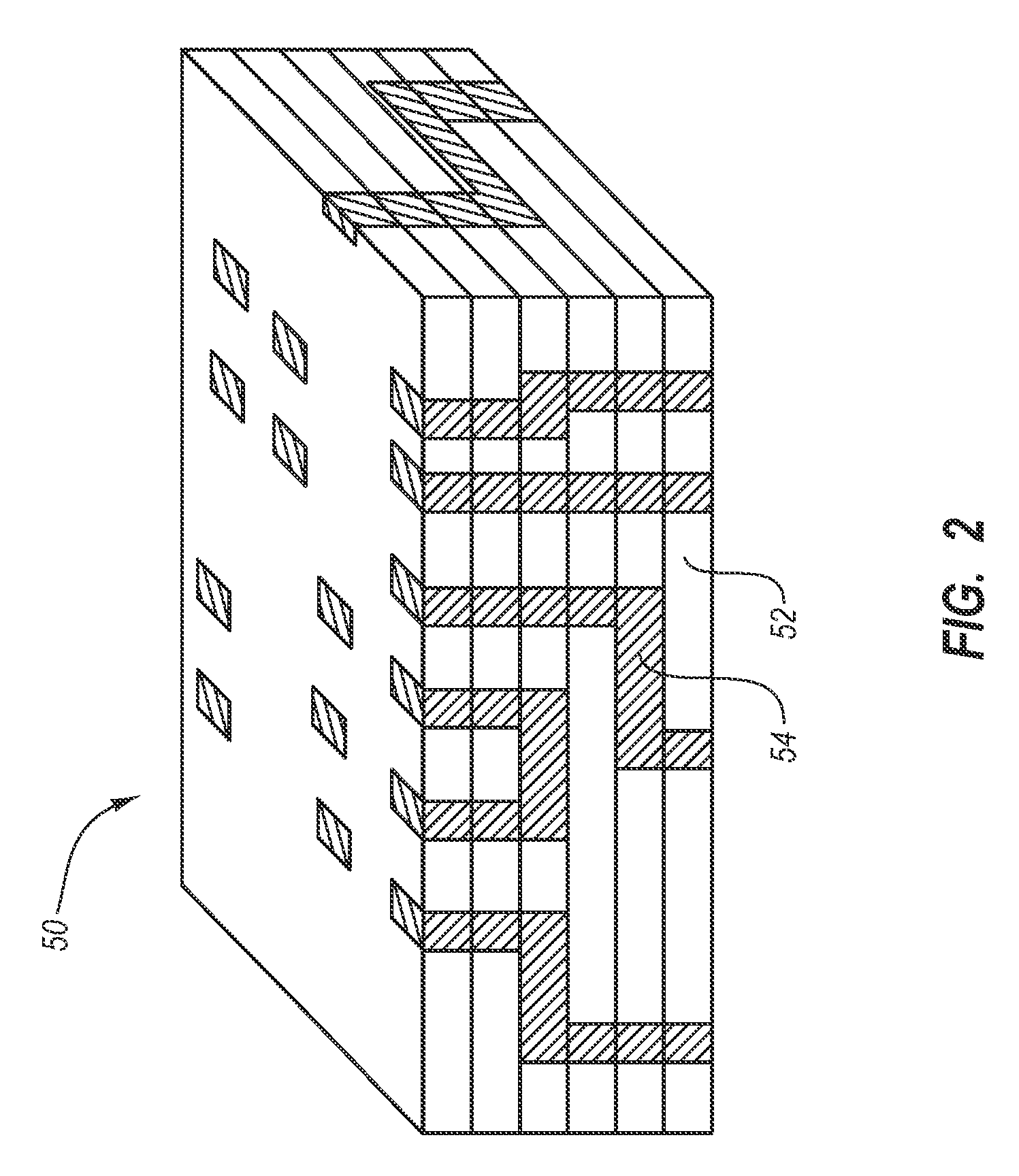
[0027] The present invention generally relates to a drug eluting medical devices, endoprostheses, stents, and the like that have an elution rate controlling matrix that contains an active agent and that is disposed within a porous material. Also, the present invention relates to methods of manufacturing and using the medical devices of the invention in treating and / or preventing diseases in animals, such as mammals. The medical devices of the invention are constructed of materials suitable for use in animals, and include at least one elution rate controlling matrix and a therapeutically effective amount of at least one biologically active agent. The medical devices of the invention also include at least one porous material associated with a supporting structure, where the elution rate controlling matrix containing the active agent is disposed within the pores of the porous material. The pores of the porous material can be dimensioned and configured to cooperate with the elution rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com