Method for synthesizing cladribine through nitration-chlorination method
A new method, chlorination technology, applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems of high cost, low synthesis yield, small reaction scale, etc., and achieve low cost, cheap and easy raw materials The effect of high yield and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention will be described in detail below in conjunction with the embodiments.
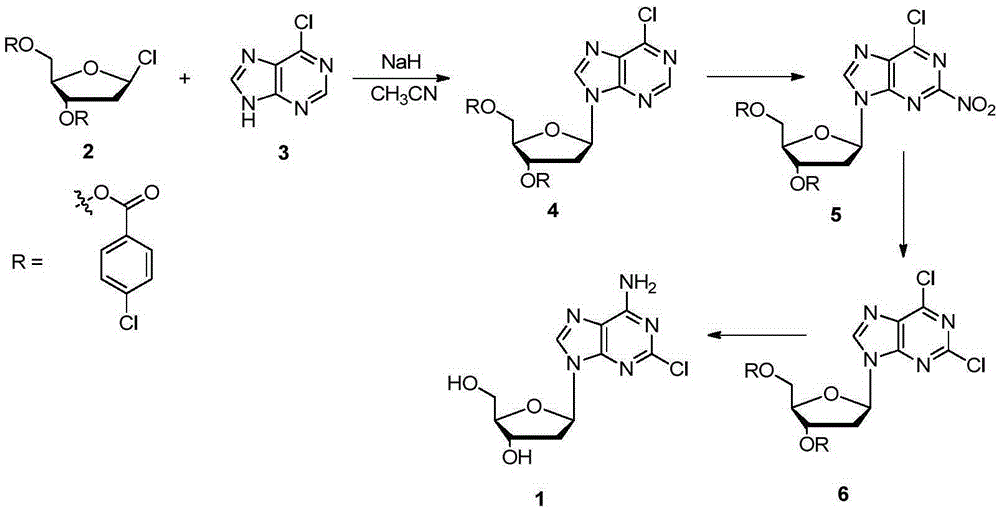
[0014] The method for synthesizing cladribine by the nitration-chlorination method comprises the following steps: taking 6-chloropurine and 1-chloro-2,3,5-tri-O-p-chlorobenzoyl-D-ribose as raw materials, and Sodium hydride is used as the base, and acetonitrile is used as the reaction solvent to obtain the β-isomer; the obtained β-isomer does not need to remove the protecting group, and reacts with trifluoromethanesulfonic anhydride after separation to introduce a nitro group at the 2-position; NH 4 Cl is converted into chlorine atoms in ethanol solution; finally, the two-step reaction of protecting group removal and chlorine atom ammonolysis is completed in methanol solution saturated with ammonia gas to obtain cladribine. The synthetic route is as follows:
[0015]
[0016] in:
[0017] At room temperature, add 6-chloropurine (3, 3.85g, 25mmol) into the three-necked fla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com