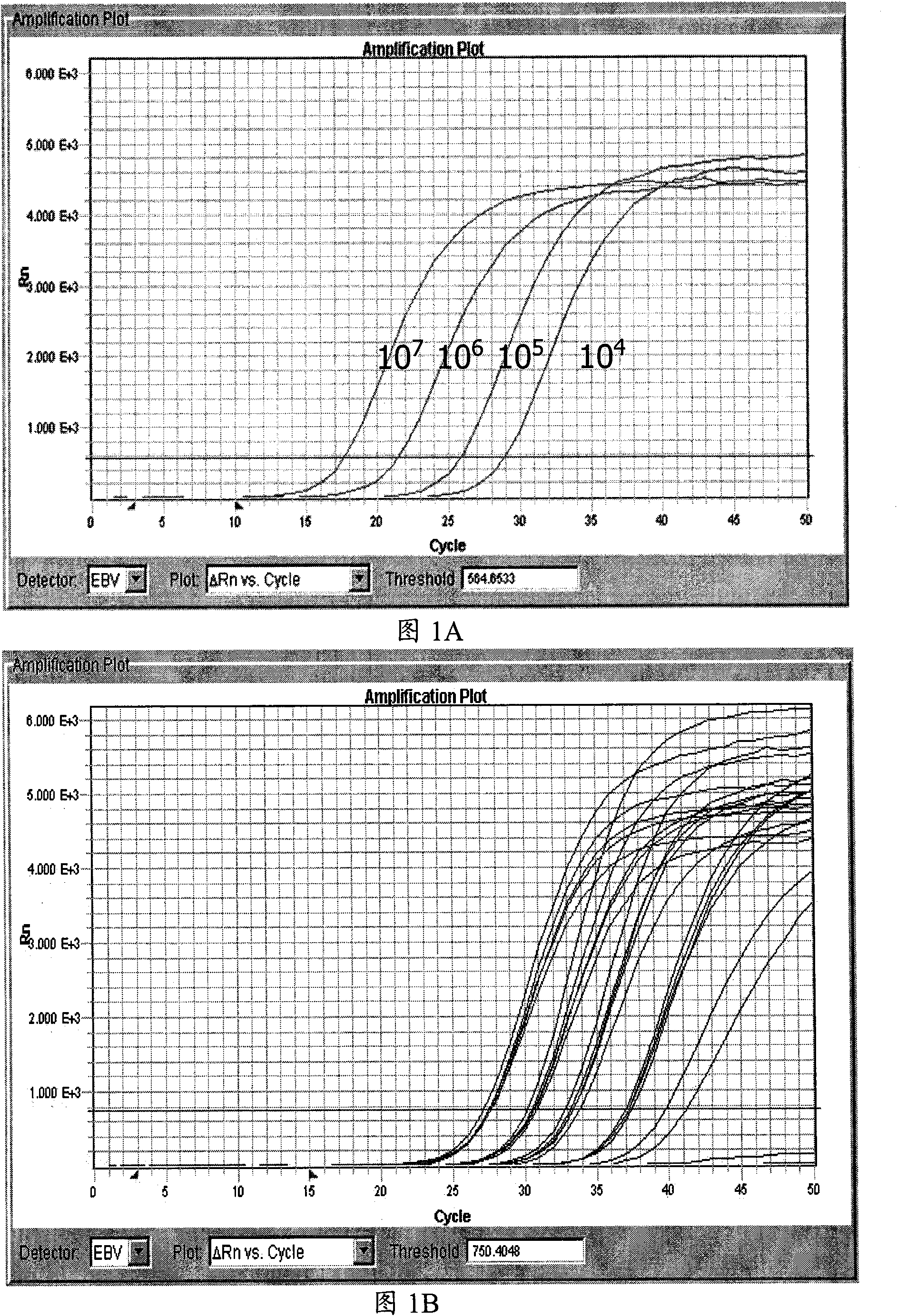
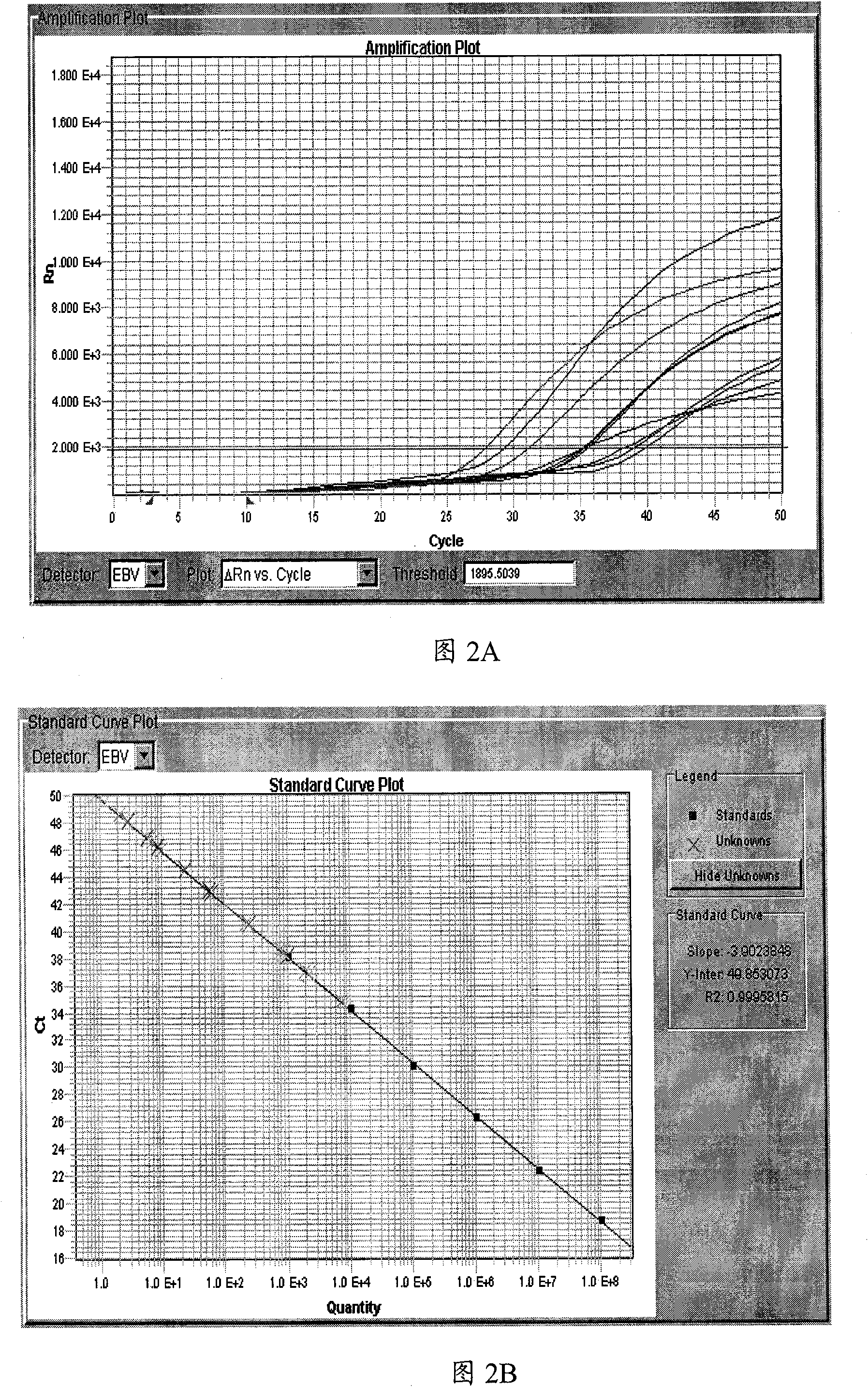
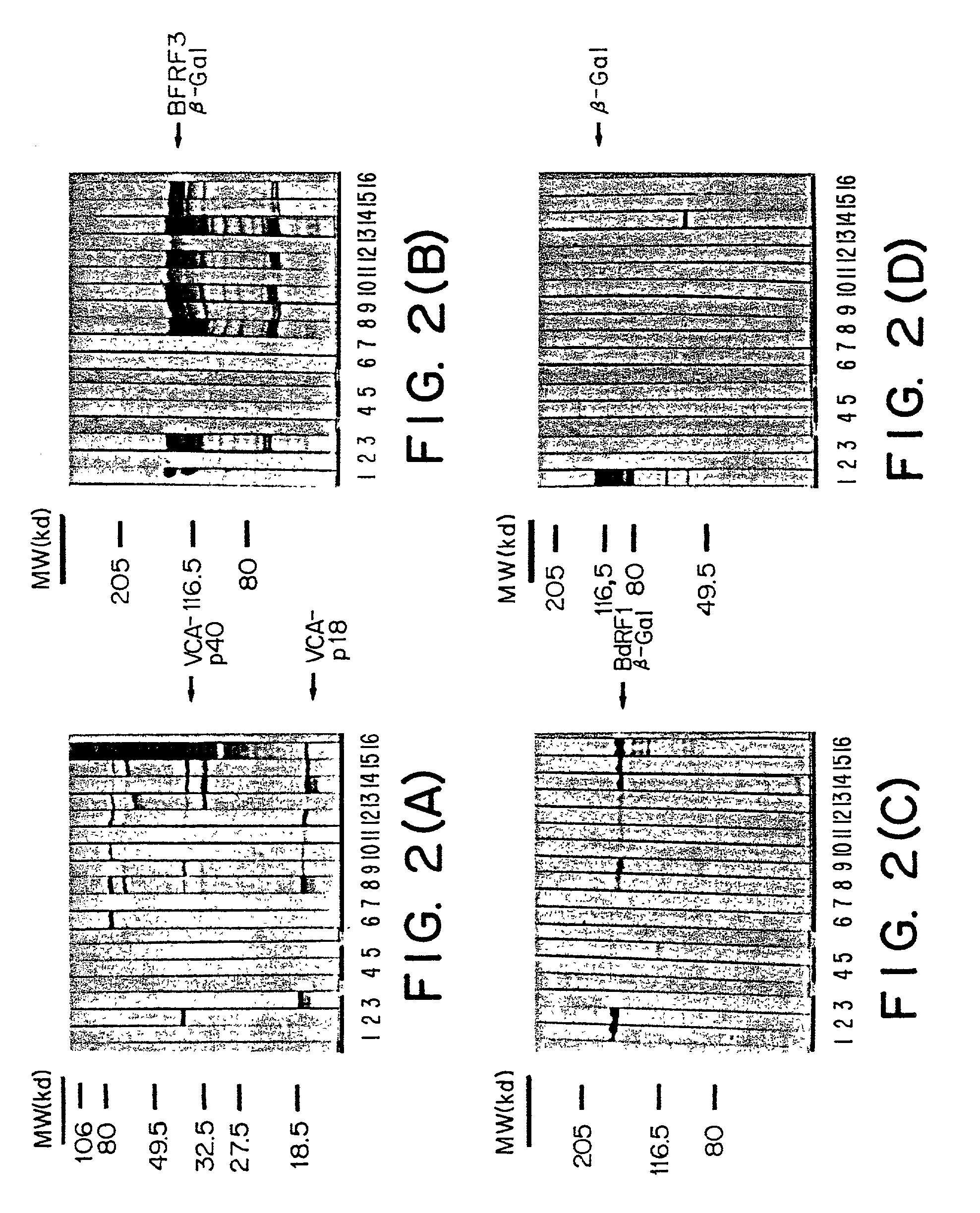
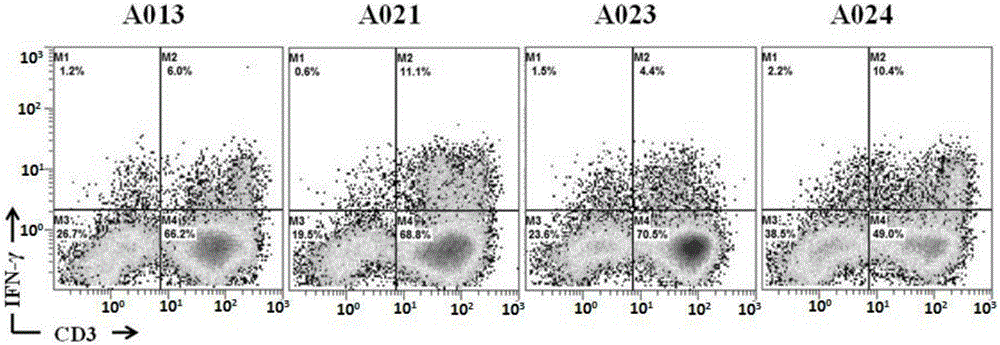
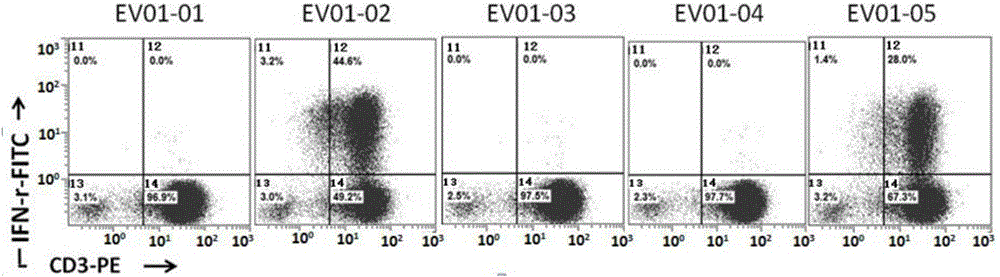
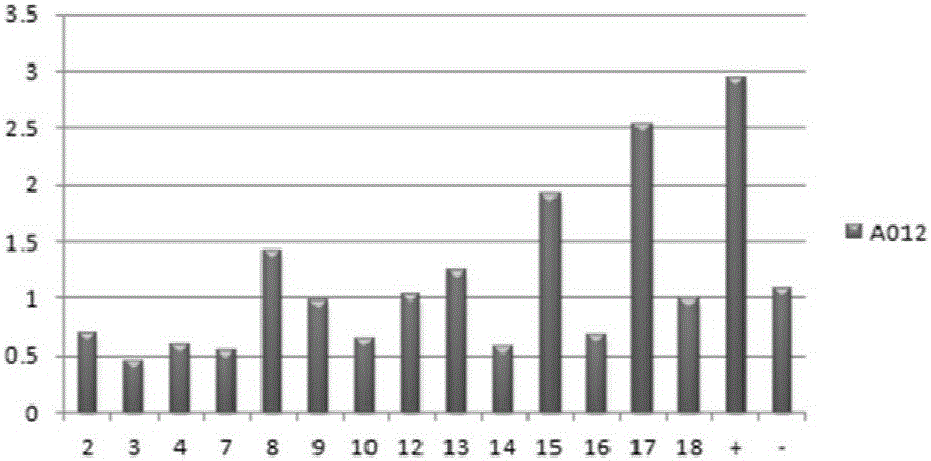
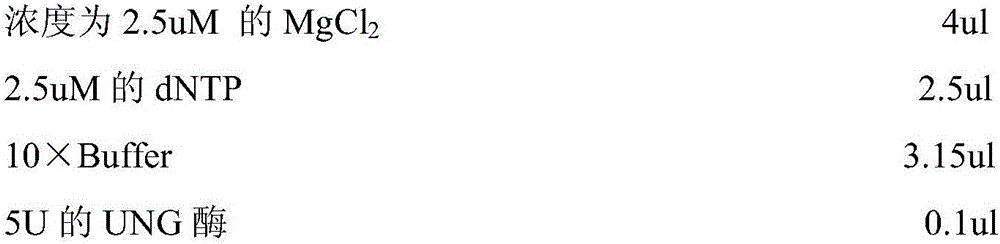Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Epstein barr" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
About Epstein Barr. “Epstein Bar Virus (EBV) is a herpes like virus thought to be the cause of infectious mononucleosis and Burkitt’s lymphoma. It is contracted through the cells in the lining of the mouth and throat, and can therefore be spread by sharing utensils, kissing, and unsanitary habits.
