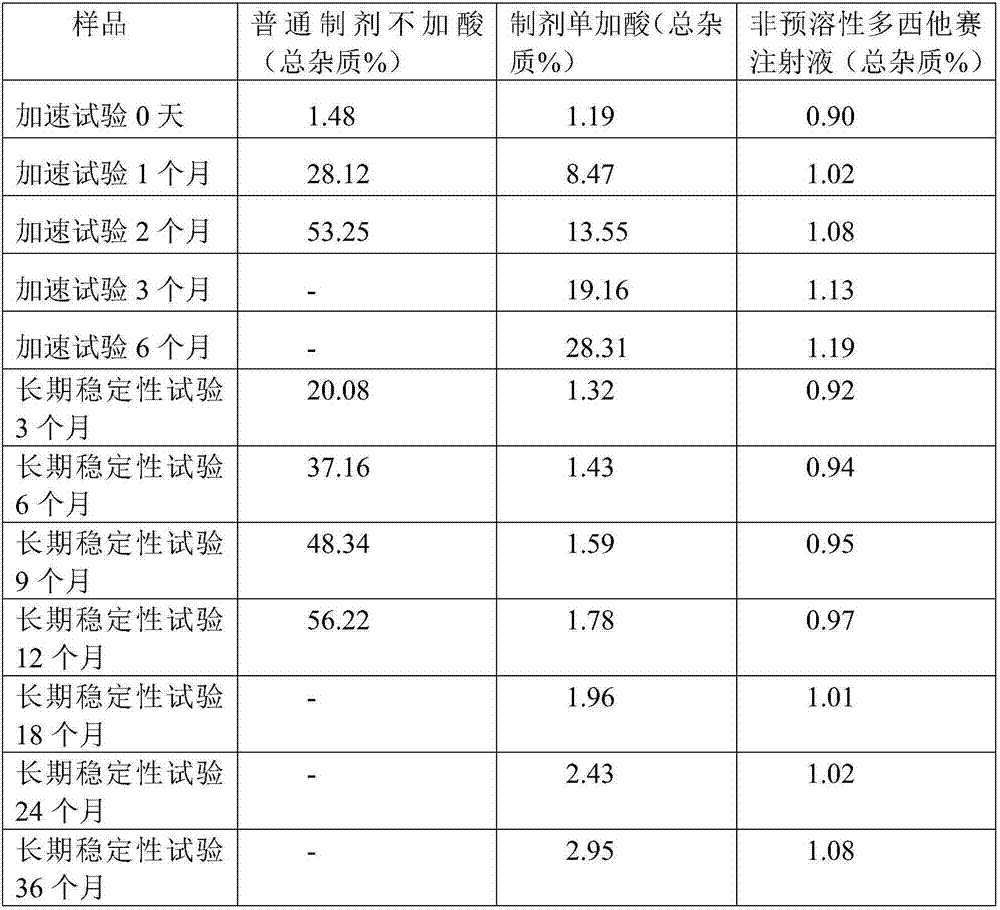
Non-predissolved Docetaxel injection
A technology of docetaxel and injection, applied in the direction of organic active ingredients, medical preparations of non-active ingredients, drug combinations, etc., can solve the problems of long time, increased bacterial contamination, large energy consumption, etc., to reduce and Effects of investment, increased safety, increased speed and degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, 0.8g citric acid, 0.16g sodium citrate are dissolved in 45g absolute ethanol, while stirring, add 1g docetaxel, 30g polysorbate 80 for injection, 1g active carbon for injection successively, after feeding , Stir and mix for 30 minutes, fully dissolve, first filter with titanium rod filter 30um, titanium rod filter 3um to remove carbon, then filter with 1.0μm polytetrafluoroethylene microporous membrane, 0.22μm polytetrafluoroethylene microporous Membrane sterilization and filtration, and finally, after filtration with a 0.22um microporous membrane, the filtrate was divided into vials, stoppered, capped, labeled, and packaged to make 100ml of non-predissolved docetaxel injection.
Embodiment 2
[0033] Embodiment 2, with 0.6g citric acid, 0.12g sodium citrate are dissolved in 37.5g dehydrated alcohol, add 2g docetaxel, 42.5g polysorbate 80 for injection, 2g active carbon for injection successively while stirring, After the feeding is completed, stir and mix for 30 minutes to fully dissolve. First, use a titanium rod filter 30um, a titanium rod filter 3um to remove carbon and filter, and then filter with a 1.0μm polytetrafluoroethylene microporous filter membrane, and a 0.22μm polytetrafluoroethylene filter. Sterile filtration with ethylene microporous membrane, and finally filter with 0.22um common microporous membrane, divide the filtrate into vials, cork, cap, label, pack, and make 100ml non-predissolved docetaxel Injection.
Embodiment 3
[0034] Embodiment 3, 0.4g citric acid, 0.08g sodium citrate are dissolved in 30g dehydrated alcohol, add 3g docetaxel, 55g polysorbate 80 for injection, 3g active carbon for injection successively while stirring, after feeding , Stir and mix for 30 minutes, fully dissolve, first filter with titanium rod filter 30um, titanium rod filter 3um to remove carbon, then filter with 1.0μm polytetrafluoroethylene microporous membrane, 0.22μm polytetrafluoroethylene microporous Membrane sterilization and filtration, and finally, after filtration with a 0.22um microporous membrane, the filtrate was divided into vials, stoppered, capped, labeled, and packaged to make 100ml of non-predissolved docetaxel injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com