Method for purifying docetaxel
A docetaxel and purification method technology, applied in the field of drug synthesis, can solve problems such as difficult purification, cumbersome operation, complex structure, etc., achieve high yield and improve product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
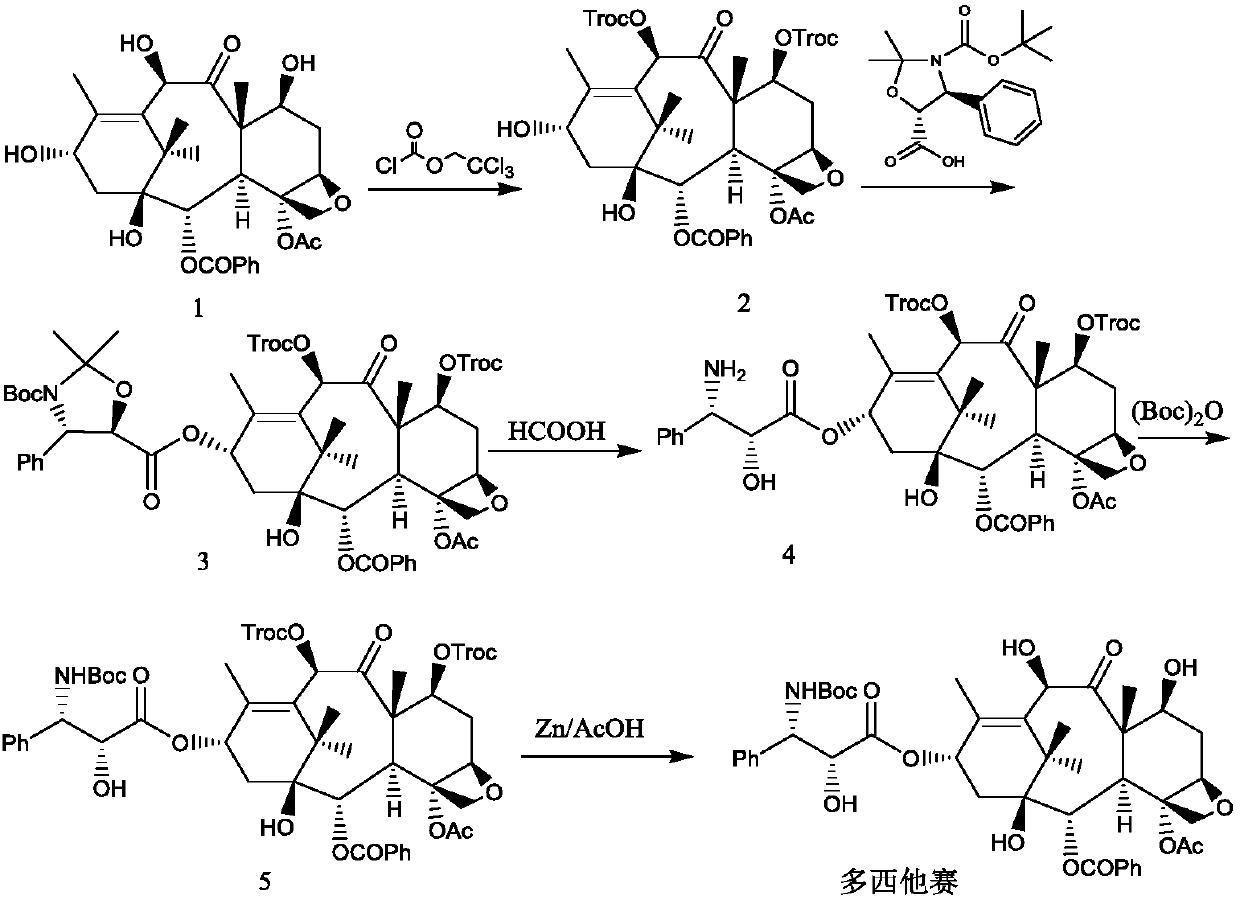
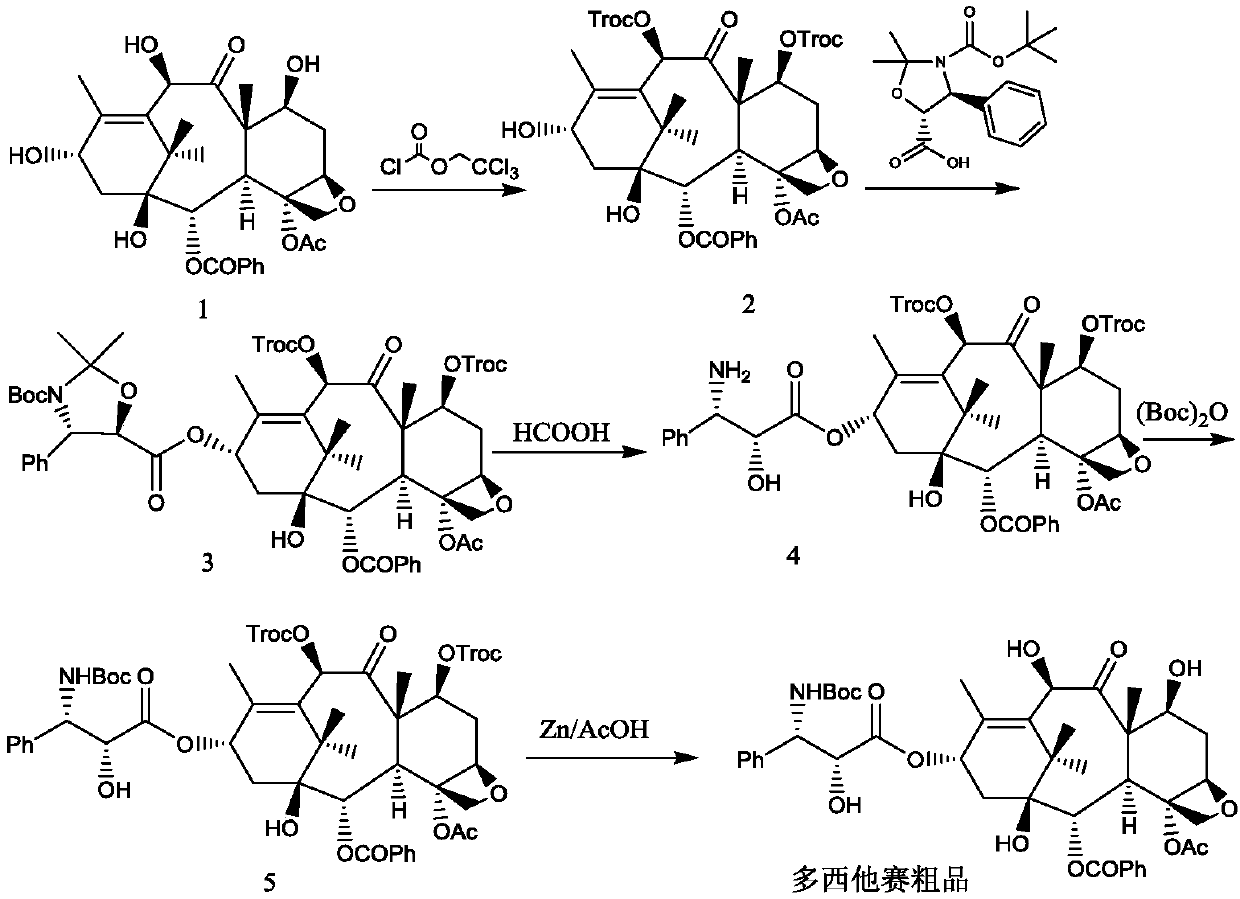
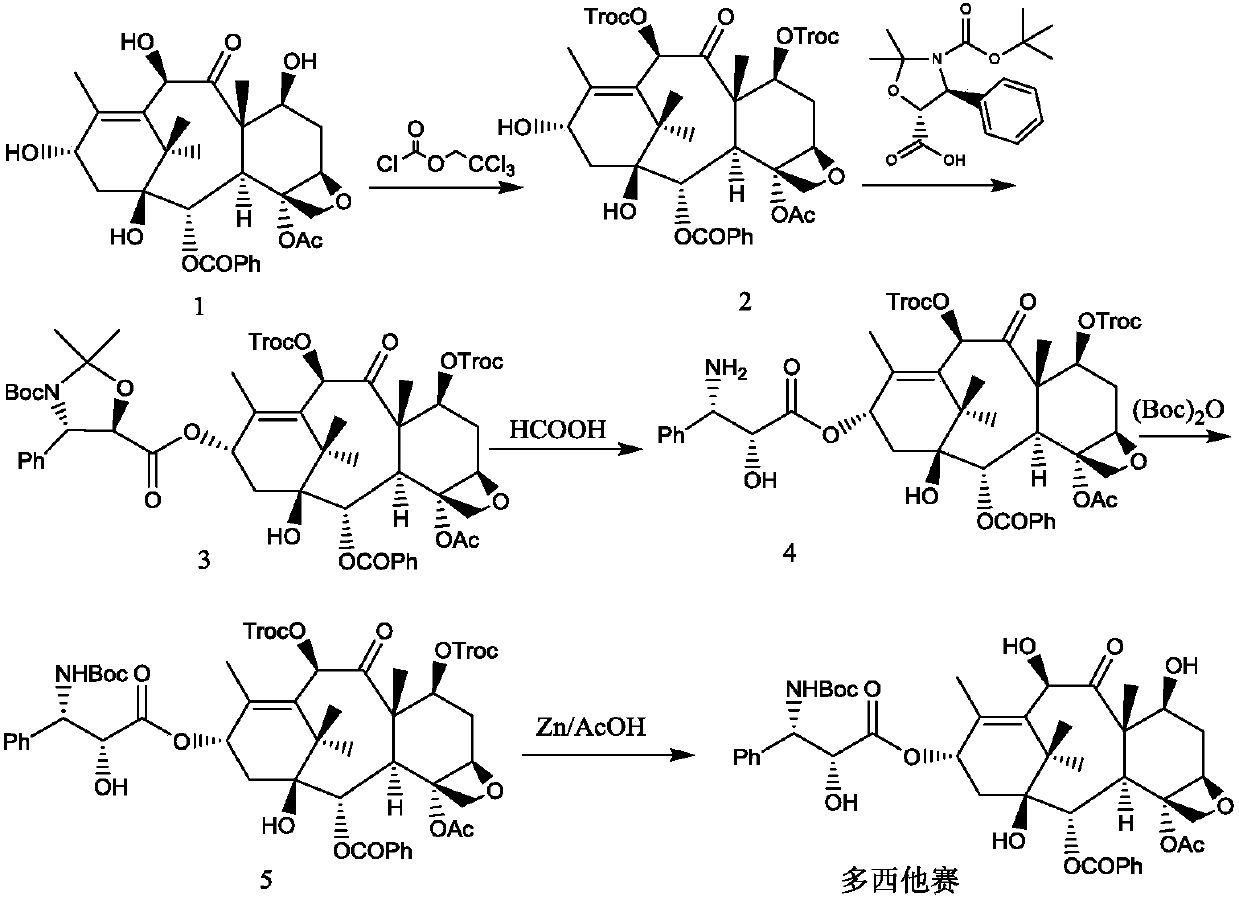
[0038] Reference Example 1: Intermediate 2
[0039]
[0040] Add 900 g of 10-deacetylbaccatin III (compound 1) and 4.5 kg of dried pyridine into the reaction flask under stirring, and control the temperature of the feed liquid at 0-10°C, slowly add trichloroethyl chloroformate dropwise into the feed liquid 980.1 g of ester, after dropping, keep the feed liquid temperature at 0-10° C. for 2 hours, monitor by TLC, stop the reaction after the reaction is complete.
[0041] Add 7.2L of dichloromethane, control the temperature of the feed liquid at 10-25°C, slowly add 6mol / L hydrochloric acid dropwise, adjust the pH of the feed liquid to 2-3, let stand and separate the layers, back-extract the water phase with 3.6L of