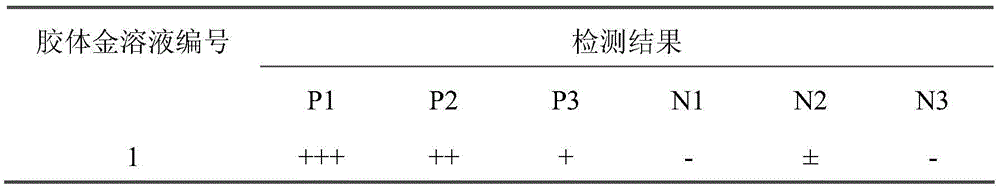
IgA (Immunoglobulin A) antibody detection reagent kit (colloidal gold method) for EB (Epstein-Barr) viruses and preparation method thereof
The invention relates to an IgA (Immunoglobulin A) antibody detection reagent kit (a colloidal gold method) for EB (Epstein-Barr) viruses and a preparation method thereof. The reagent kit comprises recombination antigen EB-NA1 coated by a nitrocellulose membrane detection line, a goat-anti-mouse IgG antibody coated on a quality control line and a mouse-anti-human IGA monoclonal antibody marked by colloidal gold and coated on a gold mark pad. The preparation method comprises the steps of: preparing a reaction membrane and a mouse-anti-human IGA monoclonal antibody gold combo pad, cutting and assembling to prepare the product. The invention has the advantages that: the IgA antibody detection reagent kit for the EB viruses has the characteristics of fast, simple and convenient detection, and high accuracy and sensitivity; the integrated operation time only requires 20 minutes to judge and read results; the colloidal gold is used for fast detecting test paper; a multi-epitope recombination antigen is used as a raw material; the method has the characteristics of simple and convenient operation, low cost, good specificity, high sensitivity, single portion detection and easy popularization; and the detection and control effect to the EB viruses is obvious.
Owner:北京中检安泰诊断科技有限公司
Ring-expanded nucleosides and nucleotides
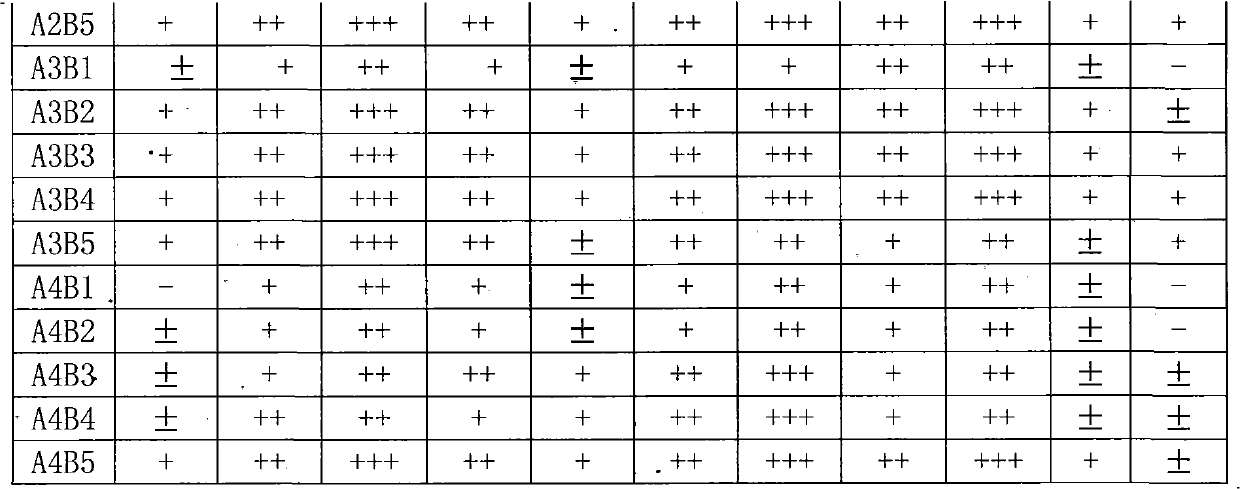
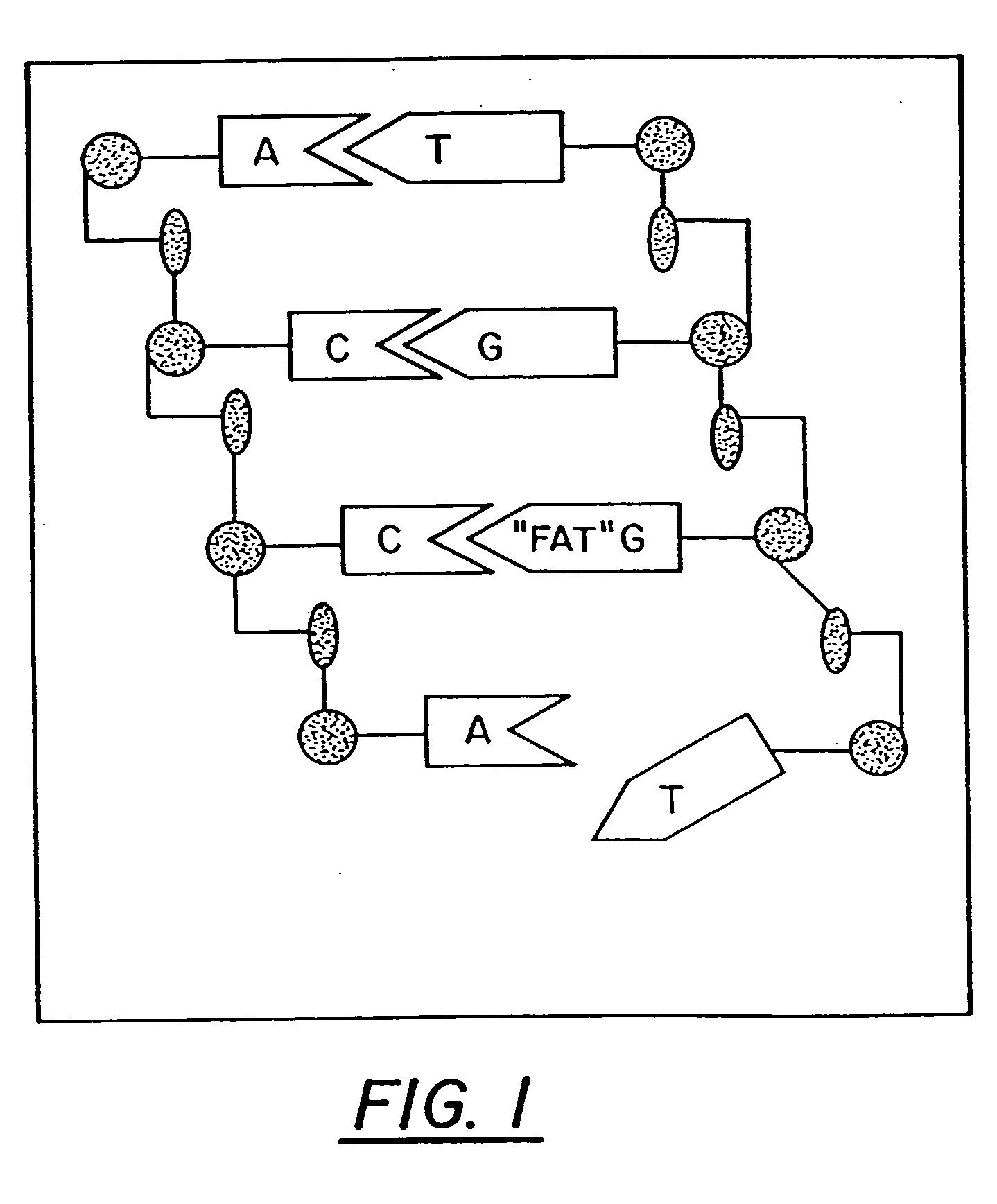
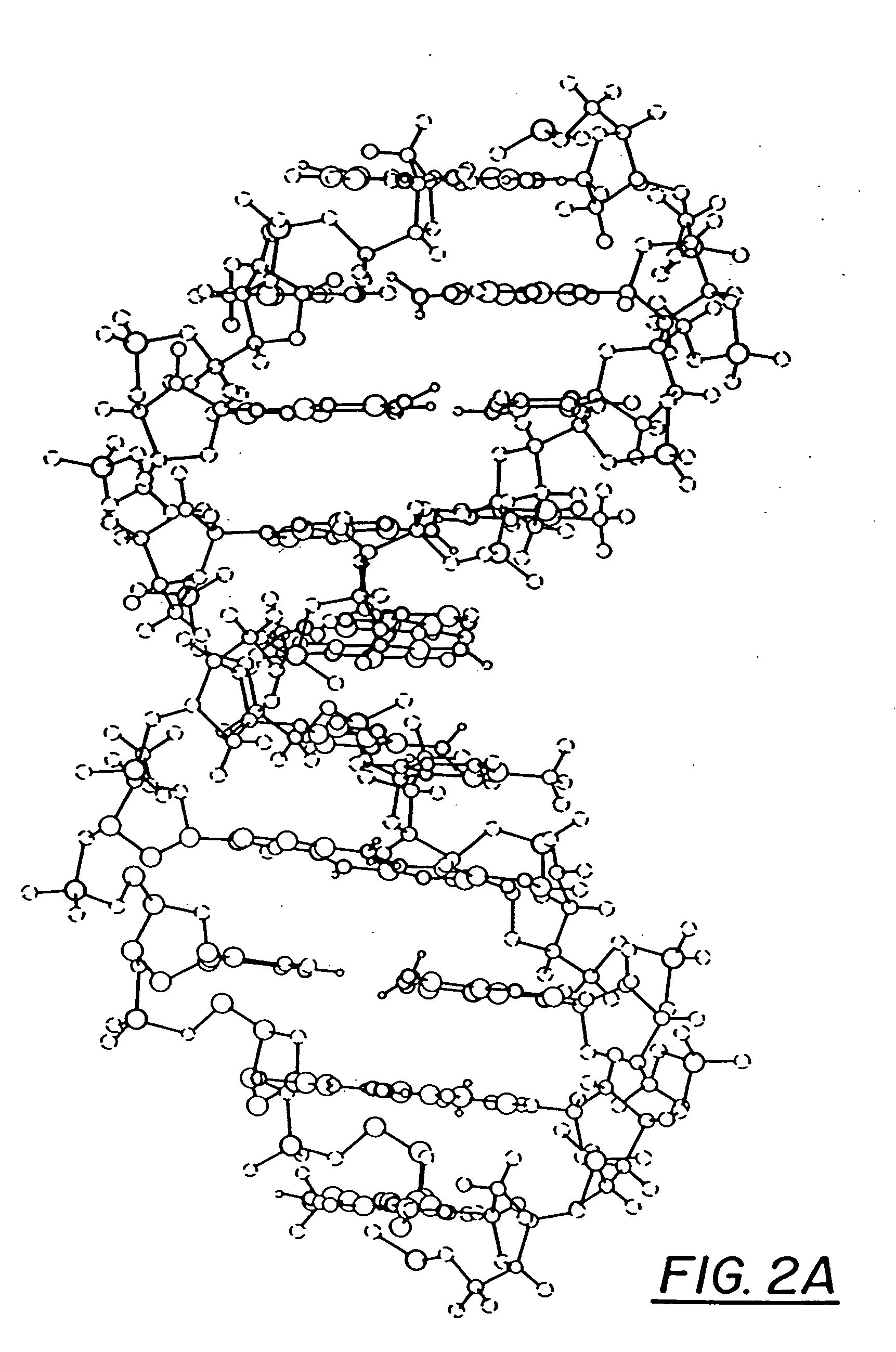
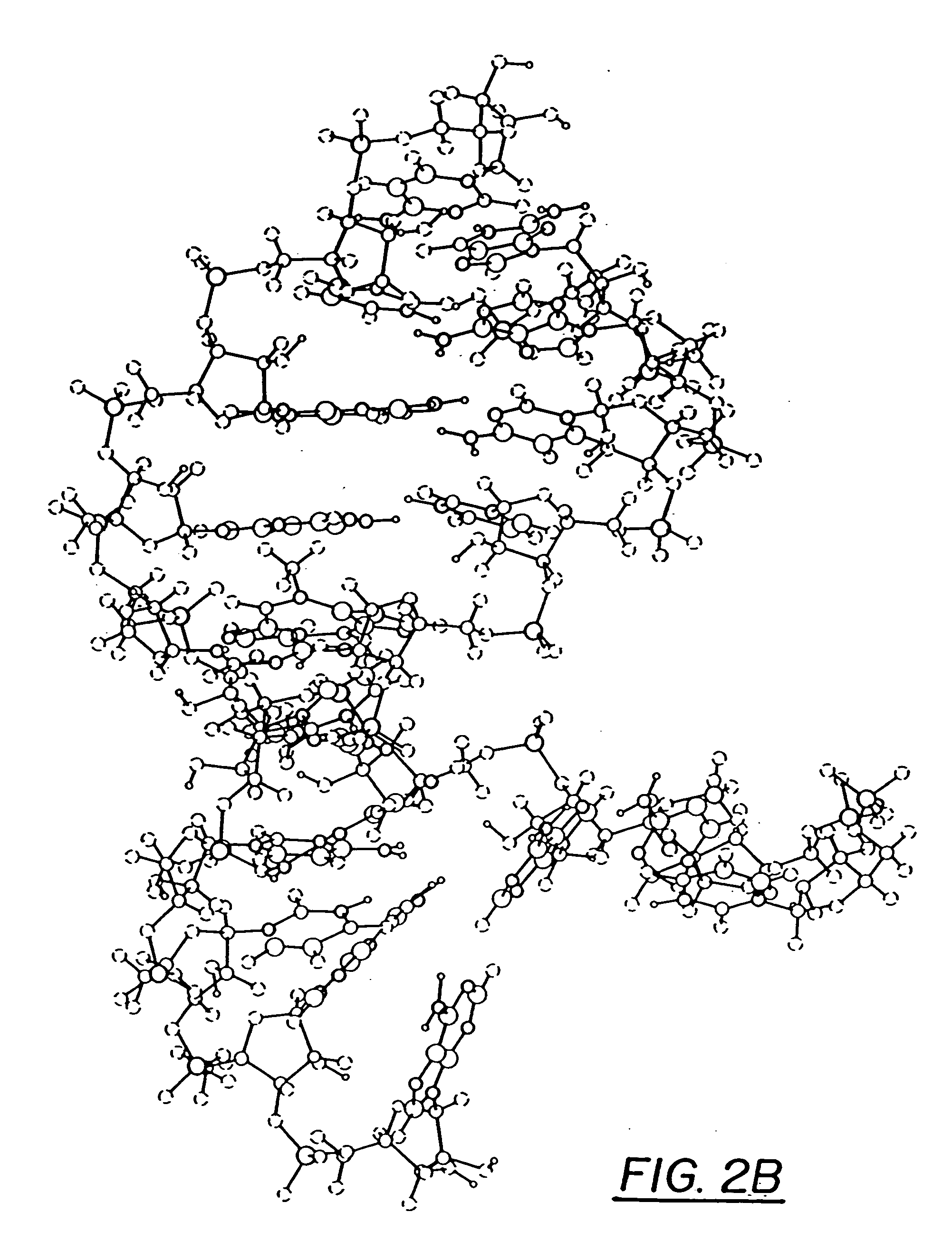
InactiveUS20060241065A1Good treatment effectImprove therapeutic indexBiocideSugar derivativesNucleotidePurine
The present invention relates to compositions comprising analogues of purine nucleosides containing a ring-expanded (“fat”) heterocyclic ring, in place of purine, and an unmodified or modified sugar residue, pharmaceutically acceptable derivatives of such compositions, as well as methods of use thereof. In particular, these compositions may be utilized in the treatment of certain cancers, bacterial, fungal, parasitic, and viral infections, including, but not limited to, Acquired Immunodeficiency Syndrome (AIDS), hepatitis, Epstein-Barr and cytomegalovirus.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY +1
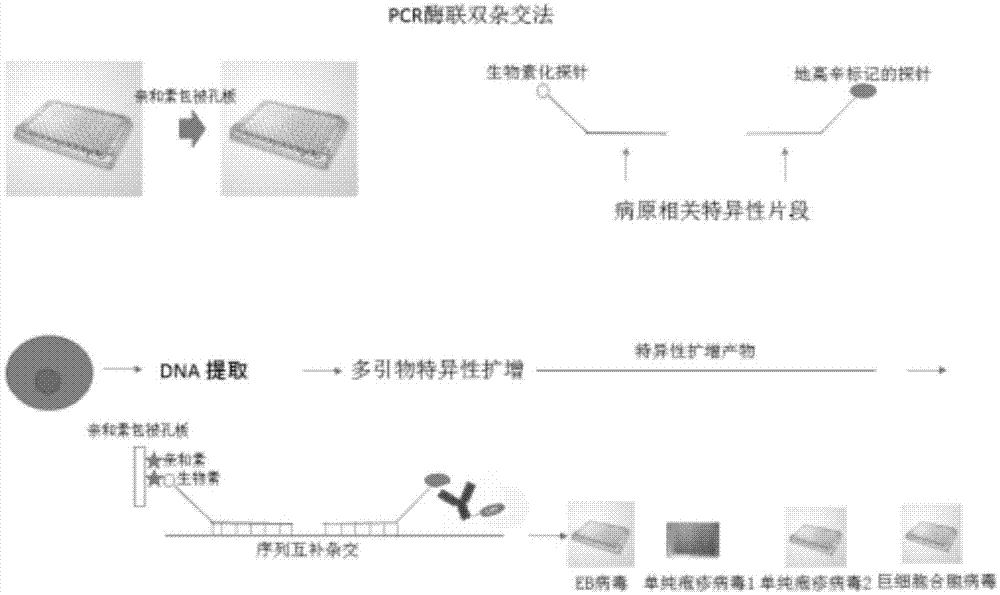
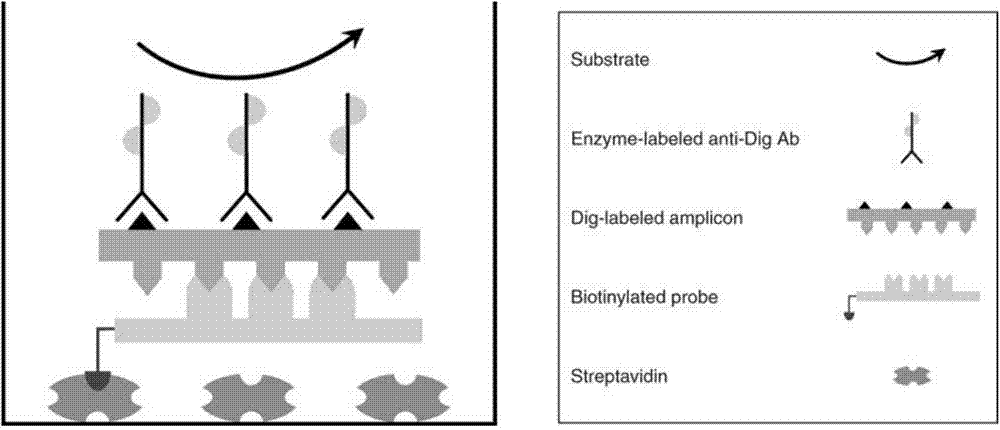
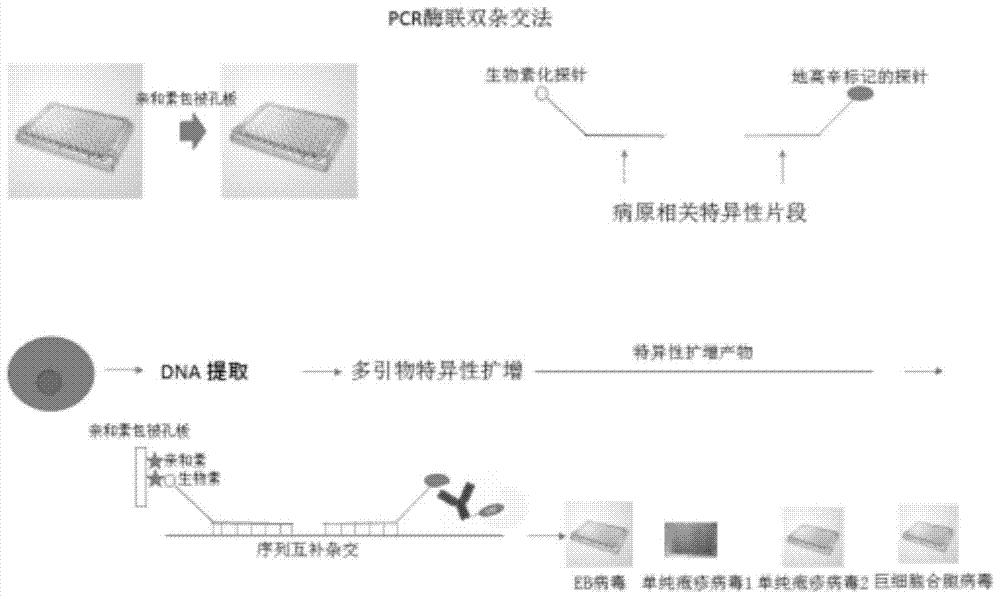
Method for detecting pathogenic microorganisms by using PCR (polymerase chain reaction) enzyme-linked double-cross method
ActiveCN104726606AFlexible and convenient to useFlexible solid phase junctionMicrobiological testing/measurementDNA hybridisationMultinucleate giant cell
The invention provides a method for detecting pathogenic microorganisms by using a PCR (polymerase chain reaction) enzyme-linked double-cross method, belongs to the technical field of clinical medical microorganism detection, and particularly relates to a synchronous qualitative and quantitative detection method for various microorganisms. In the method, DNA (deoxyribonucleic acid) amplification, DNA hybridization and enzyme-linked immunosorbent assay reaction are used, a solid-phase surface enveloping technology is adopted, a specificity conservative segment is amplified, an amplified product is combined to a capturing probe and is adsorbed to a solid-phase surface, far-end hybridization of an enzyme-linked probe is carried out, and a quantitative color product is generated by specific antibody recognition and reaction of enzyme and a substrate. The detected pathogenic microorganisms comprise EB (Epstein Barr) viruses, giant cell and cell viruses, herpes simplex viruses 1 and herpes simplex viruses 2. Various microorganisms of a clinical sample are detected simultaneously, and four types of viruses are screened by a reaction. The method has the advantages that the time and the cost are saved, application is flexible, the repeatability, the stability, the sensitivity and the specificity are high, and the method is simple and is easy to implement. Moreover, the method is suitable for widely detecting pathogenic microorganisms and is particularly suitable for a basic medical institution.
