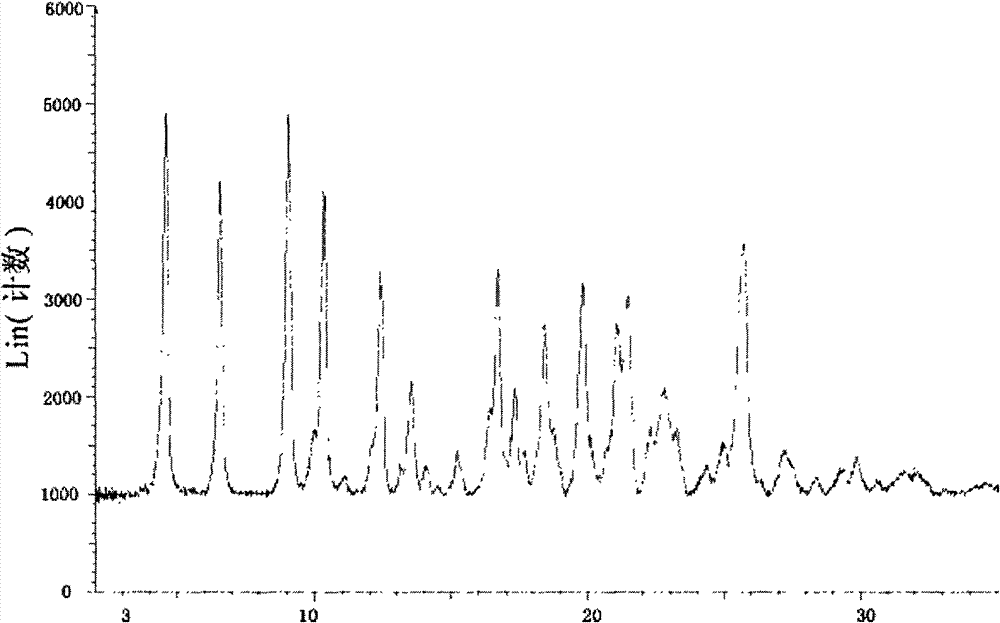
Flumatinib mesylate crystal form A and preparation method and use thereof
A technology of flumatinib mesylate and its crystal form is applied in the application field of preparing medicines for the treatment of chronic myeloid leukemia, and can solve the problems of inability to combine with Gleevec, structural changes, and the occurrence of chronic myeloid leukemia patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Put 1.0 g of flumatinib mesylate and 8.0 ml of isopropanol in a reaction flask, heat to reflux, and dissolve completely quickly, then naturally cool to room temperature under stirring conditions, stir for 24 hours, precipitate a solid, filter, Drying in vacuo yielded Form A.
Embodiment 2
[0018] Put 100g of flumatinib mesylate and 800ml of isopropanol in a reaction flask, heat to reflux, dissolve completely quickly, cool to room temperature naturally under stirring conditions, stir for 24 hours, precipitate solid, filter, and vacuum dry Form A was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com