Application of cladabine in preparation of medicine for preventing or treating psoriasis
A technology for preparing medicines and psoriasis, which is applied to cladribine single-dose preparations and drug combinations. In the field of preparation of medicines for the prevention or treatment of psoriasis, cladribine can solve the difficulties in the treatment of psoriasis that cannot be completely cured , easy to relapse and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: the establishment of psoriasis mouse model
[0087] (1) The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (80mg / kg), and the back hair was shaved with a children's hair shaver to form an exposed area with a size of about 2cm×3cm, and the hair removal area was treated with depilatory cream;
[0088] (2) After feeding in a single cage for 1 day, apply 62.5 mg / day imiquimod cream (5% mass fraction) for 14 days;
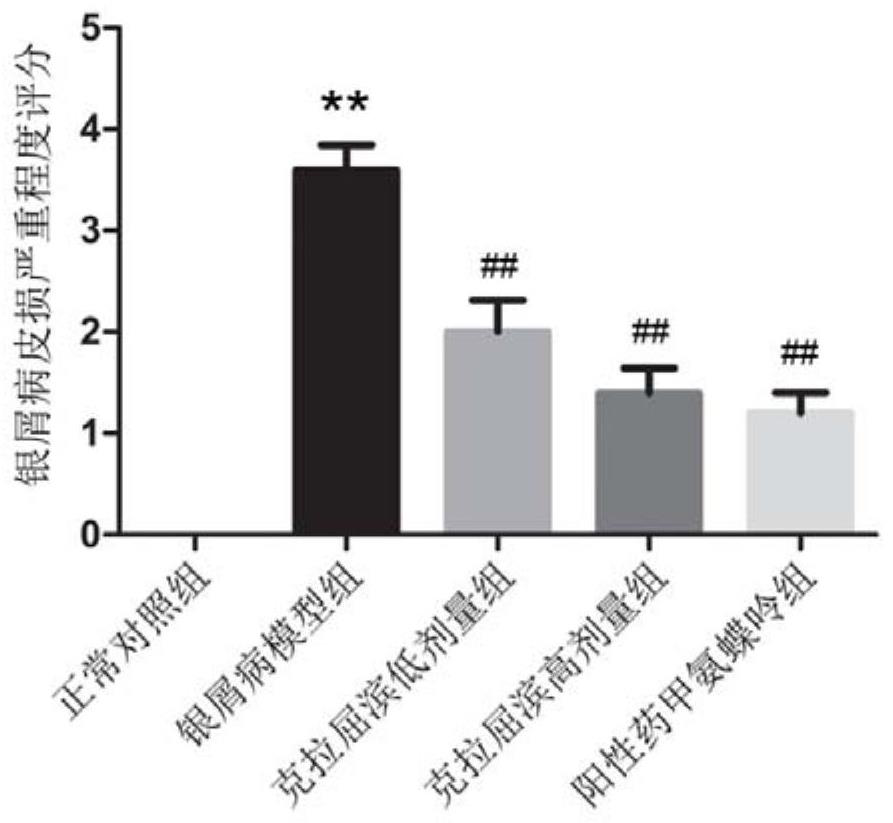
[0089](3) According to the PASI score of the control group to determine whether the model is successful or not, it was found that there was a significant difference in the PASI score of the psoriasis mouse model (pfigure 1 shown.
Embodiment 2
[0090] Embodiment 2: the in vitro experiment of mouse model
[0091] (1) Randomly divide the psoriasis mouse model successfully constructed in Example 1 into a model group, a cladribine high-dose group (abbreviated as a high-dose group), a cladribine low-dose group (abbreviated as a low-dose group), and a positive Drug methotrexate group, 10 mice in each group, and 10 normal mice were selected as the normal control group, marked and reared in a single cage in an independent ventilated cage;
[0092] (2) Carry out group treatment to mice, the opportunity of this treatment is the first day after the psoriasis mouse model of embodiment 1 is constructed successfully, wherein, cladribine high-dose group uses cladribine with 30mg / kg / The injection amount of d is carried out intravenous injection to mice, cladribine low-dose group is carried out intravenous injection to mice with cladribine injection amount with 20mg / kg / d, positive drug methotrexate group is with methotrexate The mi...
Embodiment 3
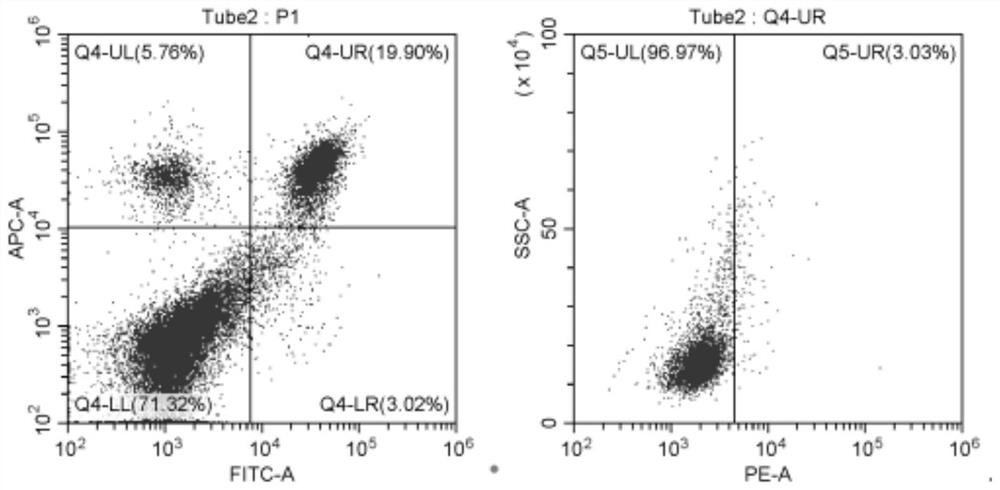
[0098] Example 3: Flow Cytometry Detection
[0099] Th1, Th2, Th17, Treg cytokines in the mouse serum extracted at 25 days in Example 2 were detected by flow cytometry, see for details Figure 3-28 . in, Figure 8 , 14 Among -15, 21, 27-28, * means there is a significant difference compared with the normal control group (p<0.05), ** means there is a very significant difference compared with the normal control group (p<0.01), # means There is a significant difference (p<0.05) compared with the psoriasis model group, ## indicates that there is a very significant difference (p<0.01) compared with the psoriasis model group.
[0100] For the detection results of Th1 cells, see Figure 3-8 . The results showed that compared with the normal control group, the proportion of Th1 cells in the psoriasis model was significantly increased. The ratio is reduced. Therefore, cladribine can significantly alleviate the phenomenon of increased Th1 cells caused by psoriasis.
[0101] For ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com