Owner:NANJING AIPEIJIE BIOLOGICAL TECH CO LTD
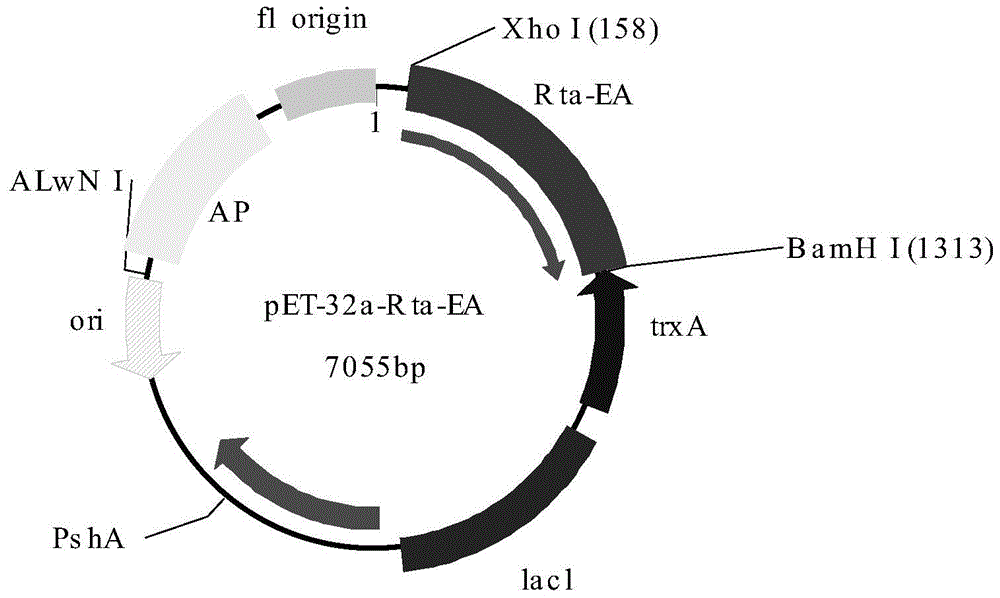
Artificial antigen and kit for joint detection of Rta protein antibody of epstein-barr (EB) virus and early antigen ethyl acrylate (EA) antibody of EB virus
ActiveCN104086657ASimple stepsImprove understandingMicroorganism based processesBiological testingTrue positive rateNasopharyngeal carcinoma
The invention relates to the technical fields of biotechnology, medical immunology and in vitro serological diagnosis, and particularly relates to an artificial antigen and a kit for joint detection of an Rta protein antibody of an epstein-barr (EB) virus and an early antigen ethyl acrylate (EA) antibody of the EB virus. The Rta protein antibody of the EB virus and the early antigen EA antibody existing in a to-be-detected sample can be accurately, efficiently, sensitively and specifically detected by the artificial antigen provided by the invention. By adopting the kit prepared from the artificial antigen, compared with the traditional detection method, the sensitivity and the specificity of nasopharynx cancer diagnosis are improved, the detection time is shortened, a patient can be timely treated, and the pain is relieved.
Owner:同昕生物技术(北京)有限公司
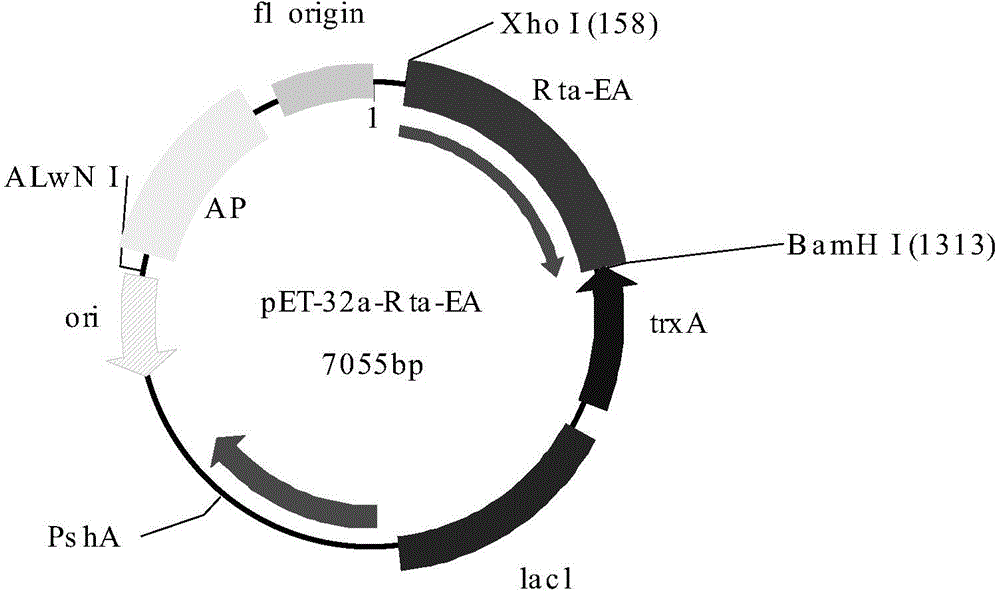
Reaction carrier and kit for detecting Epstein Barr (EB) virus replication and transcription activator (Rta)-immunoglobulin G (IgG) antibody
The invention discloses a reaction carrier and a kit for detecting an Epstein Barr (EB) virus replication and transcription activator (Rta)-immunoglobulin G (IgG) antibody. The reaction carrier for immunological detection of the EB virus Rta-IgG antibody is coated with gene recombinant EB virus Rta protein, and the gene recombinant EB virus Rta protein is obtained by expression of Chinese hamster ovary (CHO) cell line CHO / RTA with the preservation number of China General Microbiological Culture Collection Center (CGMCC) 6955. The kit comprises the reaction carrier. Clinical experiments show that the kit for the quantitative detection of the EB virus Rta-IgG is easy and convenient to operate, high in sensitivity and good in specificity.
Owner:同昕生物技术(北京)有限公司
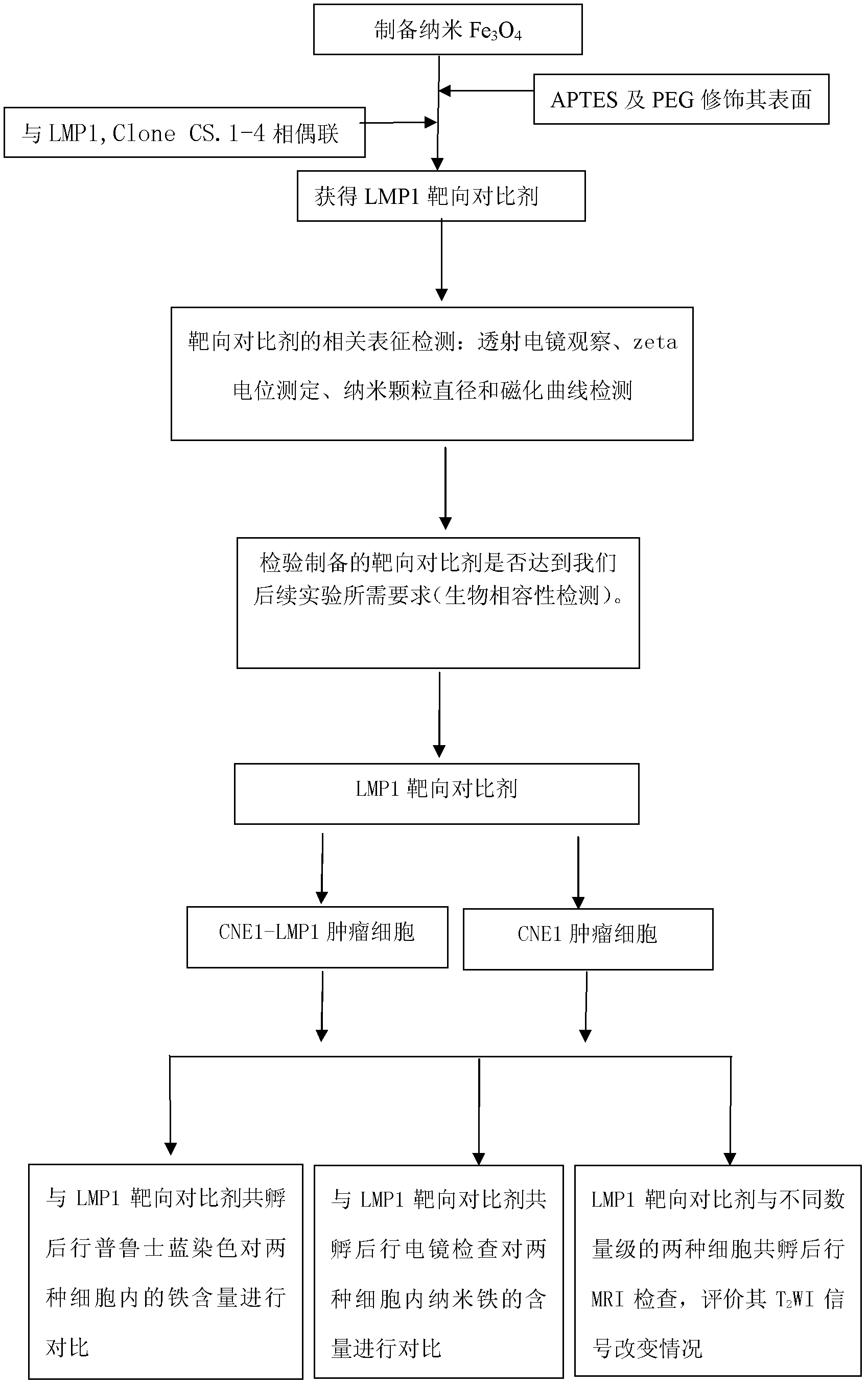
Nasopharyngeal carcinoma targeted magnetic resonance contrast agent and preparation method thereof
ActiveCN102552944AImprove securityMorphology has no obvious effectIn-vivo testing preparationsLatent Membrane Protein-1Biocompatibility Testing
The invention discloses a nasopharyngeal carcinoma targeted magnetic resonance contrast agent and a preparation method thereof. The method comprises the following steps: synthesizing superparamagnetic Fe3O4 with grain diameter of between 10 and 15nm by adopting a chemical coprecipitation method, coating with APTES ((3-aminopropyl)triethoxysilane) or connecting superparamagnetic Fe3O4 to 10-15nm Fe3O4 to carry out surface amination on Fe3O4 to obtain Fe3O4-APTES surface-modified micro-particles; and connecting Fe3O4-APTES and EB (Epstein-Barr) virus latent membrane protein 1 monoclonal antibody (LMP1, Clone CS.1-4) with polyethylene glycol (PEG) as a connecting arm to obtain Fe3O4-APTES-PEG-LMP1, Clone CS.1-4 colloidal solution with stable dispersion. Compared with a conventional contrast agent, the contrast agent has the advantages of low toxicity, high stability, good biocompatibility, high sensitivity and good specific targeting property to LMP1<+>-nasopharyngeal carcinoma, and can be used for nasopharyngeal carcinoma screening and specific magnetic resonance diagnosis.
