Primer, reagent kit and method for detecting EB (Epstein-Barr) virus
An Epstein-Barr virus and kit technology, which is applied in the directions of biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc., to achieve the effect of saving time, fast detection process, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 : DNA sample preparation
[0041] In this embodiment, a human blood sample is used to prepare a DNA sample used in the detection method of the present invention, which is described in detail as follows.
[0042] 1. Extraction reagent:
[0043] Solution A: cell lysate, 15ml / bottle, 200μl each time.
[0044]
[0045] Component Final Concentration
[0046]
[0047] Tris-HCl (pH8.0) 0.01mol / l
[0048] NaCl 0.1mol / l
[0049] EDTA (pH8.0) 0.01mol / l
[0050] SDS 2% (g / ml)
[0051]
[0052] Solution B: Nucleic acid binding solution, 25ml / bottle, 400μl each time.
[0053]
[0054] Component Final Concentration
[0055]
[0056] GuSCN 5mol / l
[0057] Tris-HCl (pH6.4) 0.05mol / l
[0058] EDTA(pH8.0) 0.02mol / l
[0059] Triti...
Embodiment 2
[0083] Example 2 : Determination of internal reference primer sequence
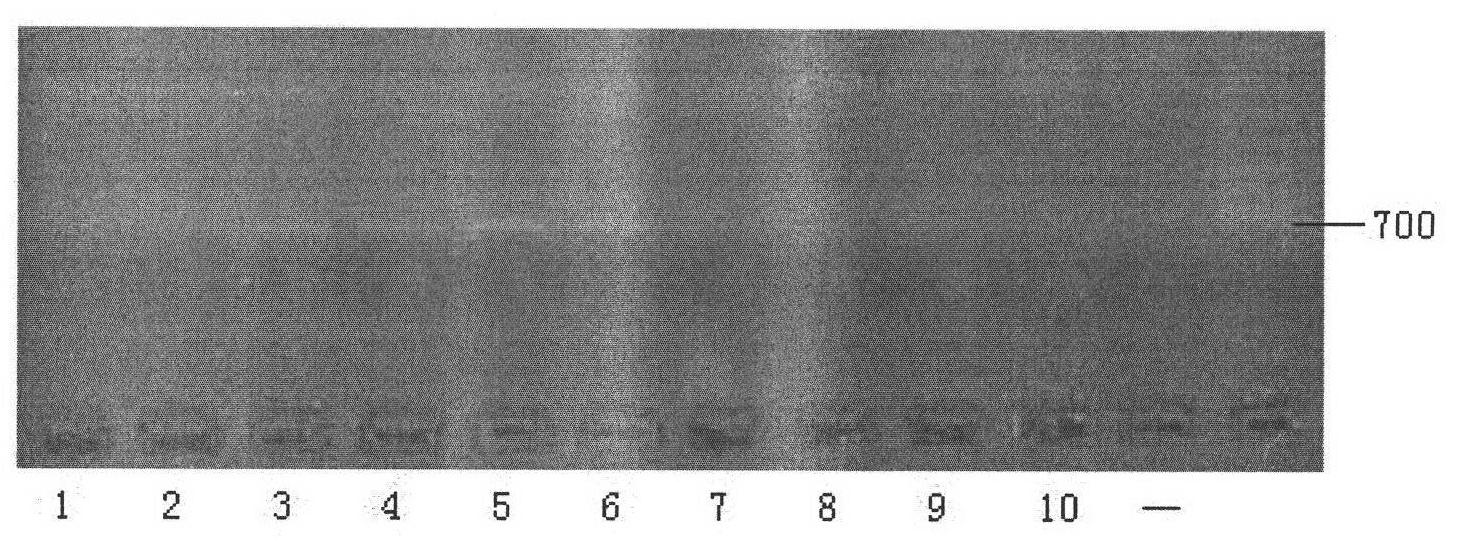
[0084] In this example, two pairs of DNA amplification primers designed according to the human DNA conserved sequence β-globin-specific gene were used to amplify the DNA sample prepared in Example 1, and the amplification effects of the two pairs of primers were detected by gel electrophoresis to screen out Internal reference primers.
[0085] 1. Single primer PCR amplification
[0086] 1) Prepare a 25 μl reaction system in a 200 μL Eppendorf tube as follows, in which the extracted DNA samples are DNA from 10 different blood samples:
[0087]
[0088] Component Volume / μl
[0089]
[0090] wxya 2 O 18.3
[0091] 5U / μl Taq enzyme 0.2
[0092] Taq enzyme 10×buffer 2.5
[0093] 10μM internal reference upstream and downstream primers 0.5 each
[0094] 2.5mM dNTP2
[0095] Extracted DNA sample 1
[0096] ...
Embodiment 3
[0127] Example 3 : Amplify DNA using a primer mix
[0128] In this example, the primer composition provided by the present invention was used to amplify the DNA sample extracted in Example 1, and gel electrophoresis was used to detect the correctness of the PCR product.
[0129] 1. PCR amplification with mixed primers
[0130] a) Prepare a 25 μl reaction system in a 200 μL Eppendorf tube:
[0131]
[0132] Composition Volume (μl)
[0133]
[0134] wxya 2 O 10.3
[0135] 2×buffer 12.5
[0136] Primer composition 1.2
[0137] Extracted clinical sample DNA 1
[0138]
[0139] Note: 2×buffer contains Tris-HCl (pH 8.4) 40mM, MgCl 2 20mM, KCl 50mM, (NH) 2 SO 4 10mM, each dNTP 0.2mM, Taq DNA polymerase 0.08U / μL, the primer composition contains: B297 upper and lower primers 0.2μM each, EBV upper and lower primers 0.4μM each. The specific seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com