dichloromethane, and combine The organic phase was washed with purified water, collected, dried over anhydrous sodium sulfate, and suction filtered. The filtrate was collected and concentrated under reduced pressure to about 1 / 3 of the volume of the raw material s...
reference example 2
[0042] Reference Example 2: Intermediate 3
[0043]
[0044] Add 15.1L of dichloromethane, 1370g of intermediate 2, (4S,5R)-3-tert-butoxycarbonyl-2,2-dimethyl-4-phenyloxazolidine-5-acid in sequence into a 30L glass reaction tank 589.8g and 97.3g of 4-dimethylaminopyridine were cooled to 5-15°C under the protection of nitrogen. Control the feed liquid temperature at 15-25°C, and add 787.8 g of N,N'-dicyclohexylcarbodiimide in batches. After the injection was completed, after stirring and reacting at 15-25°C for 1 hour, the reaction progress was tracked by TLC, and the reaction was stopped when the intermediate 2 basically reacted completely. Suction filtration, dichloromethane 2.7L wash filter cake. The filtrate was concentrated under reduced pressure at 20-30°C to nearly dryness. Add 7.1 L of anhydrous methanol, and concentrate under reduced pressure at 10-20°C until there is basically no dichloromethane in the feed solution. Stir and crystallize at -5 to 5°C for 1 hour...
reference example 3
[0045] Reference Example 3: Intermediate 4
[0046]
[0047] Add 1456g of crushed intermediate 3 and 14.6L of formic acid into a 30L glass reaction tank, and stir and react at 25-35°C. After reacting for 1 hour, TLC followed the reaction process, and stopped the reaction when the raw materials basically reacted completely. Add 26.2kg of ethyl acetate and saturated sodium chloride solution, then slowly add 5.5% sodium carbonate aqueous solution, after the addition, let it stand for stratification, extract the aqueous phase with 8.6kg of ethyl acetate in two equal parts, combine the organic layers, add 7.6 % sodium carbonate aqueous solution, adjust the pH to 7-8, wash the organic phase once with saturated sodium chloride solution, collect the organic phase, and dry over anhydrous sodium sulfate. Suction filtration, the filtrate was collected, and the filtrate was concentrated to about 14.6 L under reduced pressure at 45-50°C to obtain an ethyl acetate solution of intermedia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com