Owner:CENT SOUTH UNIV
Hypersensitivity Epstein-Barr (EB) virus fluorescence quantitative polymerase chain reaction (PCR) kit for locked nucleotide acid (LNA) and detection method and application thereof
InactiveCN101899528AShort detection timeEasy to operateMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
The invention provides a hypersensitivity Epstein-Barr (EB) virus fluorescence quantitative polymerase chain reaction (PCR) kit for locked nucleotide acid (LNA). The kit comprises LNA-TaqMan reaction liquid, a positive control sample, a forward primer, a reverse primer, and an LNA-TanMan fluorescent probe, wherein the forward primer is 5'-AATTTTTTCTGCTAAGCCCAACA-3'; the reverse primer is 5'-ACGGGTGGGTGTGTGTAGTGT-3'; and the LNA-TanMan fluorescent probe is 5'-FAM-CCACCACACCCAGGC-MGB3'. The invention also provides a hypersensitivity EB virus fluorescence quantitative PCR detection method for the LNA and the application of the kit in the detection of EB viruses, the diagnosis and treatment of nasopharyngeal darcinoma, prognosis judgment and the monitoring of recurrence and transfer after the treatment of the nasopharyngeal darcinoma. The kit of the invention has the advantages of rapidness, high sensitivity, simple operation process, large detection sample size and safety.
Owner:广州达健生物科技有限公司
Preparation method of EB (Epstein-Barr) virus antigen and quick detection kit for detecting EB virus antibody prepared from antigen
InactiveCN106188248AHigh antigen yieldImproving immunogenicityVirus peptidesDsDNA virusesEscherichia coliInclusion bodies
The invention relates to an EB virus gene engineering artificial expression antigen and a method for preparing the antigen. The method comprises the following steps: artificially synthesizing a fused EB virus capsid proteantigen gene sequence, establishing a prokaryotic expression vector, expressing the EB virus capsid proteantigen in Escherichia coli, and renaturating the inclusion body by a dialyssis process, a gradient dilution process and gelchromatography to obtain the recombinant EB virus capsid proteantigen with the three-dimensional structure and immunocompetence. The invention also relates to a quick detection method for detecting an EB virus antibody. The method comprises the following step: using the EB virus capsid proteantigen. The invention also relates to a quick detection kit for EB virus antibody detection. The kit comprises the EB virus capsid proteantigen which can be directly used for whole blood detection. The kit comprises a rheumatism factor treatment pad which can be used for removing rheumatism factors in a sample and directly detecting IgM in the sample. The invention provides an EB virus antigen which has high specificity. The invention also provides a method for preparing the antigen, a method for quickly detecting the EB virus antibody and a kit for quickly detecting the EB virus antibody.
Owner:LANZHOU YAHUA BIOTECH
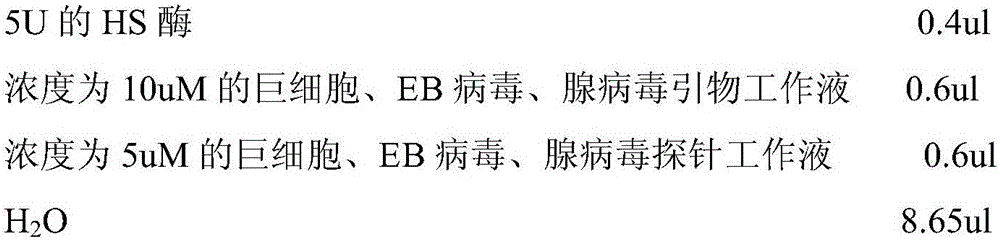
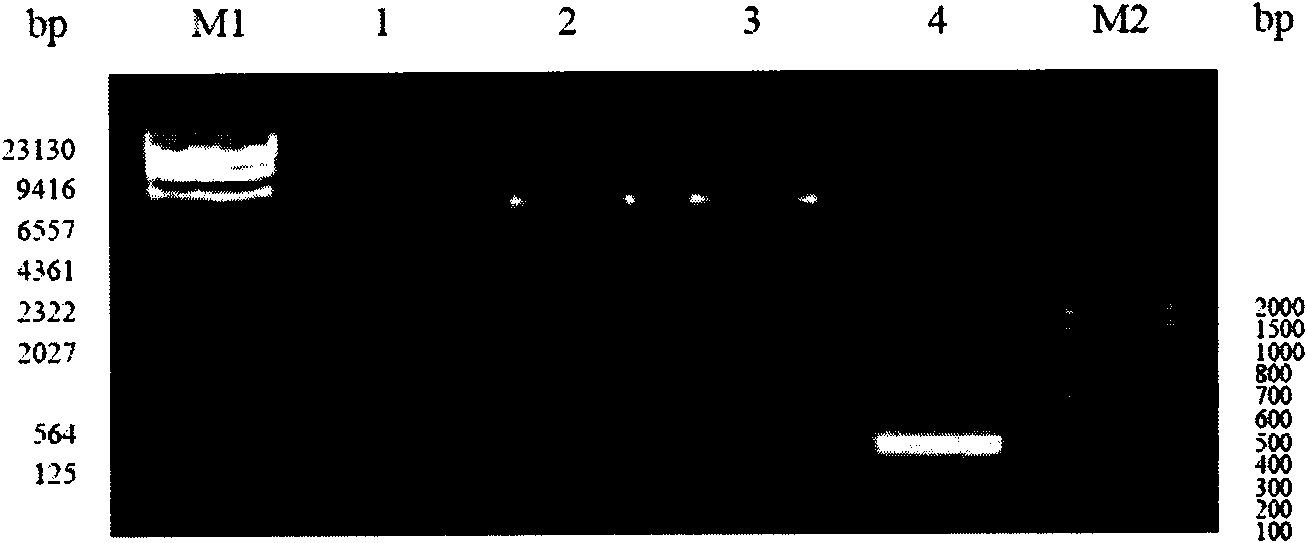
Primer-probe assembly for detecting cytomegalovirus, EB (epstein-barr) virus and adenovirus
InactiveCN106191308AAvoid pollutionHigh degree of automationMicrobiological testing/measurementMicroorganism based processesFluorescenceRespiratory infection
The invention relates to primers and probes for detection of various respiratory viruses and provides a primer-probe assembly for detecting cytomegalovirus, EB (epstein-barr) virus and adenovirus. Sequences of a probe and a primer pair for detecting the cytomegalovirus, the EB virus and the adenovirus are as shown in SEQ ID NO:1-9. The invention further provides a kit for simultaneous detection of the cytomegalovirus, the EB virus and the adenovirus. By combination of multiplex PCR (polymerase chain reaction) and Taqman fluorescent probe technique, simultaneous detection of various pathogens possibly causing acute respiratory infection can be realized in one-time detection, and quickness in diagnosis and treatment guide is realized. In addition, shortening of operation time and reduction of raw material consumption are realized, and high sensitivity and specificity, effectiveness in solving of PCR pollution, high automation degree and the like are achieved.
Owner:ZHEJIANG UNIV
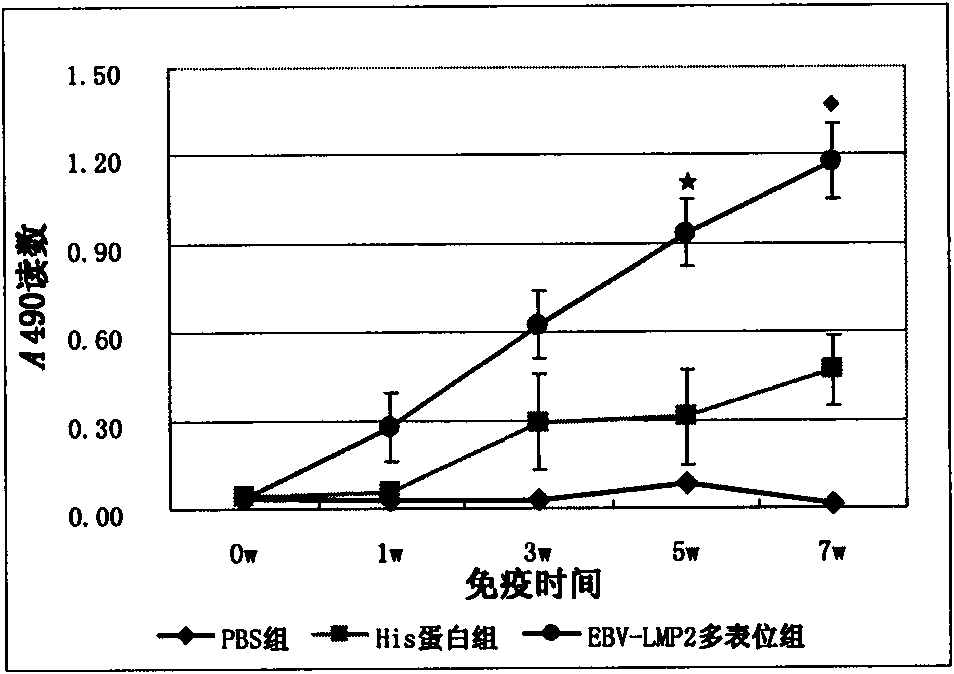
Multi-epitope recombinant protein of epstein-barr (EB) virus latent membrane protein 2 and application thereof
The invention relates to preparation and application of multi-epitope recombinant protein of epstein-barr (EB) virus latent membrane protein 2. The invention discloses the multi-epitope recombinant protein rich in a plurality of CTL epitopes, Th epitopes and B cell epitopes obtained by screening based on the full-length EB virus latent membrane protein 2. The invention also discloses the coding nucleic acid of the protein, and comprises a nucleic acid recombinant vector and a host cell. The invention also discloses the application of the protein in the aspects of preventing, treating and diagnosing EB virus infection and related disease thereof. The protein of the invention has very strong immunogenicity and antigenicity and good application prospect.
Owner:WENZHOU MEDICAL UNIV
Primer, reagent kit and method for detecting EB (Epstein-Barr) virus
InactiveCN102168149AIncreased sensitivityAvoid false negativesMicrobiological testing/measurementDNA/RNA fragmentationForward primerNucleotide
The invention provides a primer, reagent and method for detecting EB (Epstein-Barr) virus. A forward primer nucleotide sequence of the primer is SEQ ID NO: 1, and a reverse primer nucleotide sequence of the primer is SEQ ID NO: 2. The invention also provides a combination, tool, reagent and method for detecting the EV virus, wherein the combination comprises the primer. Through the combination, the tool, the reagent and the method, rapid detection can be performed at the gene level, sensitivity is high and specificity is good, and the advantages of time saving, mature technology and stable detection results are achieved.
Owner:宁波基内生物技术有限公司
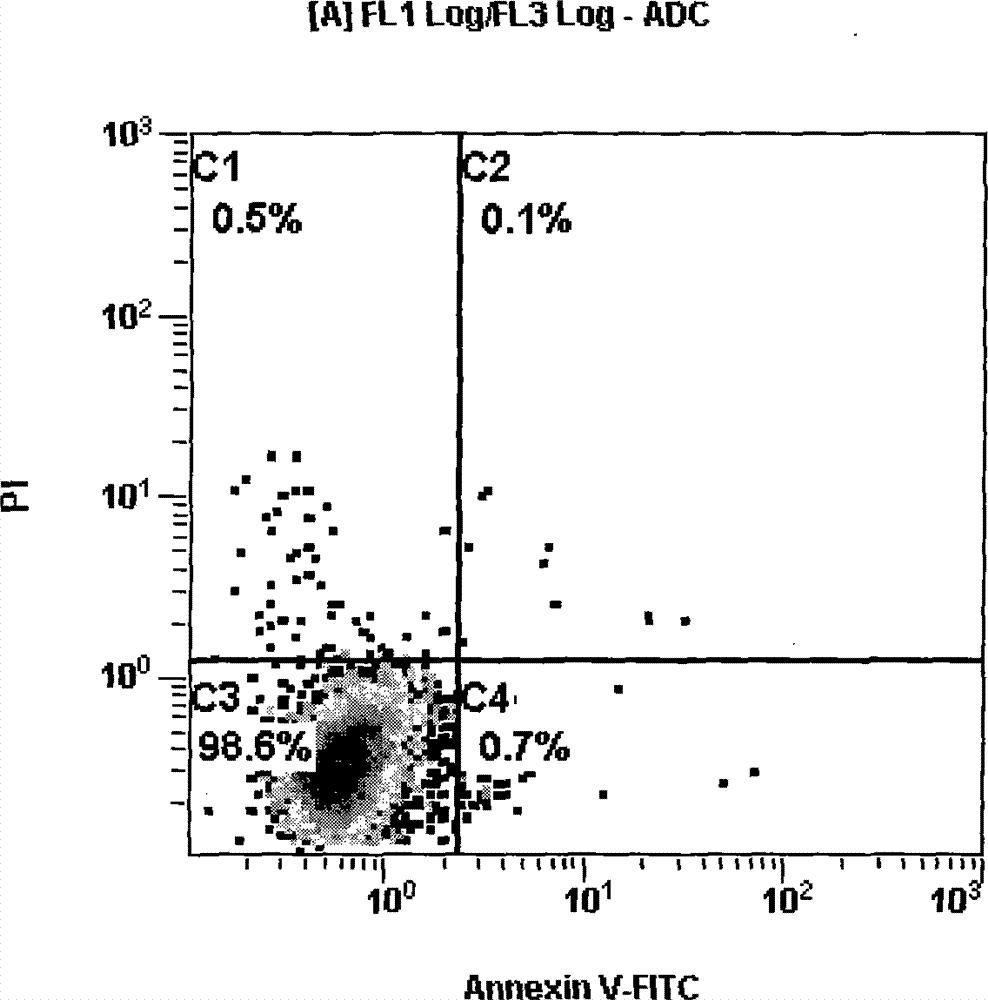
Identification method of EB (Epstein-Barr) virus transformation cell apoptosis of excellent ice athletes
The invention discloses an identification method of EB (Epstein-Barr) virus transformation cell apoptosis of excellent ice athletes. The implementation steps of the identification method of the EB virus transformation cell apoptosis of the excellent ice athletes are shown in a specific implement mode part. The identification method provided by the invention provides a researching mode for the EB virus transformation cell apoptosis of the excellent ice athletes, the EB virus transformation cell apoptosis conditions of the excellent ice athletes are subjected to quantitative analysis, according to the identification method, viable apoptotic cells, non-viable apoptotic cells and non-viable non-apoptotic cells can be distinguished quantitatively, and the effect and the accuracy are reliable through the electron microscope inspection and Tunel method inspection.
Owner:HARBIN INST OF PHYSICAL EDUCATION
Peptides and nucleic acid sequences related to the Epstein Barr Virus
InactiveUS7507804B2Effective protectionEasy to insertAntibody mimetics/scaffoldsVirus peptidesAnti-EBV AntibodyViral nucleic acid
The present invention relates to peptides immunochemically reactive with antibodies to the Epstein-Barr virus (EBV), nucleic acid sequences encoding these peptides, monoclonal antibodies against these peptides, cell lines capable of producing monoclonal antibodies and anti-idiotype antibodies. The invention also relates to recombinant vector molecules comprising a nucleic acid sequence according to the invention and host cells transformed or transfected with these vector molecules. The invention is further concerned with immunological reagents and methods for the detection of EBV or anti-EBV antibodies and a method for the amplification and detection of Epstein Barr viral nucleic acid.
