Industrial synthesis method of water-soluble docetaxel derivative
A technology of docetaxel and synthetic method, which is applied in the direction of organic chemistry, can solve the problems of high production cost, achieve the effects of reducing solvent consumption, ensuring product quality, product yield and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
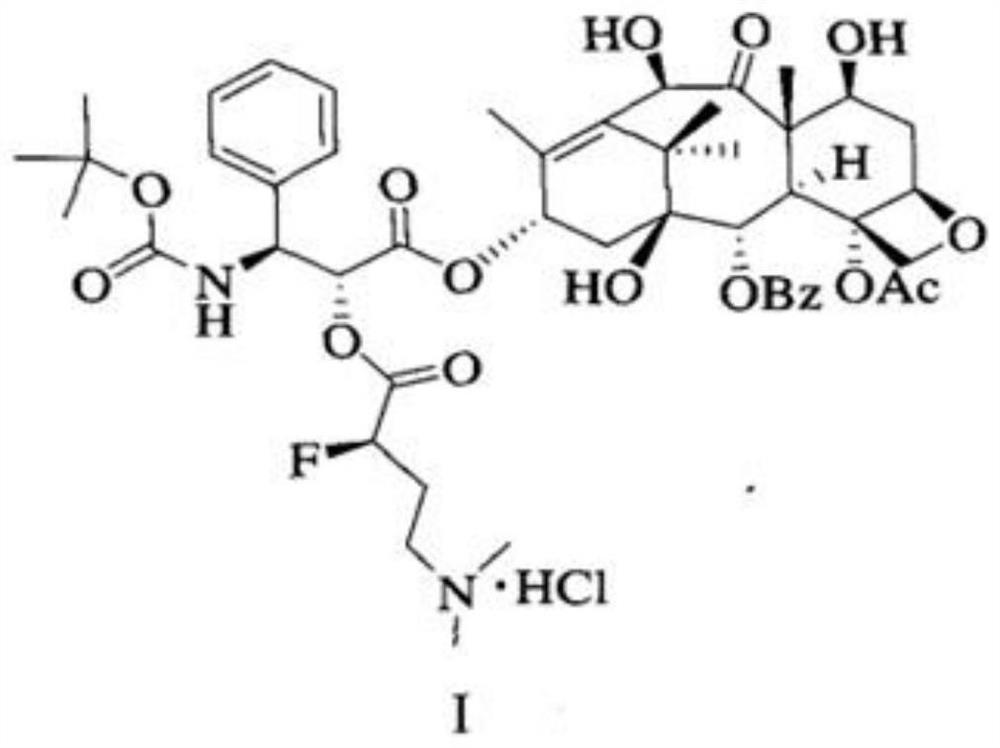
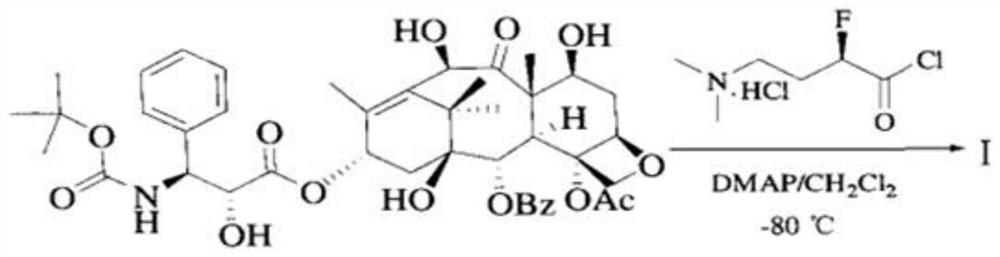
[0088] An industrial synthesis method of a water-soluble docetaxel derivative, comprising:
[0089] (R)-4-dimethylamino-2-fluoro-butyric acid hydrochloride synthesis steps; and water-soluble docetaxel synthesis steps.
[0090] (R)-4-dimethylamino-2-fluoro-butyric acid hydrochloride synthetic steps are as follows:
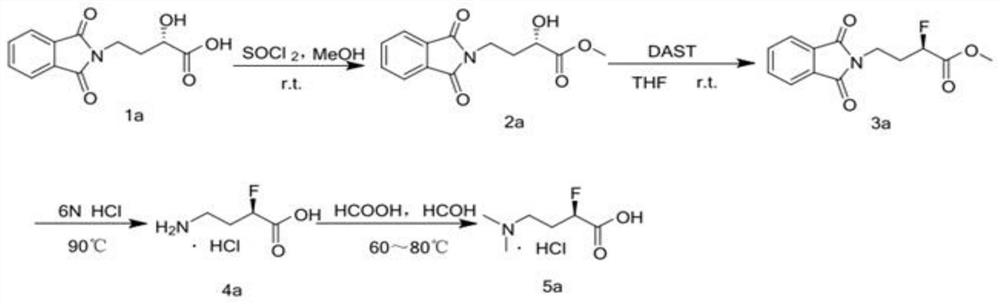
[0091] S1: Put α(-S)-hydroxy-γ-phthalimide-butyric acid into 2000L No. 1 reaction kettle, pump methanol into it, stir for 30min, then add thionyl chloride and stir at room temperature React to complete;
[0092] S2: the reaction solution is sent to No. 1 concentrator for concentration, centrifugal precipitation, and solvent recovery to obtain dry extract A;
[0093] S3: the dry extract A is loaded into No. 1 extraction kettle of 3000L, pumped into ethyl acetate for extraction, then pumped into saturated sodium bicarbonate solution for washing 2 times, and collecting and merging the washing liquid;
[0094] S4: pump the washing liquid into No. 1 liquid-liquid cent...
Embodiment
[0361] (R)-4-dimethylamino-2-fluoro-butyric acid hydrochloride synthetic steps are as follows:
[0362] (1), 2a synthetic process steps
[0363] a. Put 83.66 kg of α(-S)-hydroxyl-γ-phthalimide-butyric acid into a 2000L No. 1 reaction kettle, pump 1004L of methanol, stir for 30min, and then add dichloromethylene Sulfone 36.42L stirred and reacted at room temperature until complete;
[0364] b. Send the reaction solution to No. 1 concentrator for concentration, centrifuge precipitation, and recover the solvent to obtain dry filter cake A;
[0365] c. Put the dry filter cake A into No. 1 extraction kettle of 3000L, pump into 1673L of ethyl acetate for extraction, then pump into 502L of saturated sodium bicarbonate solution to wash twice, and collect the combined washing liquid;
[0366] d. Pump the washing liquid into the No. 1 liquid-liquid centrifugal extractor to separate the organic phase and the aqueous phase;
[0367] e. The organic phase was filtered, and the filtrate w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com