Owner:AKZO NOBEL NV
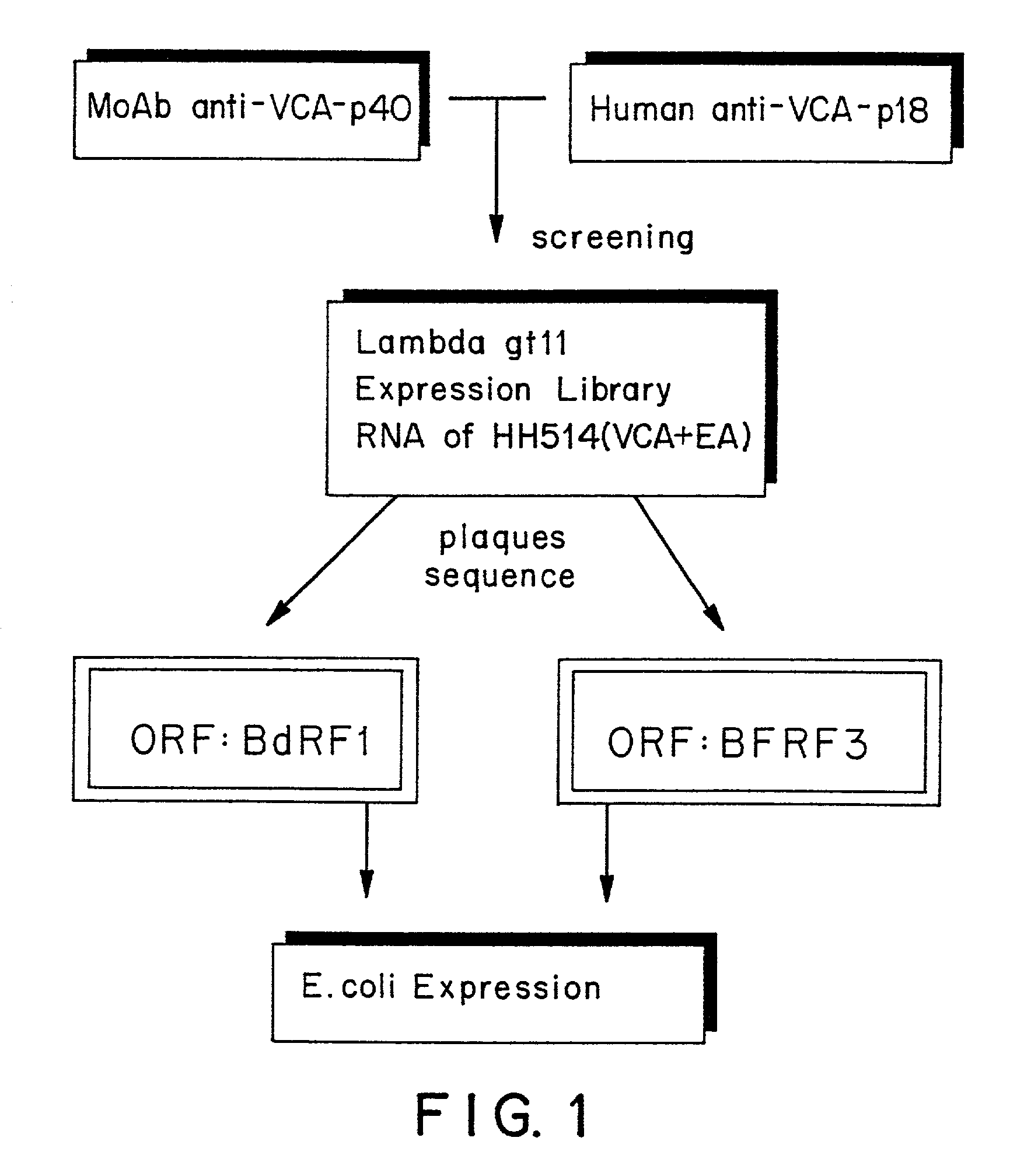
Method for detecting specificity of Epstein-Barr viruses through Epstein-Barr virus p18-p23 fused capsid antigen
The invention discloses a method for detecting specificity of Epstein-Barr viruses (EBV) through an Epstein-Barr virus p18-p23 fused capsid antigen. The method is characterized in that an overlap extension polymerase chain reaction (PCR) technology is adopted; a coding gene p23 and a coding gene p18 are fused in vitro by a coding sequence of an intermediate head (Gly4Ser)3; a fused protein is expressed and the expressed fused protein is purified and is subjected to specificity identification through an immunoblotting technology; the purified fused protein is coated on an enzyme-linked immuno sorbent assay (ELISA) reaction plate and component content and reaction conditions are optimized; commercial horseradish peroxidase-labelled goat anti-human IgM and IgG and a series of other reagents are prepared; and EBV-viral cuspid antigen (VCA) specific IgM and IgG indirect ELISA diagnostic kit is obtained by assembling.
Owner:邱清芳
Novel EB (Epstein-Barr) virus EBNA1 epitope peptide and application thereof in diagnosis, treatment and prevention of EBV related diseases
InactiveCN105693828AHigh affinitySignificant application valueVirus peptidesAntiviralsDiseaseT lymphocyte
The invention provides novel EB (Epstein-Barr) virus EBNA1 and application thereof in the diagnosis, treatment and prevention of EBV related diseases and discloses HLA-A*0201 limiting epitope peptide of EBNA1 in novel V-val subtype EB virus, this peptide having an amino acid sequence shown as GIALAVPQC; and discloses a method for preparing T-lympphocyte specifically reacting with EBNA1 by using the epitope peptide; and application of cells cultured by this method in the preparation of pharmaceutical compositions for preventing and treating EBV related diseases.The invention also relates to application of HLA-A*0201 limiting epitope peptide of the EBNA1 in the in-vitro diagnostic reagents and immunotherapy or preventive vaccines for EBV related diseases.Compared with corresponding sequences of common B95-8 viral strains, this epitope peptide has higher affinity with HLA-A*0201 molecules.
Owner:深圳市中美康士生物科技有限公司
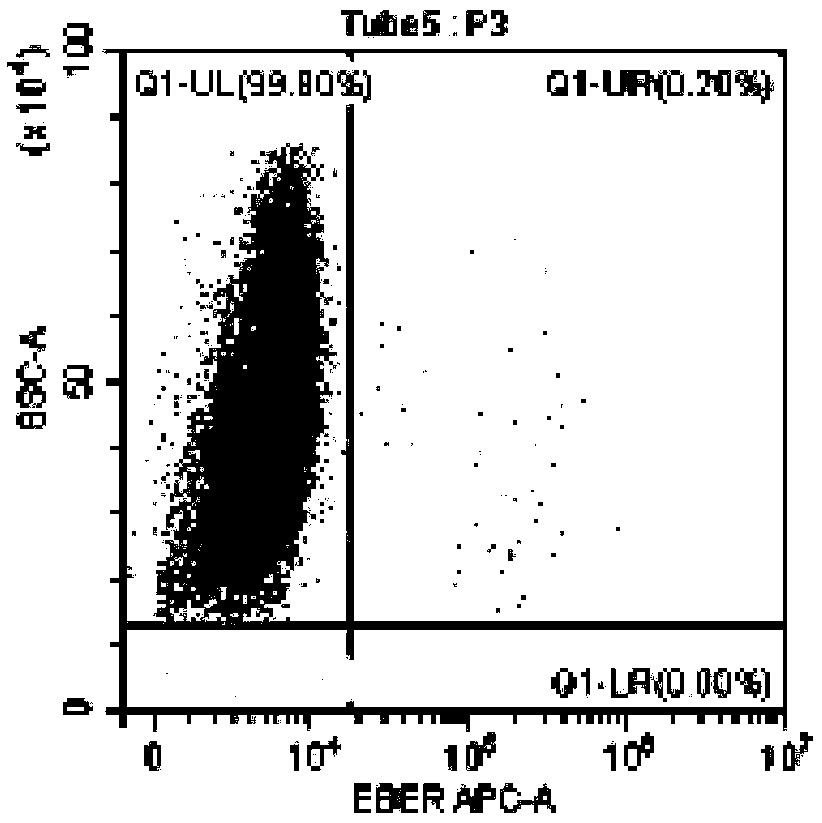
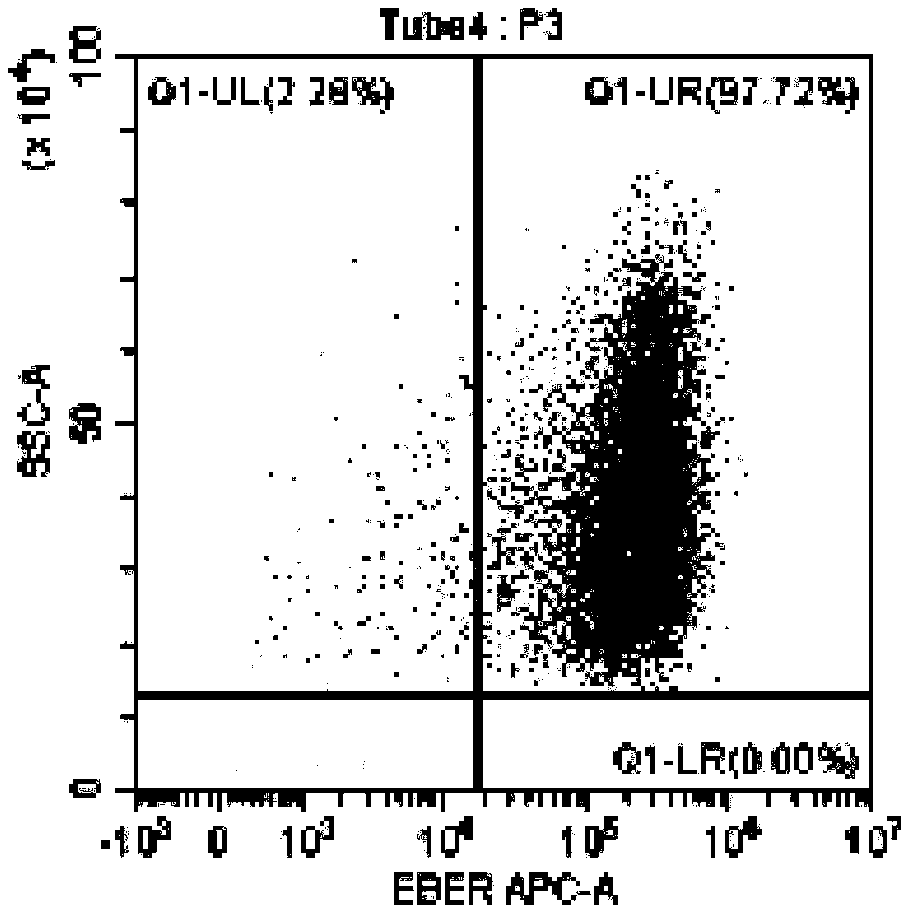
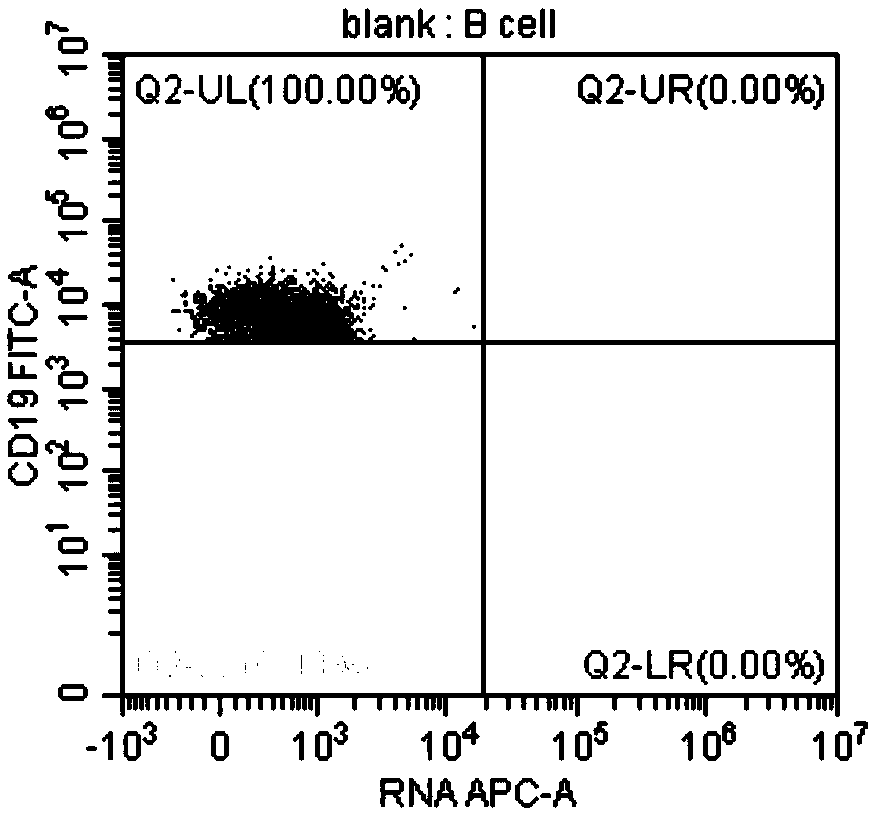
Method for identifying lymphocyte subpopulations infected by EB (epstein-barr) virus and proportion of injected cells in lymphocyte subpopulations and application of method
InactiveCN109136333APrecision therapyEffective treatmentMicrobiological testing/measurementInfected cellDisease
The invention provides a method for identifying lymphocyte subpopulations infected by an EB (epstein-barr) virus and a proportion of injected cells in the lymphocyte subpopulations and application ofthe method, and relates to the technical field of medical test. According to the method, a probe is mixed with a mononuclear cell extracted from preprocessed peripheral blood, and after hybridization,a flow cytometry measurement method is adopted to acquire and analyze to identify the lymphocyte subpopulations infected by the EB virus and the proportion of the injected cells in the lymphocyte subpopulations. The method has the advantages that the type of cells infected by the EBV (epstein-barr virus) can be found out accurately, the proportion of the infected cells in the lymphocyte subpopulations can be found out, according to the type of the cells infected by the EBV and the proportion of the infected cells, a targeted therapeutic schedule can be beneficially made to effectively and accurately treat diseases caused by EBV injection, and accordingly the method plays important roles in implement of accurate therapy and prognosis improvement of a patient.
Owner:北京倍科为生物技术有限公司
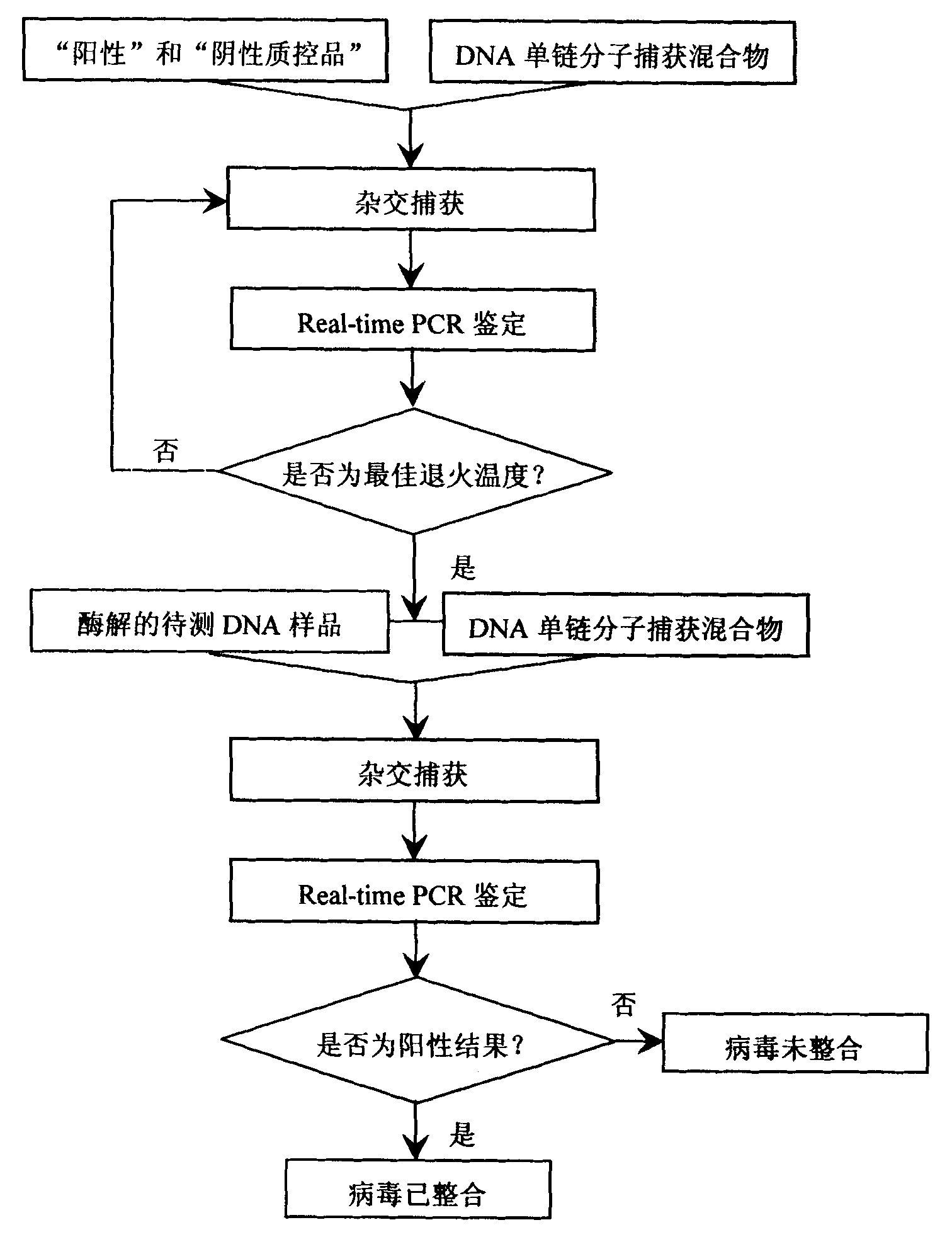
Kit for detecting integrated viruses of genome in hybrid capture
InactiveCN101974652AMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingBiomedicine
The invention belongs to the field of biomedicine, in particular to a kit for detecting integrated viruses of a genome in hybrid capture. The kit comprises the following main components: (1) a solid phase medium fixed with a capture mixture of human genome DNA (Deoxyribonucleic Acid) single-chain molecules, (2) positive and negative quality control materials for identifying the hybrid capture efficiency and specificity, and (3) a primer pair and a molecular probe for carrying out real-time PCR (Polymerase Chain Reaction) identification to the captured mixture of the target DNA molecules, obtained by capture. By utilizing all or partial components in the invention, a complete integrated genome virus detection kit or an integrated genome virus detection kit with the type specificity can be assembled. The obtained virus detection kit can be used for detecting and identifying the integrated state of the genome in a certain common integrated and oncogenic viruses, such as human hepatitis B viruses, human papilloma viruses, Epstein-Barr viruses and human herpes simplex viruses, in tissue or cytology specimens.
Owner:何以丰
Recombinant vaccinia virus carrying EB virus latent membrane antigen 2 gene and application of recombinant vaccinia virus
InactiveCN107488677AEasy to take offEasy to markAntiviralsBlood/immune system cellsLymphocyteMembrane antigen
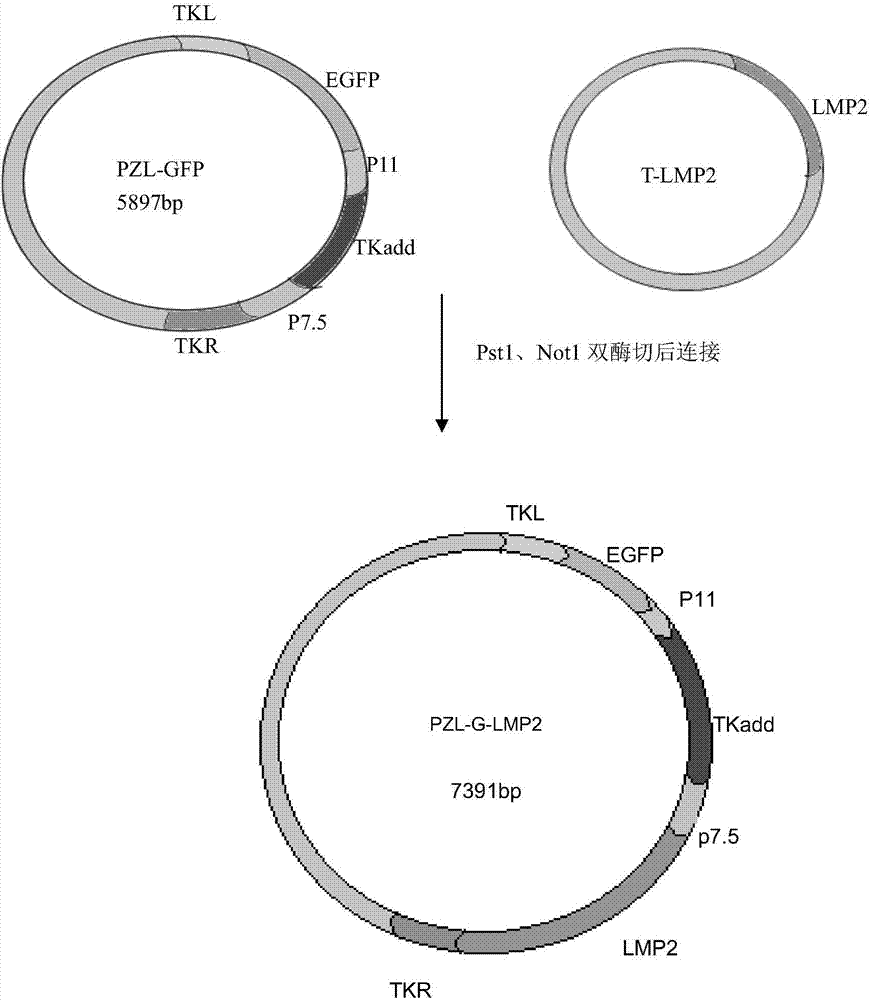
The invention provides a recombinant vaccinia virus carrying an EB (epstein-barr) virus latent membrane antigen 2 gene and an application of the recombinant vaccinia virus. A preparation method of the recombinant virus comprises the steps of constructing a joint recombinant plasmid and MVA (modified vaccinia virus ankara) infection BHK-21 cell carrying an EBV (epstein-barr virus) LMP2 exogenous gene and a green fluorescin exogenous gene, and obtaining an MVA recombinant virus (MVA-LMP2A) only carrying the EBV LMP2 exogenous gene. Mouse spleen lymphocytes immunized by MVA-LMP2A are detected by an IFN (interferon)-gamma immunodotting method, and a result shows that MVA-LMP2A can well induce EBV LMP2 specific immune response generated in a body of a mouse.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Nasopharyngeal carcinoma targeted magnetic resonance contrast agent and preparation method thereof
ActiveCN102552944BImprove securityMorphology has no obvious effectIn-vivo testing preparationsLatent Membrane Protein-1Polyethylene glycol
The invention discloses a nasopharyngeal carcinoma targeted magnetic resonance contrast agent and a preparation method thereof. The method comprises the following steps: synthesizing superparamagnetic Fe3O4 with grain diameter of between 10 and 15nm by adopting a chemical coprecipitation method, coating with APTES ((3-aminopropyl)triethoxysilane) or connecting superparamagnetic Fe3O4 to 10-15nm Fe3O4 to carry out surface amination on Fe3O4 to obtain Fe3O4-APTES surface-modified micro-particles; and connecting Fe3O4-APTES and EB (Epstein-Barr) virus latent membrane protein 1 monoclonal antibody (LMP1, Clone CS.1-4) with polyethylene glycol (PEG) as a connecting arm to obtain Fe3O4-APTES-PEG-LMP1, Clone CS.1-4 colloidal solution with stable dispersion. Compared with a conventional contrast agent, the contrast agent has the advantages of low toxicity, high stability, good biocompatibility, high sensitivity and good specific targeting property to LMP1<+>-nasopharyngeal carcinoma, and can be used for nasopharyngeal carcinoma screening and specific magnetic resonance diagnosis.
Owner:CENT SOUTH UNIV
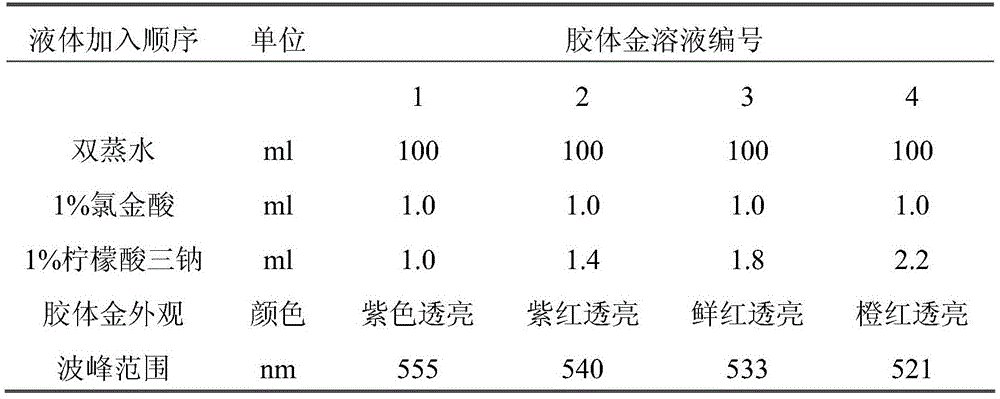
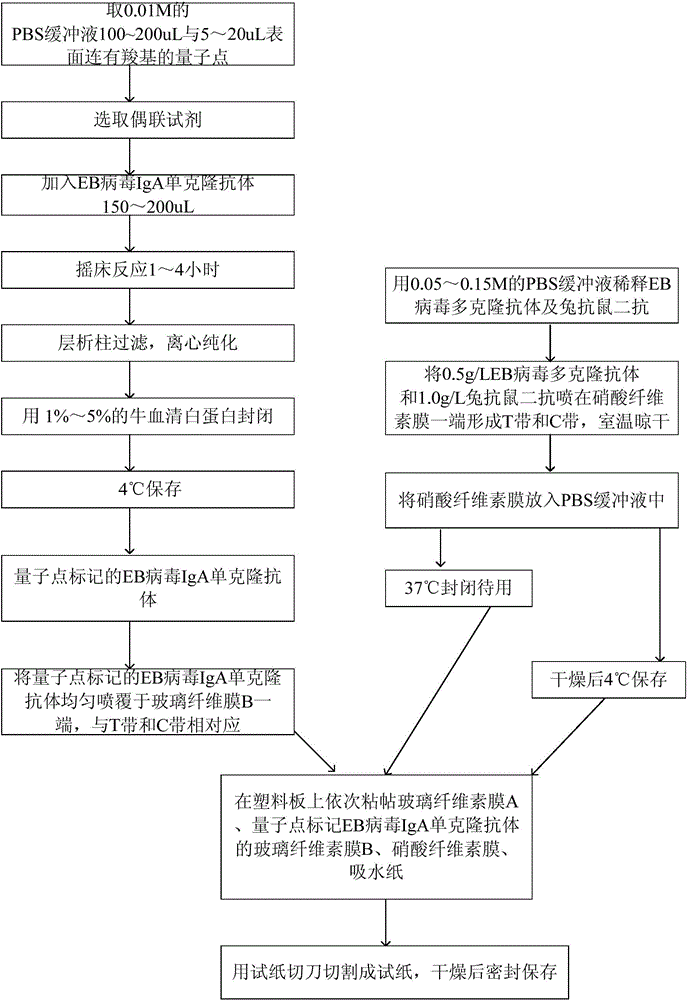
Method for detecting EB (Epstein-Barr) virus, quantum dot labelled immunochromatographic test strip and preparation method thereof
ActiveCN103543262AHigh detection sensitivityHigh detection sensitivity than a rapid detection method commonly used at present - the detection sensitivity of colloidal goldBiological material analysisCelluloseGlass fiber
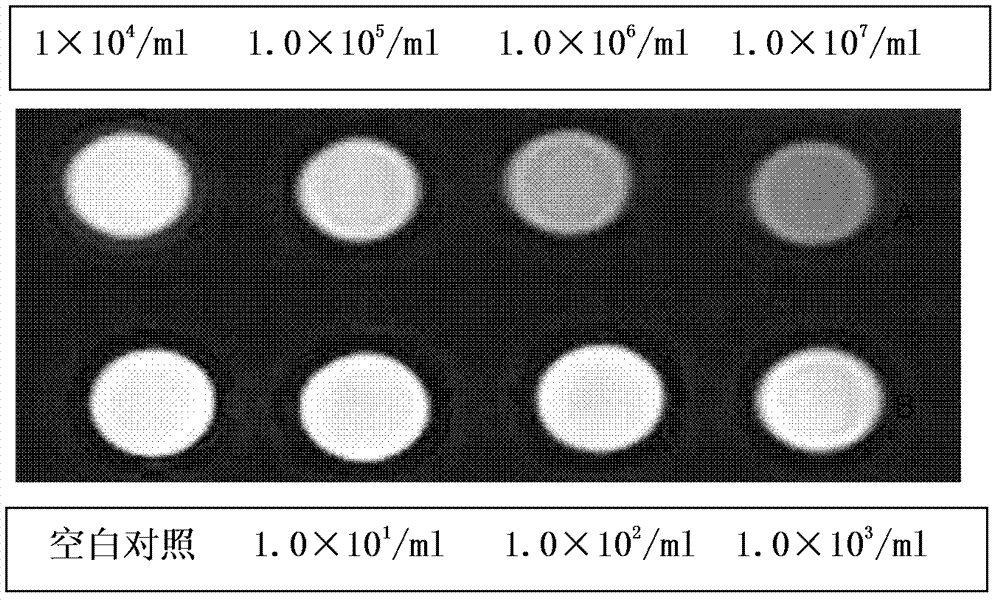
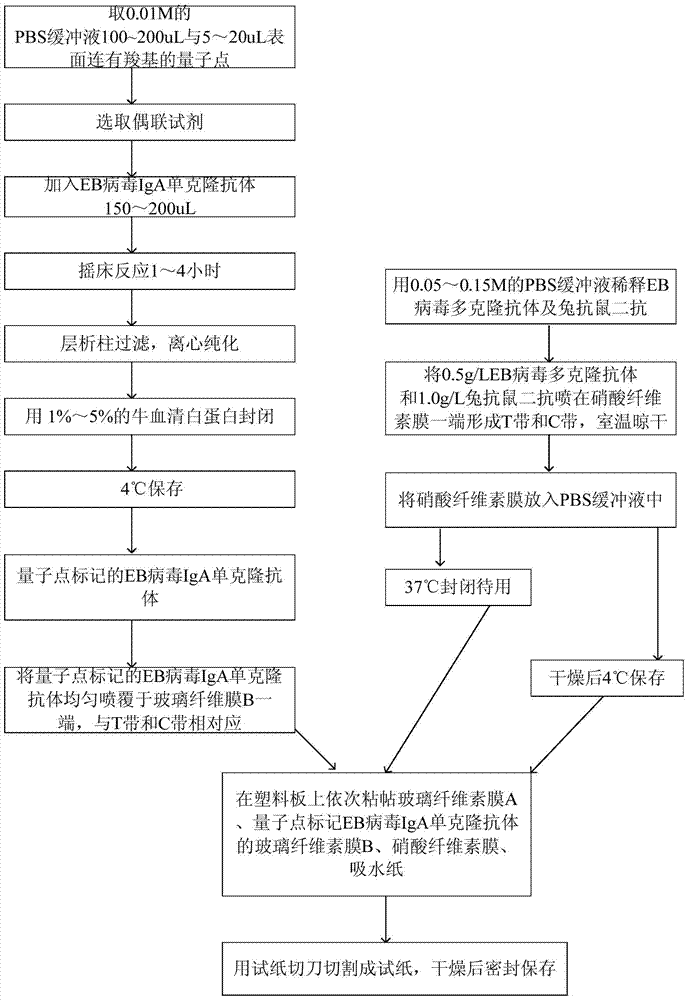
The invention relates to a medical immunodetection method and in particular relates to a method for detecting an EB (Epstein-Barr) virus by an immunological method by using a quantum dot labelled immunochromatographic test strip. The quantum dot labelled immunochromatographic test strip is characterized in that a glass cellulose membrane A, a glass cellulose membrane B of a quantum dot labelled EB virus IgA (immunoglobulin A) monoclonal antibody, a nitrocellulose membrane and absorbent paper are stuck to a plastic board from bottom to top in sequence, wherein one end of the nitrocellulose membrane is provided with an EB virus polyclonal antibody and a rabbit anti-mouse second antibody, thereby forming a detection zone T and a quality control zone C; the quantum dot labelled EB virus IgA monoclonal antibody is arranged at the other end of the glass cellulose membrane B, corresponds to the detection zone T and the quality control zone C and is arranged at one end of a sampling point. The detection sensitivity of the method is about 1000 times higher than that of the detection method frequently used at present.
Owner:北京华卫天和生物科技有限公司
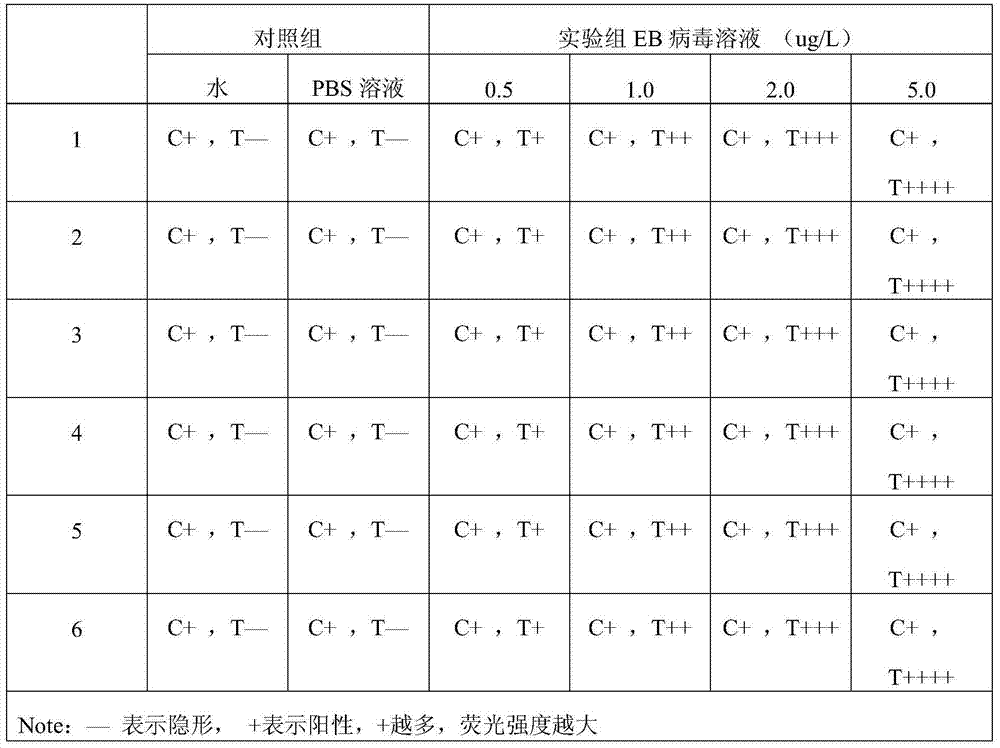
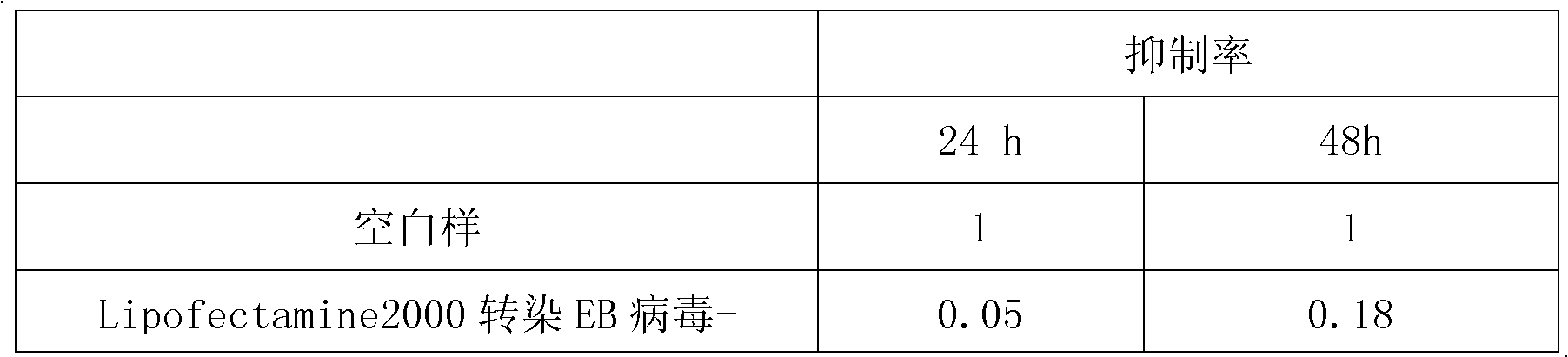
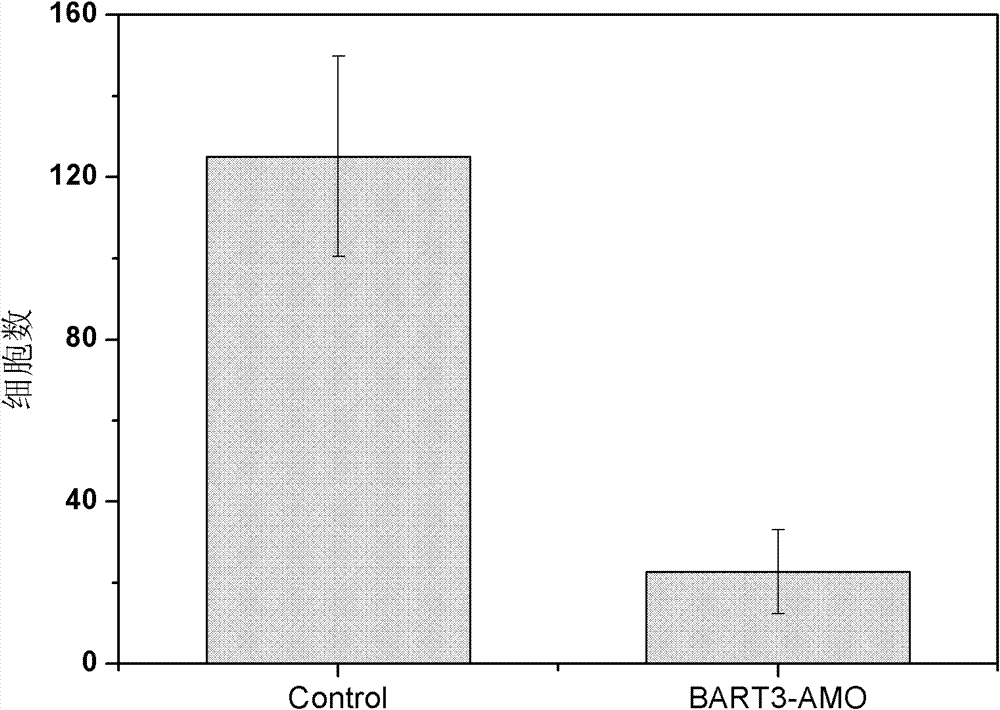
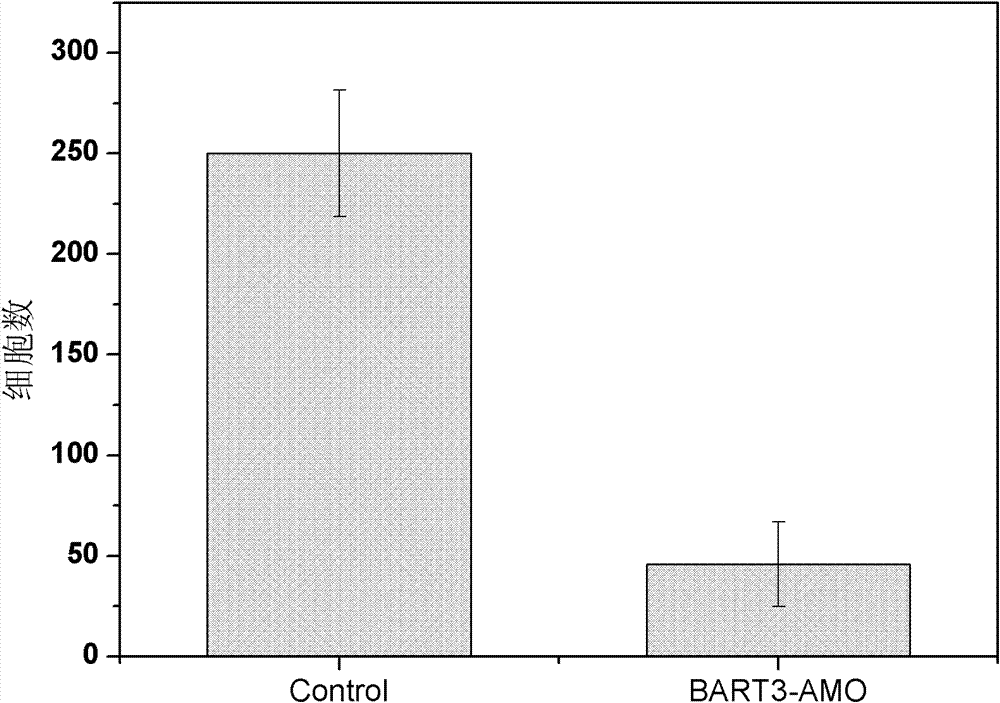
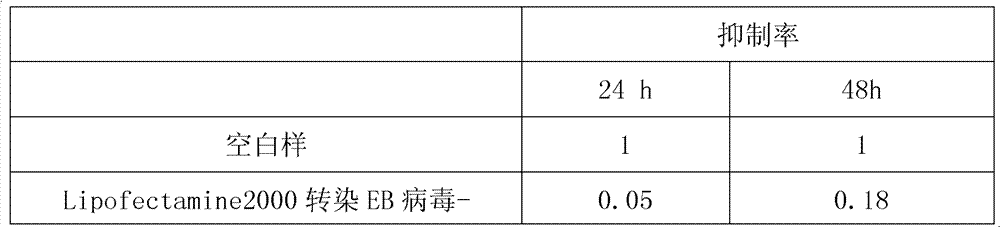
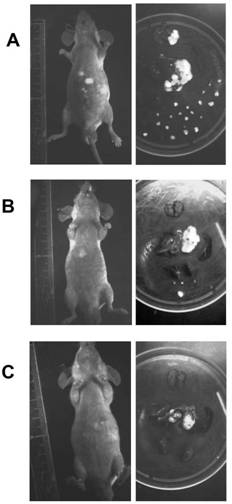
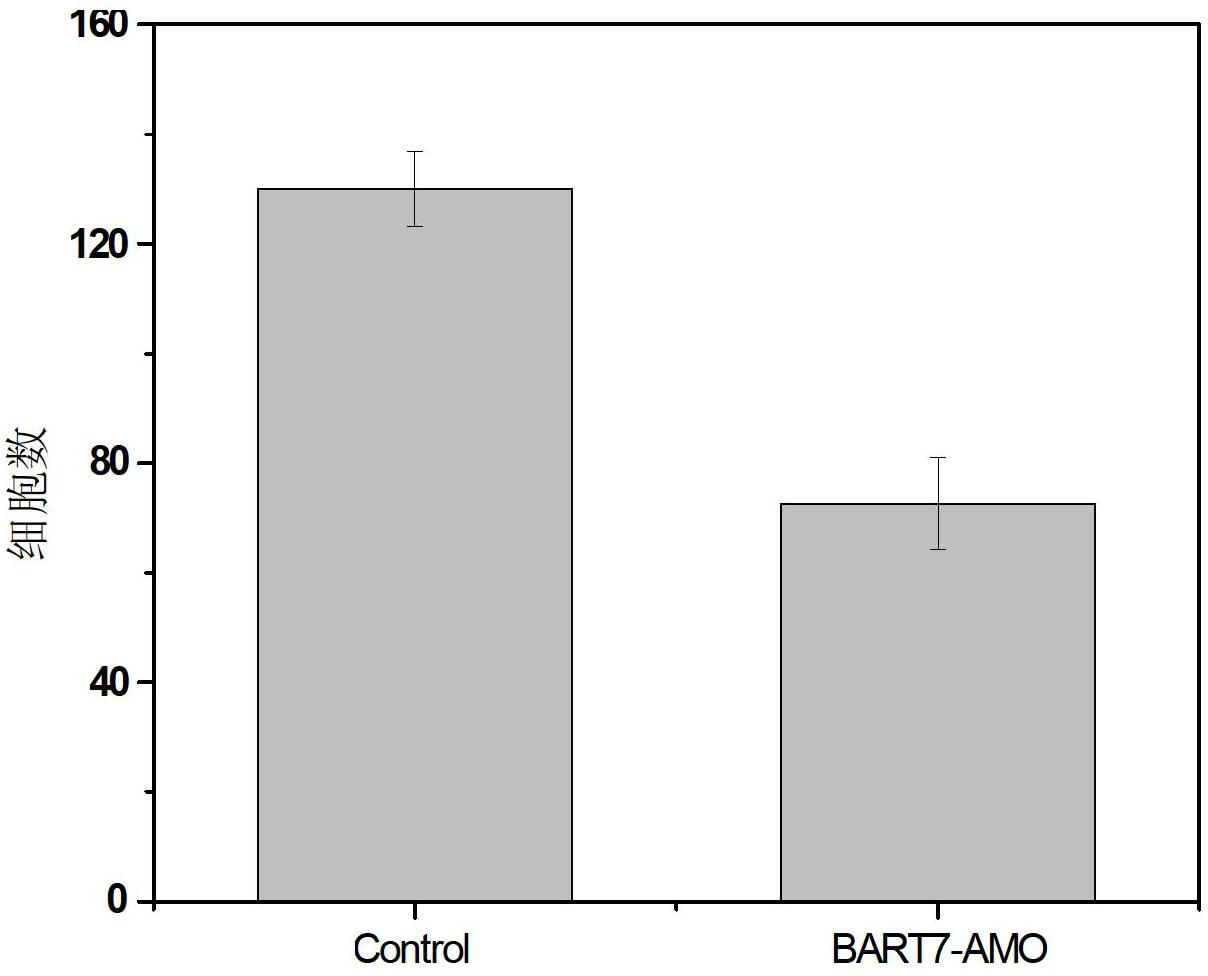
Application of EB virus miR-BART3 antisense oligonucleotides in preparing medicament for treating nasopharyngeal darcinoma
InactiveCN102188719AProlong the action timeNon-immunogenicOrganic active ingredientsGenetic material ingredientsNasopharyngeal carcinomaMiRNA binding
The invention relates to a medical application of EB (Epstein-Barr) virus miR-BART3 antisense oligonucleotides, in particular relating to an application of EB virus miR-BART3 antisense oligonucleotides in preparing a medicament for treating nasopharyngeal darcinoma, wherein the sequence of the EB virus miR-BART3 antisense oligonucleotides is ACACCUGGUGACUAGUGGUGCG (SEQ ID NO:1). The EB virus miR-BART3 antisense oligonucleotides can be effectively combined with mature miRNA of the EB virus to block the expression of the miRNA and corresponding regulation action thereof, thus restraining the invasion and metastasis of nasopharyngeal darcinoma cells; and the miRNA has no immunogenifcity, thus being favorable for being further applied in preventing the recurrence and metastasis of the nasopharyngeal darcinoma. The medicament can be used for protecting the antisense oligonucleotides from being degraded by nuclease and prolonging the action time, has higher transfection effciciency than that of commodity liposome, and is favorable for further practical clinical development and application.
Owner:SOUTHERN MEDICAL UNIVERSITY
Artificial antigen and kit for combined detection of eb virus rta protein antibody and eb virus early antigen ea antibody
ActiveCN104086657BSimple stepsImprove understandingMicroorganism based processesBiological testingTrue positive rateNasopharyngeal carcinoma
The invention relates to the technical fields of biotechnology, medical immunology and in vitro serological diagnosis, and particularly relates to an artificial antigen and a kit for joint detection of an Rta protein antibody of an epstein-barr (EB) virus and an early antigen ethyl acrylate (EA) antibody of the EB virus. The Rta protein antibody of the EB virus and the early antigen EA antibody existing in a to-be-detected sample can be accurately, efficiently, sensitively and specifically detected by the artificial antigen provided by the invention. By adopting the kit prepared from the artificial antigen, compared with the traditional detection method, the sensitivity and the specificity of nasopharynx cancer diagnosis are improved, the detection time is shortened, a patient can be timely treated, and the pain is relieved.
Owner:同昕生物技术(北京)有限公司
Compounds with EB (Epstein-Barr) virus and Kaposi's sarcoma-associated herpesvirus (KSHV) resisting functions in Hypericum japonicum, and preparation method and application thereof
The invention provides compounds with EB (Epstein-Barr) virus and Kaposi's sarcoma-associated herpesvirus (KSHV) resisting activities and sources thereof, and a separation purification method and application of the compounds. The compounds 1-18 are separated and purified from Hypericum japonicum, wherein the compounds 1-10 are separated and purified from Hypericum japonicum collected in Dabieshan in Qichun County of Hubei Province in October, and the compounds 11-18 are separated and purified from Hypericum japonicum collected in Lushan in Lushan City of Jiangxi province. The research of the antivirus activities of the 18 compounds on the two tumorigenic herpesviruses detects that the compounds 1, 3, 4, 7 and 8 have inhibiting activities for DNA replication of EB virus; and the compounds 3, 6 and 13-17 have inhibiting activities for replication of KSHV in the pyrolysis stage.
Reaction carrier and kit for detecting Epstein Barr (EB) virus replication and transcription activator (Rta)-immunoglobulin G (IgG) antibody
The invention discloses a reaction carrier and a kit for detecting an Epstein Barr (EB) virus replication and transcription activator (Rta)-immunoglobulin G (IgG) antibody. The reaction carrier for immunological detection of the EB virus Rta-IgG antibody is coated with gene recombinant EB virus Rta protein, and the gene recombinant EB virus Rta protein is obtained by expression of Chinese hamster ovary (CHO) cell line CHO / RTA with the preservation number of China General Microbiological Culture Collection Center (CGMCC) 6955. The kit comprises the reaction carrier. Clinical experiments show that the kit for the quantitative detection of the EB virus Rta-IgG is easy and convenient to operate, high in sensitivity and good in specificity.
Owner:同昕生物技术(北京)有限公司
Application of EB virus miR-BART3 antisense oligonucleotides in preparing medicament for treating nasopharyngeal darcinoma
InactiveCN102188719BProlong the action timeNon-immunogenicGenetic material ingredientsGene therapyNasopharyngeal carcinomaMiRNA binding
The invention relates to a medical application of EB (Epstein-Barr) virus miR-BART3 antisense oligonucleotides, in particular relating to an application of EB virus miR-BART3 antisense oligonucleotides in preparing a medicament for treating nasopharyngeal darcinoma, wherein the sequence of the EB virus miR-BART3 antisense oligonucleotides is ACACCUGGUGACUAGUGGUGCG (SEQ ID NO:1). The EB virus miR-BART3 antisense oligonucleotides can be effectively combined with mature miRNA of the EB virus to block the expression of the miRNA and corresponding regulation action thereof, thus restraining the invasion and metastasis of nasopharyngeal darcinoma cells; and the miRNA has no immunogenifcity, thus being favorable for being further applied in preventing the recurrence and metastasis of the nasopharyngeal darcinoma. The medicament can be used for protecting the antisense oligonucleotides from being degraded by nuclease and prolonging the action time, has higher transfection effciciency than that of commodity liposome, and is favorable for further practical clinical development and application.
Owner:SOUTHERN MEDICAL UNIVERSITY
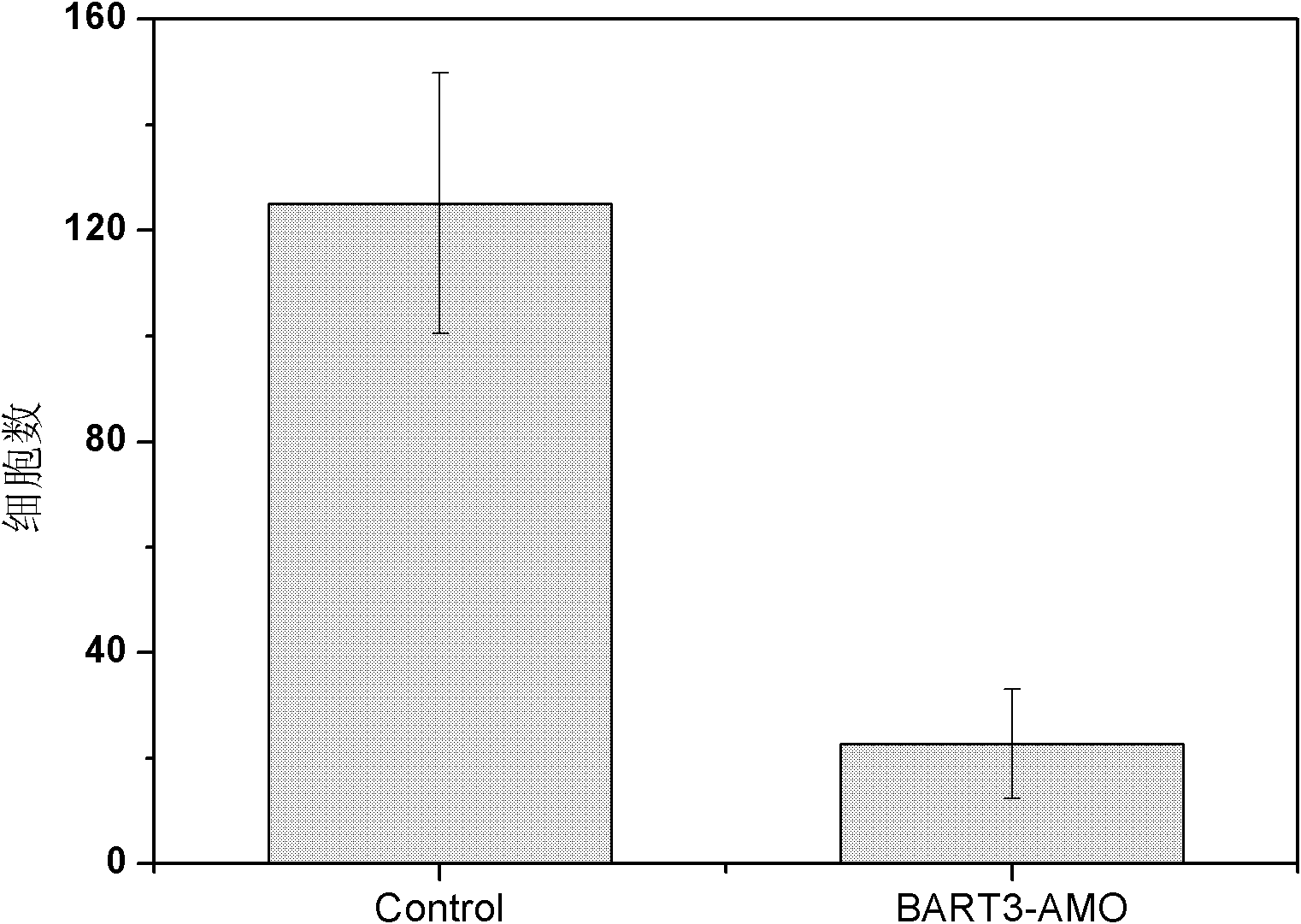
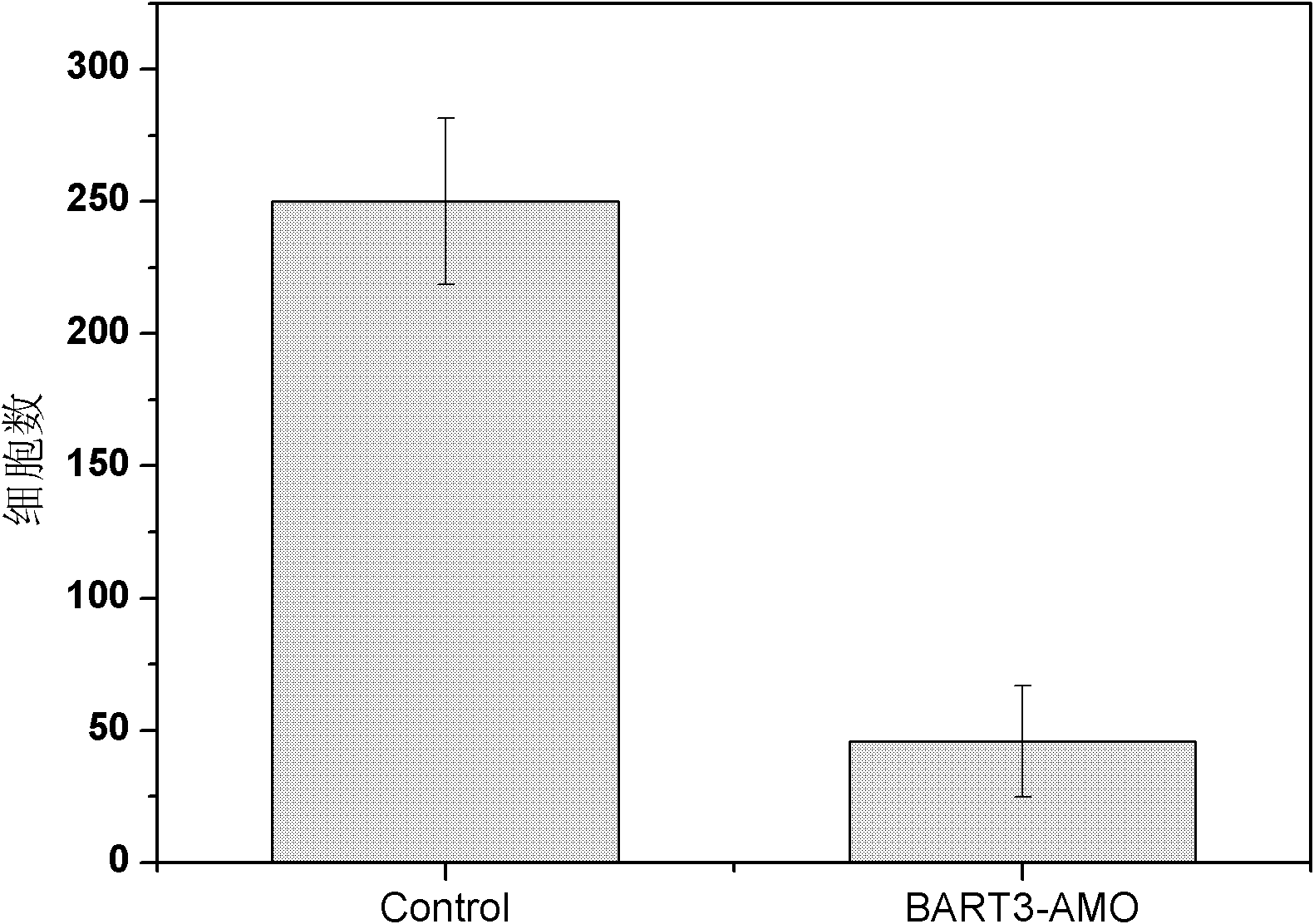
Application of Epstein barr (EB) virus miR-BART7 antisense oligonucleotide in preparing medicine capable of treating nasopharynx cancer
InactiveCN102641509AProlong the action timeNon-immunogenicPowder deliveryGenetic material ingredientsNucleotideNasopharyngeal carcinoma
The invention relates to a medicine application of Epstein barr (EB) virus miR-BART7 antisense oligonucleotide, in particular to an application of EB virus miR-BART7 antisense oligonucleotide in preparing medicine capable of treating nasopharynx cancer. The antisense oligonucleotide has a sequence of CCCUGGACACUGGACUAUGAUG (SEQ ID NO:1). The antisense oligonucleotide disclosed by the invention can be effectively combined with the mature mi ribonucleic acid (RNA) of the EB virus to block EB virus miR-BART7 expression and the corresponding regulation action of the EB virus miR-BART7 so as to prevent the nasopharynx cancer cell from invading and transferring. The miRNA does not have immunogenicity and is favorable for further use for preventing the nasopharynx cancer from recurring and transferring. According to the medicine which embodies the application disclosed by the invention, the antisense oligonucleotide can be prevented from degrading by nuclease, the action time is prolonged, and the medicine has higher transfection efficiency than a commercial liposome and is favorable for further clinic practical development and application.
Owner:SOUTHERN MEDICAL UNIVERSITY
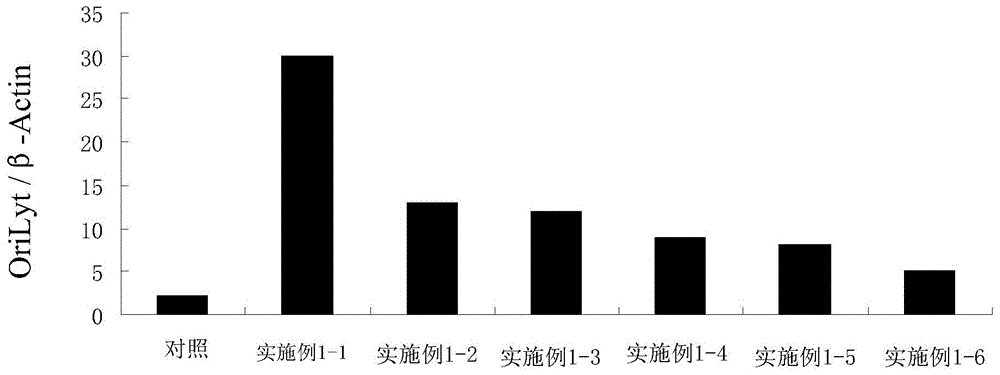
Application of camptothecin in preparation of medicine resisting to EB (epstein-barr) virus in lysis period
InactiveCN104666301AImprove replication efficiencyInhibition of replicationOrganic active ingredientsAntiviralsInfected cellLysis
The invention relates to the field of antiviral drugs and in particular discloses application of camptothecin in preparation of a medicine resisting to an EB (epstein-barr) virus in a lysis period and application of camptothecin in preparation of a medicine used for preventing cancer caused by EB virus infection. According to the application, early-stage EB virus infected cells and immediate early-stage EB virus infected cells are constructed and are treated by the camptothecin. The application of the camptothecin in preparation of the medicine resisting to the EB virus in the lysis period has the beneficial effects that a new medical application of the camptothecin is explored, and a new application field is expanded; and combination between Zta protein of the EB virus in the lysis period and OriLyt can be obviously inhibited, so that the camptothecin has a good medicinal prospect.
Owner:SHENZHEN INST OF ADVANCED TECH
Epstein-Barr virus vca/na1-iga antibody combined detection reagent and preparation method thereof
The invention provides an EB (epstein-barr) virus VCA / NA1-IgA antibody joint detection reagent. The EB virus VCA / NA1-IgA antibody joint detection reagent at least comprises a reaction membrane, an antigen pad and a gold label pad, wherein a detection line and a quality control line are labeled on the reaction membrane; the detection line contains a rat anti-human IgA monoclonal antibody; the quality control line contains biotin; the antigen pad contains a recombinant EB virus VCA antigen labeled with the biotin and a recombinant EB virus NA1 antigen labeled with the biotin; and the gold label pad contains an avidin compound containing a colloid gold label. The joint detection reagent provided by the invention is used for carrying out joint detection on VCA and NA1 so that the cost can be saved; the application and the popularization of the detection reagent are facilitated and the detection cost is reduced; and the EB virus VCA / NA1-IgA antibody joint detection reagent has the characteristics of rapidness, simplicity and convenience, accuracy and high sensitivity.
Owner:中山生物工程有限公司
Method for detecting EB (Epstein-Barr) virus, quantum dot labelled immunochromatographic test strip and preparation method thereof
ActiveCN103543262BHigh detection sensitivityNarrow emission peakBiological material analysisCelluloseGlass fiber
The invention relates to a medical immunodetection method and in particular relates to a method for detecting an EB (Epstein-Barr) virus by an immunological method by using a quantum dot labelled immunochromatographic test strip. The quantum dot labelled immunochromatographic test strip is characterized in that a glass cellulose membrane A, a glass cellulose membrane B of a quantum dot labelled EB virus IgA (immunoglobulin A) monoclonal antibody, a nitrocellulose membrane and absorbent paper are stuck to a plastic board from bottom to top in sequence, wherein one end of the nitrocellulose membrane is provided with an EB virus polyclonal antibody and a rabbit anti-mouse second antibody, thereby forming a detection zone T and a quality control zone C; the quantum dot labelled EB virus IgA monoclonal antibody is arranged at the other end of the glass cellulose membrane B, corresponds to the detection zone T and the quality control zone C and is arranged at one end of a sampling point. The detection sensitivity of the method is about 1000 times higher than that of the detection method frequently used at present.
Owner:北京华卫天和生物科技有限公司
A kind of pcr enzyme-linked two-hybrid method detects pathogenic microorganism detection method
ActiveCN104726606BConvenient and flexible useFlexible solid phase junctionMicrobiological testing/measurementMultinucleate giant cellDNA hybridisation
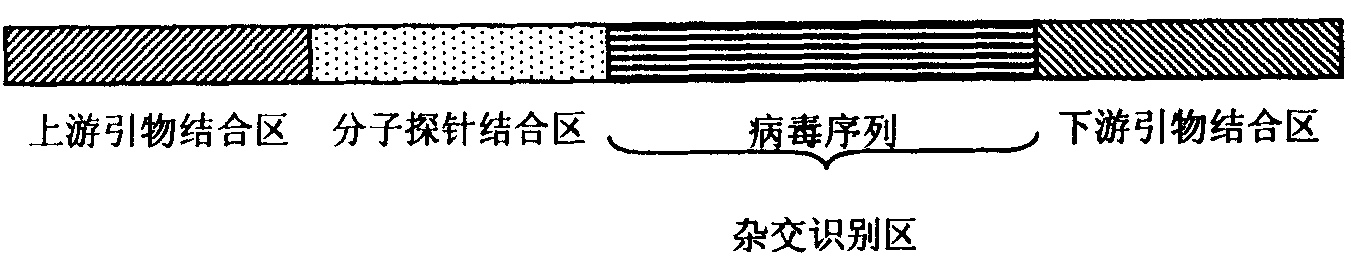
The invention provides a method for detecting pathogenic microorganisms by using a PCR (polymerase chain reaction) enzyme-linked double-cross method, belongs to the technical field of clinical medical microorganism detection, and particularly relates to a synchronous qualitative and quantitative detection method for various microorganisms. In the method, DNA (deoxyribonucleic acid) amplification, DNA hybridization and enzyme-linked immunosorbent assay reaction are used, a solid-phase surface enveloping technology is adopted, a specificity conservative segment is amplified, an amplified product is combined to a capturing probe and is adsorbed to a solid-phase surface, far-end hybridization of an enzyme-linked probe is carried out, and a quantitative color product is generated by specific antibody recognition and reaction of enzyme and a substrate. The detected pathogenic microorganisms comprise EB (Epstein Barr) viruses, giant cell and cell viruses, herpes simplex viruses 1 and herpes simplex viruses 2. Various microorganisms of a clinical sample are detected simultaneously, and four types of viruses are screened by a reaction. The method has the advantages that the time and the cost are saved, application is flexible, the repeatability, the stability, the sensitivity and the specificity are high, and the method is simple and is easy to implement. Moreover, the method is suitable for widely detecting pathogenic microorganisms and is particularly suitable for a basic medical institution.
Owner:NANJING AIPEIJIE BIOLOGICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com